APTES-Modified SBA-15 as a Non-Toxic Carrier for Phenylbutazone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
Mesoporous Material Preparation and Surface Modification
2.2. Phenylbutazone Adsorption Studies
2.3. Characterization Methods
2.3.1. Powder X-ray Diffraction (XRD)
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Nitrogen Adsorption/Desorption
2.3.4. Transmission and Scanning Electron Microscopy (TEM/SEM)
2.3.5. Fourier Transformed Infrared Spectroscopy (FTIR)
2.3.6. Spectrophotometry
2.3.7. Solid-State 1H-NMR
2.4. Drug Release Studies
2.5. Cytotoxicity Study
2.5.1. Cell Culture
2.5.2. Cell Viability
2.6. Statistical Analysis
3. Results and Discussion
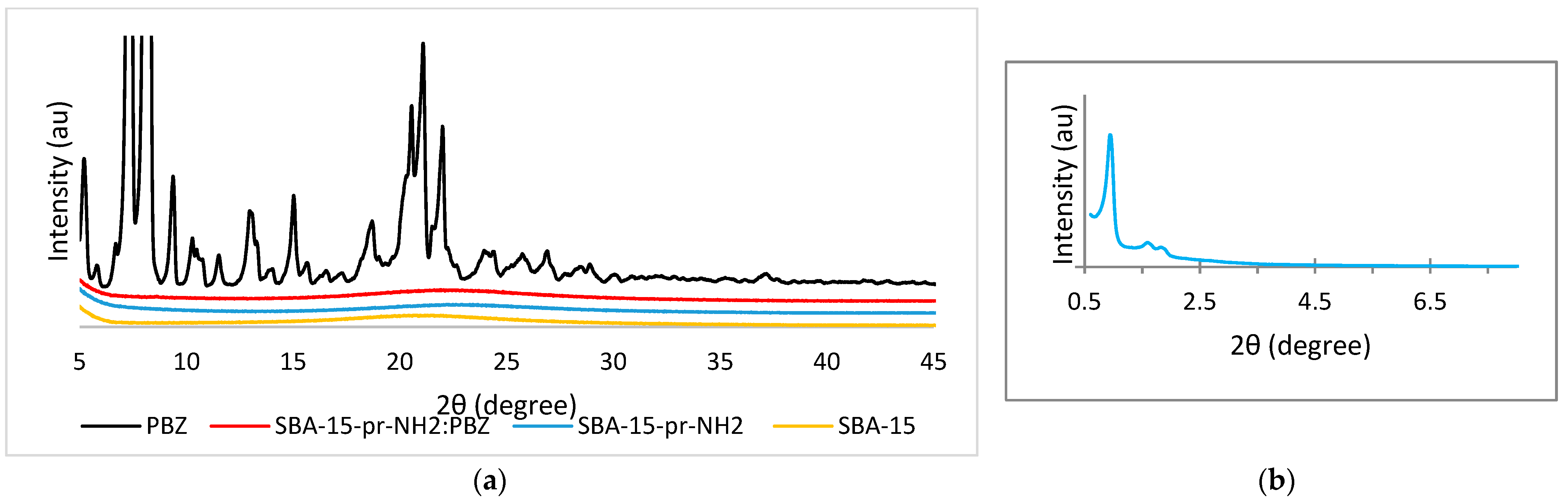
3.1. Powder X-ray Diffraction
3.2. Differential Scanning Calorimetry
3.3. Nitrogen Adsorption/Desorption
3.4. Transmission Electron Microscopy
3.5. Scanning Electron Microscopy
3.6. Fourier Transformed Infrared Spectroscopy
3.7. Proton Nuclear Magnetic Resonance
3.8. Validation of UV Method
3.9. Drug Release Study
3.10. Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhary, V.; Sharma, S. An Overview of Ordered Mesoporous Material SBA-15: Synthesis, Functionalization and Application in Oxidation Reactions. J. Porous Mater. 2017, 24, 741–749. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravikovitch, P.I.; Neimark, A.V. Characterization of Micro- and Mesoporosity in SBA-15 Materials from Adsorption Data by the NLDFT Method. J. Phys. Chem. B 2001, 105, 6817–6823. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous Silica Nanoparticles for Drug and Gene Delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, L.; Monduzzi, M.; Salis, A. Adsorption and Release of Ampicillin Antibiotic from Ordered Mesoporous Silica. J. Colloid Interface Sci. 2017, 497, 217–225. [Google Scholar] [CrossRef]
- Alazzawi, H.F.; Salih, I.K.; Albayati, T.M. Drug Delivery of Amoxicillin Molecule as a Suggested Treatment for Covid-19 Implementing Functionalized Mesoporous SBA-15 with Aminopropyl Groups. Drug Deliv. 2021, 28, 856–864. [Google Scholar] [CrossRef]
- Krajnović, T.; Maksimović-Ivanić, D.; Mijatović, S.; Drača, D.; Wolf, K.; Edeler, D.; Wessjohann, L.A.; Kaluđerović, G.N. Drug Delivery System for Emodin Based on Mesoporous Silica SBA-15. Nanomaterials 2018, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Mellaerts, R.; Aerts, C.A.; Van Humbeeck, J.; Augustijns, P.; Van den Mooter, G.; Martens, J.A. Enhanced Release of Itraconazole from Ordered Mesoporous SBA-15 Silica Materials. Chem. Commun. 2007, 13, 1375–1377. [Google Scholar] [CrossRef]
- Žid, L.; Zeleňák, V.; Almáši, M.; Zeleňáková, A.; Szücsová, J.; Bednarčík, J.; Šuleková, M.; Hudák, A.; Váhovská, L. Mesoporous Silica as a Drug Delivery System for Naproxen: Influence of Surface Functionalization. Molecules 2020, 25, 4722. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.G.; Doustkhah, E.; Kirillova, M.V.; Rostamnia, S.; Mahmoudi, G.; Kirillov, A.M. Combining Ethylenediamine and Ionic Liquid Functionalities within SBA-15: A Promising Catalytic Pair for Tandem Cu–AAC Reaction. Appl. Catal. A Gen. 2017, 548, 96–102. [Google Scholar] [CrossRef]
- Worboys, M.; Toon, E. Phenylbutazone (Bute, PBZ, EPZ): One Drug across Two Species. Hist. Philos. Life Sci. 2018, 40, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Batchelor, H.; Hanson, P.; Perrie, Y.; Mohammed, A.R. Physicochemical Characterisation, Drug Polymer Dissolution and in Vitro Evaluation of Phenacetin and Phenylbutazone Solid Dispersions with Polyethylene Glycol 8000. J. Pharm. Sci. 2011, 100, 4281–4294. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.J.; Hanrahan, J.P.; Tobin, J.M.; Finch, C.V.; Parkes, G.M.B.; Ahmad, S.A.; Mohammad, F.; Saleem, M. Enhancing the Dissolution of Phenylbutazone Using Syloid® Based Mesoporous Silicas for Oral Equine Applications. J. Pharm. Anal. 2018, 8, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Speybroeck, M.V.; Barillaro, V.; Thi, T.D.; Mellaerts, R.; Martens, J.; Humbeeck, J.V.; Vermant, J.; Annaert, P.; Den Mooter, G.V.; Augustijns, P. Ordered Mesoporous Silica Material SBA-15: A Broad-Spectrum Formulation Platform for Poorly Soluble Drugs. J. Pharm. Sci. 2009, 98, 2648–2658. [Google Scholar] [CrossRef]
- Dadej, A.; Woźniak-Braszak, A.; Bilski, P.; Piotrowska-Kempisty, H.; Józkowiak, M.; Geszke-Moritz, M.; Moritz, M.; Dadej, D.; Jelińska, A. Modification of the Release of Poorly Soluble Sulindac with the APTES-Modified SBA-15 Mesoporous Silica. Pharmaceutics 2021, 13, 1693. [Google Scholar] [CrossRef] [PubMed]
- Group, I.E.W. Q 2 (R1) Validation of Analytical Procedures: Text and Methodology. Fed. Regist. 1995, 60, 11260. [Google Scholar]
- Surve, D.H.; Jindal, A.B. Development and Validation of Reverse-Phase High-Performance Liquid Chromatographic (RP-HPLC) Method for Quantification of Efavirenz in Efavirenz-Enfuvirtide Co-Loaded Polymer-Lipid Hybrid Nanoparticles. J. Pharm. Biomed. Anal. 2019, 175, 112765. [Google Scholar] [CrossRef]
- Mansilha, C.; Melo, A.; Rebelo, H.; Ferreira, I.M.P.L.V.O.; Pinho, O.; Domingues, V.; Pinho, C.; Gameiro, P. Quantification of Endocrine Disruptors and Pesticides in Water by Gas Chromatography-Tandem Mass Spectrometry. Method Validation Using Weighted Linear Regression Schemes. J. Chromatogr. A 2010, 1217, 6681–6691. [Google Scholar] [CrossRef] [Green Version]
- Stawny, M.; Gostyńska, A.; Dettlaff, K.; Jelińska, A.; Kościelniak, M.; Ogrodowczyk, M. Development, Validation, and Stability Assessment Application of RP-HPLC-DAD Method for Quantification of Ampicillin in Total Parenteral Nutrition Admixtures. Antibiotics 2019, 8, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, A.M.; Castel-Branco, M.M.; Falcão, A.C. Linear Regression for Calibration Lines Revisited: Weighting Schemes for Bioanalytical Methods. J. Chromatog.r B Analyt. Technol. Biomed. Life Sci. 2002, 774, 215–222. [Google Scholar] [CrossRef]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.P. In Vitro Dissolution Profile Comparison--Statistics and Analysis of the Similarity Factor, F2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Yeo, S. Recrystallization of Phenylbutazone Using Supercritical Fluid Antisolvent Process. Korean J. Chem. Eng. 2008, 53, 575–580. [Google Scholar] [CrossRef]
- Gallo, M.; Serpella, L.; Leone, F.; Manna, L.; Banchero, M.; Ronchetti, S.; Onida, B. Piroxicam Loading onto Mesoporous Silicas by Supercritical CO2 Impregnation. Molecules 2021, 26, 2500. [Google Scholar] [CrossRef]
- Thahir, R.; Wahab, A.W.; Nafie, N.L.; Raya, I. Synthesis of mesoporous silica sba-15 through surfactant set-up and hydrothermal process. RJC 2019, 12, 1117–1126. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, T.; Zhang, Q.; Wang, S. Inclusion of Telmisartan in Mesocellular Foam Nanoparticles: Drug Loading and Release Property. Eur. J. Pharm. Biopharm. 2010, 76, 17–23. [Google Scholar] [CrossRef]
- Dave, V.; Yadav, R.B.; Gupta, S.; Sharma, S. Guggulosomes: A Herbal Approach for Enhanced Topical Delivery of Phenylbutazone. Future J. Pharm. Sci. 2017, 3, 23–32. [Google Scholar] [CrossRef]
- Cussa, J.; Juárez, J.M.; Gómez Costa, M.B.; Anunziata, O.A. Nanostructured SBA-15 Host Applied in Ketorolac Tromethamine Release System. J. Mater. Sci. Mater. Med. 2017, 28, 113. [Google Scholar] [CrossRef]
- Azimov, F.; Markova, I.; Stefanova, V.; Sharipov, K.H. Synthesis and characterization of sba-15 and ti-sba-15 nanoporous materials for dme catalysts. J. Univ. Chem. Technol. Metall. 2012, 47, 333–340. [Google Scholar]
- Goscianska, J.; Olejnik, A.; Nowak, I. APTES-Functionalized Mesoporous Silica as a Vehicle for Antipyrine—Adsorption and Release Studies. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 187–196. [Google Scholar] [CrossRef]
- Eren, Z.S.; Tunçer, S.; Gezer, G.; Yildirim, L.T.; Banerjee, S.; Yilmaz, A. Improved Solubility of Celecoxib by Inclusion in SBA-15 Mesoporous Silica: Drug Loading in Different Solvents and Release. Microporous Mesoporous Mater. 2016, 235, 211–223. [Google Scholar] [CrossRef]
- Albayati, T.M.; Salih, I.K.; Alazzawi, H.F. Synthesis and Characterization of a Modified Surface of SBA-15 Mesoporous Silica for a Chloramphenicol Drug Delivery System. Heliyon 2019, 5, e02539. [Google Scholar] [CrossRef] [Green Version]
- Maria Chong, A.S.; Zhao, X.S. Functionalization of SBA-15 with APTES and Characterization of Functionalized Materials. J. Phys. Chem. B 2003, 107, 12650–12657. [Google Scholar] [CrossRef]
- Maczka, P.; Komsta, Ł.; Skibiński, R.; Gumieniczek, A. Theoretical Studies on Keto-Enol Tautomerism, Gas Phase Acidity and Spectral Properties of Phenylbutazone. Ann. UMCS Pharm. 2010, 23, 29–41. [Google Scholar]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948, 73, 679–712. [Google Scholar] [CrossRef]
- Abragam, A. The Principles of Nuclear Magnetism; Oxford University Press: Oxford, UK, 1961; ISBN 978-0-19-852014-6. [Google Scholar]
- Slichter, C.P. Principles of Magnetic Resonance. In Springer Series in Solid-State Sciences, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1990; ISBN 978-3-540-50157-2. [Google Scholar]
- HoŁderna-Natkaniec, K.; Jurga, K.; Braszak, A.; Walczak, A. Molecular Reorientations of N-n-Hexyltetrachlorophthalimide Studied by H-1 NMR. Acta Phys. Pol. A 2010, 117, 537–548. [Google Scholar] [CrossRef]
- Pajzderska, A.; Drużbicki, K.; Bilski, P.; Jenczyk, J.; Jarek, M.; Mielcarek, J.; Wąsicki, J. Environmental Effects on the Molecular Mobility of Ranitidine Hydrochloride: Crystalline State versus Drug Loaded into the Silica Matrix. J. Phys. Chem. C 2019, 123, 18364–18375. [Google Scholar] [CrossRef]
- Marchetti, A.; Yin, J.; Su, Y.; Kong, X. Solid-State NMR in the Field of Drug Delivery: State of the Art and New Perspectives. Magn. Reson. Lett. 2021, 1, 28–70. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Zhou, G.; Wu, Y.; Chen, J. Improving the Controlled Release of Water-Insoluble Emodin from Amino-Functionalized Mesoporous Silica. Appl. Surf. Sci. 2012, 258, 6366–6372. [Google Scholar] [CrossRef]
- Marchais, H.; Benali, S.; Irache, J.M.; Tharasse-Bloch, C.; Lafont, O.; Orecchioni, A.M. Entrapment Efficiency and Initial Release of Phenylbutazone from Nanocapsules Prepared from Different Polyesters. Drug Dev. Ind. Pharm. 1998, 24, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Almomen, A.; El-Toni, A.M.; Badran, M.; Alhowyan, A.; Abul Kalam, M.; Alshamsan, A.; Alkholief, M. The Design of Anionic Surfactant-Based Amino-Functionalized Mesoporous Silica Nanoparticles and Their Application in Transdermal Drug Delivery. Pharmaceutics 2020, 12, 1035. [Google Scholar] [CrossRef] [PubMed]
- Doadrio, A.L.; Salinas, A.J.; Sánchez-Montero, J.M.; Vallet-Regí, M. Drug Release from Ordered Mesoporous Silicas. Curr. Pharm. Des. 2015, 21, 6213–6819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Breemen, R.B.; Li, Y. Caco-2 Cell Permeability Assays to Measure Drug Absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.; Karavasili, C.; Ruparelia, K.; Haj-Ahmad, R.; Charalambopoulou, G.; Steriotis, T.; Giasafaki, D.; Cox, P.; Singh, N.; Giassafaki, L.-P.N.; et al. Electrosprayed Mesoporous Particles for Improved Aqueous Solubility of a Poorly Water Soluble Anticancer Agent: In Vitro and Ex Vivo Evaluation. J. Control. Release 2018, 278, 142–155. [Google Scholar] [CrossRef]








| Sample | Specific Surface Area (SBET) (m2/g) | Pore volume (Vp) (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| SBA-15 | 789 | 1.021 | 6.0 |
| SBA-15-pr-NH2 | 639 | 0.995 | 5.7 |
| SBA-15-pr-NH2:PBZ | 455 | 0.852 | 5.5 |
| Sample | 1 Motion | 2 Motion | 3 Motion |
|---|---|---|---|
| PBZ | |||
| SBA-15-pr-NH2:PBZ |
| Regression | Isopropanol | HCl Medium (pH 1.2) | Phosphate Buffer (pH 7.4) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wi | a | b | r | ∑ %ER | a | b | r | ∑ %ER | a | b | r | ∑ %ER | |
| Ordinary least squares | 1 | 51.37 | 0.0170 | 0.9999 | 3.81 | 48.94 | 0.0169 | 0.9991 | 6.76 | 38.06 | 0.0151 | 0.9995 | 4.23 |
| Weighted least squares | 51.29 | 0.0049 | 0.9999 | 1.38 | 49.17 | 0.0130 | 0.9991 | 5.49 | 37.97 | 0.0169 | 0.9995 | 0.59 | |
| 51.21 | 0.0058 | 0.9999 | 0.04 | 48.94 | 0.0170 | 0.9991 | 6.76 | 37.91 | 0.0178 | 0.9995 | 2.22 | ||
| 51.07 | 0.0070 | 0.9999 | 0.17 | 48.92 | 0.0169 | 0.9908 | 5.60 | 37.90 | 0.0179 | 0.9995 | 1.66 | ||
| 48.88 | 0.0179 | 0.9991 | 43.96 | 48.88 | 0.0179 | 0.9908 | 9.63 | 37.97 | 0.0169 | 0.9995 | 1.03 | ||
| 51.21 | 0.0059 | 0.9999 | 0.19 | 48.90 | 0.0176 | 0.9908 | 8.52 | 37.92 | 0.0178 | 0.9995 | 2.80 | ||
| 51.07 | 0.0070 | 0.9999 | 0.18 | 49.23 | 0.0144 | 0.9905 | 7.26 | 37.92 | 0.0177 | 0.9995 | 2.76 | ||
| Concentration (mg/mL) | Accuracy (%) (Expressed as ) (n = 6) Acceptance Limit: εr < 5% | Precision (%) (Expressed as RSD, n = 6) Acceptance Limit: RSD < 5% | LOD (mg/mL) | LOQ (mg/mL) | |
|---|---|---|---|---|---|
| OLS | WLS | ||||
| Isopropanol | 0.97 | −0.25 | 0.09 | 3.20 × 10−4 | 9.70 × 10−4 |
| HCl medium (pH 1.2) | 1.31 | 1.11 | 0.59 | 1.13 × 10−3 | 3.41 × 10−3 |
| Phosphate buffer (pH 7.4) | 0.52 | 0.55 | 0.35 | 1.04 × 10−3 | 3.16 × 10−3 |
| PBZ (pH 1.2) | SBA-15-pr-NH2:PBZ (pH 7.4) | PBZ (pH 7.4) | |
|---|---|---|---|
| SBA-15-pr-NH2:PBZ (pH 1.2) | 7.66 | 66.21 | - |
| SBA-15-pr-NH2:PBZ (pH 7.4) | - | - | 21.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadej, A.; Woźniak-Braszak, A.; Bilski, P.; Piotrowska-Kempisty, H.; Józkowiak, M.; Stawny, M.; Dadej, D.; Mrotek, M.; Jelińska, A. APTES-Modified SBA-15 as a Non-Toxic Carrier for Phenylbutazone. Materials 2022, 15, 946. https://doi.org/10.3390/ma15030946
Dadej A, Woźniak-Braszak A, Bilski P, Piotrowska-Kempisty H, Józkowiak M, Stawny M, Dadej D, Mrotek M, Jelińska A. APTES-Modified SBA-15 as a Non-Toxic Carrier for Phenylbutazone. Materials. 2022; 15(3):946. https://doi.org/10.3390/ma15030946
Chicago/Turabian StyleDadej, Adrianna, Aneta Woźniak-Braszak, Paweł Bilski, Hanna Piotrowska-Kempisty, Małgorzata Józkowiak, Maciej Stawny, Daniela Dadej, Michał Mrotek, and Anna Jelińska. 2022. "APTES-Modified SBA-15 as a Non-Toxic Carrier for Phenylbutazone" Materials 15, no. 3: 946. https://doi.org/10.3390/ma15030946
APA StyleDadej, A., Woźniak-Braszak, A., Bilski, P., Piotrowska-Kempisty, H., Józkowiak, M., Stawny, M., Dadej, D., Mrotek, M., & Jelińska, A. (2022). APTES-Modified SBA-15 as a Non-Toxic Carrier for Phenylbutazone. Materials, 15(3), 946. https://doi.org/10.3390/ma15030946









