Recent Advances in Synthesis and Degradation of Lignin and Lignin Nanoparticles and Their Emerging Applications in Nanotechnology
Abstract
1. Introduction
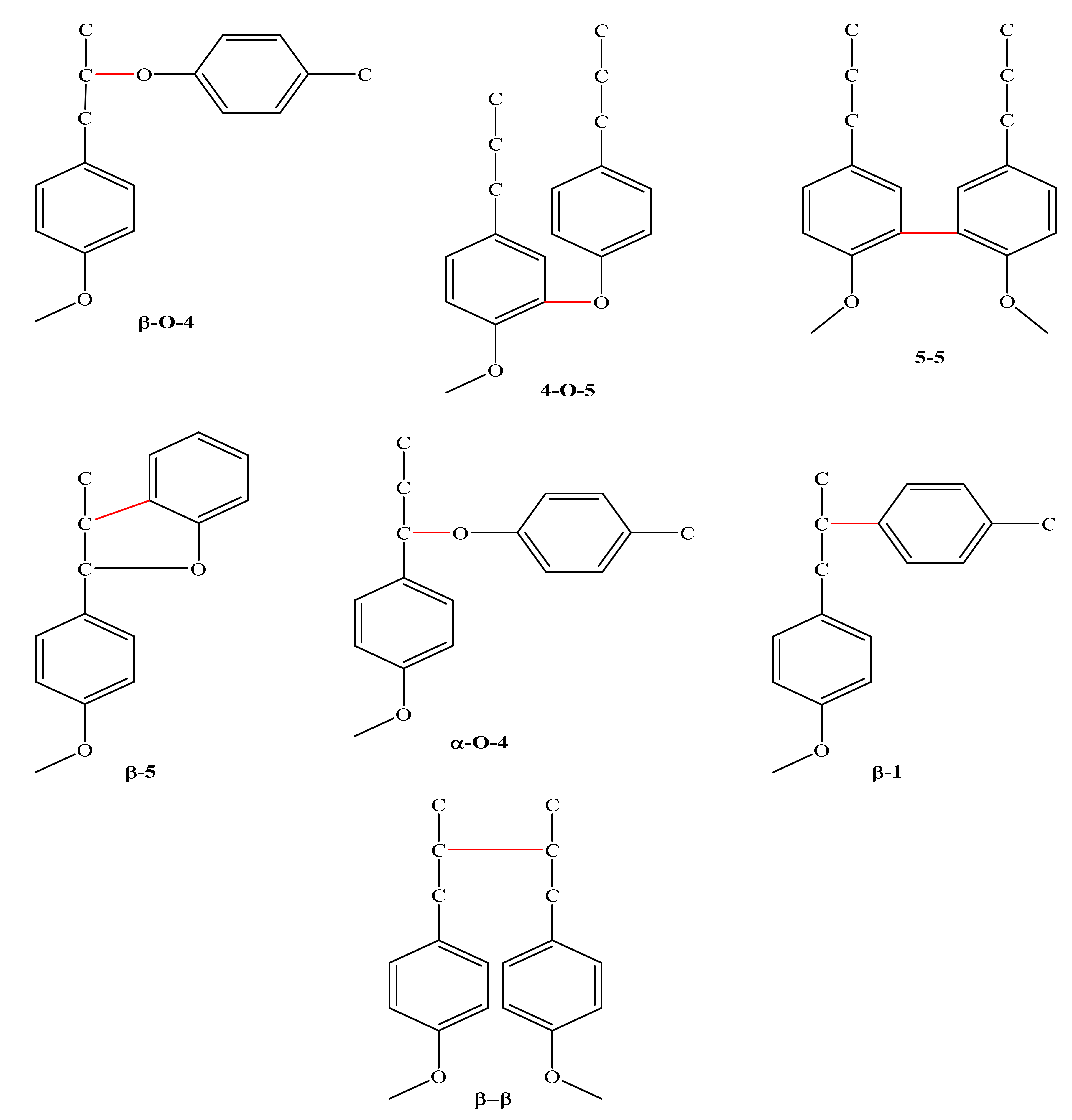
2. Structure and Biosynthesis of Lignin
3. Microbial Synthesis of Lignin Nanoparticles (LNPs)
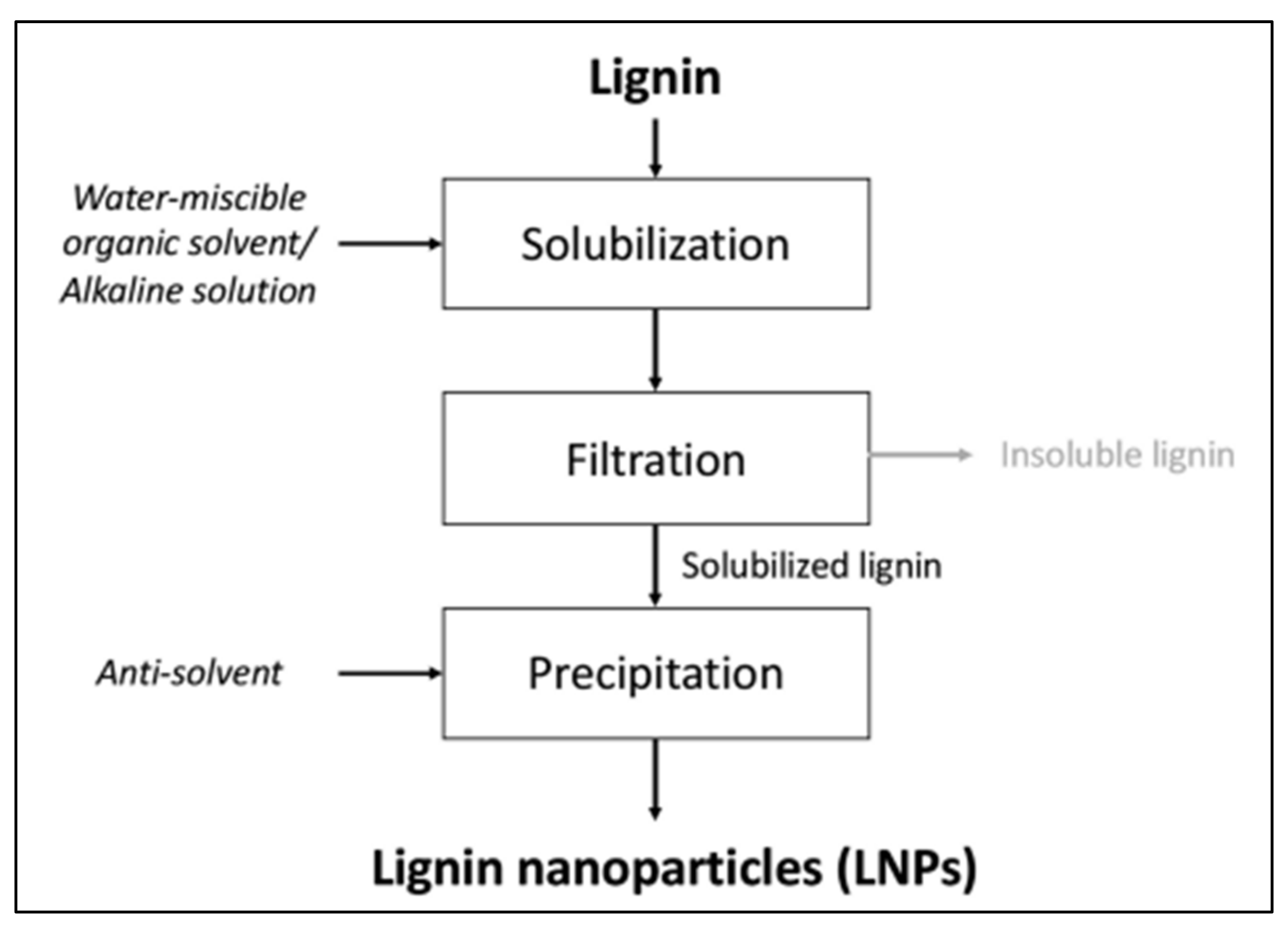
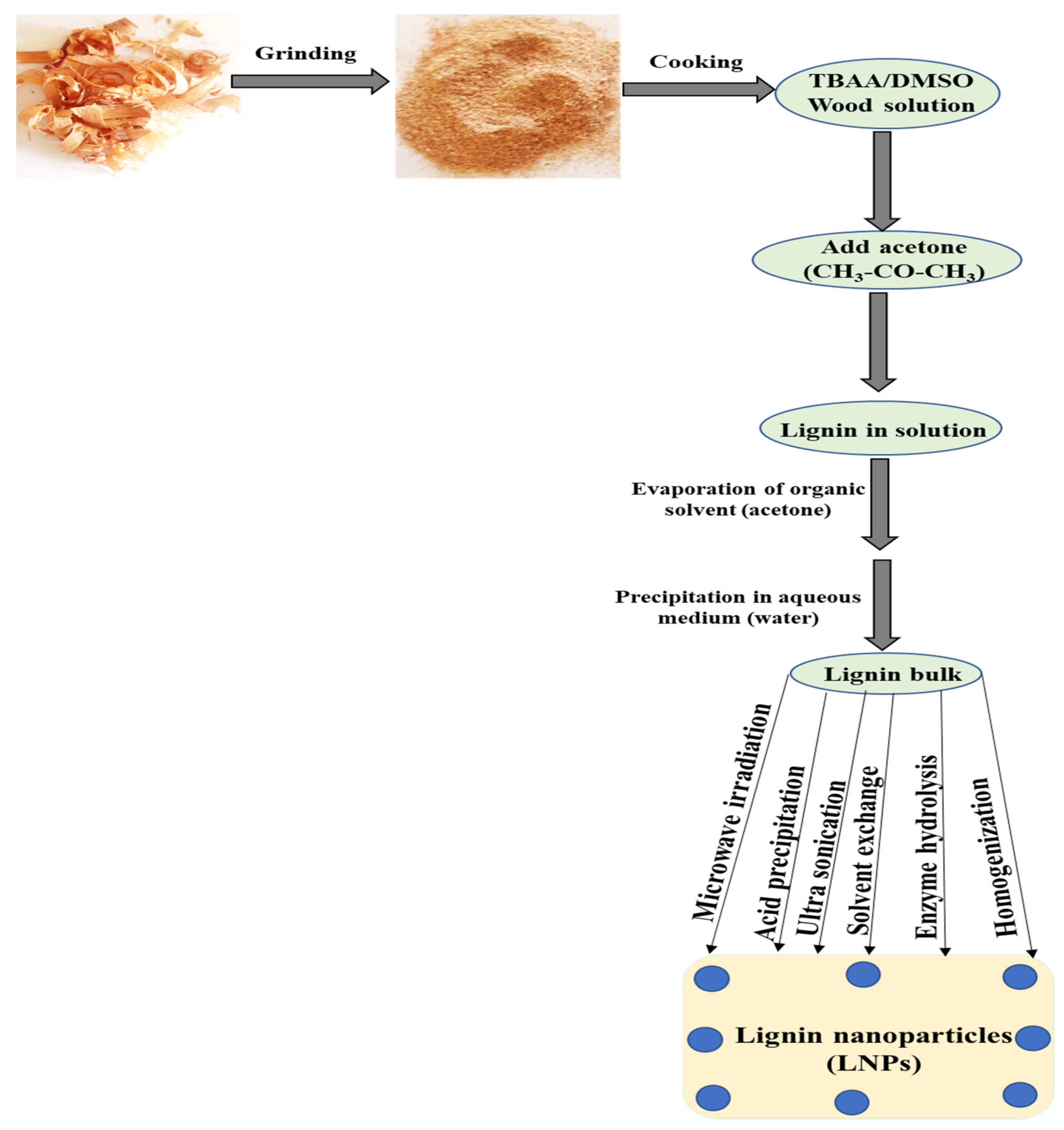
4. Different Methods for LiG NPs Synthesis
4.1. Acid Precipitation
4.2. Ultrasonication
4.3. Self-Assembly
4.4. Solvent Exchange Method
4.5. Biological Methods
4.6. Flash Nano Precipitation (FNP)
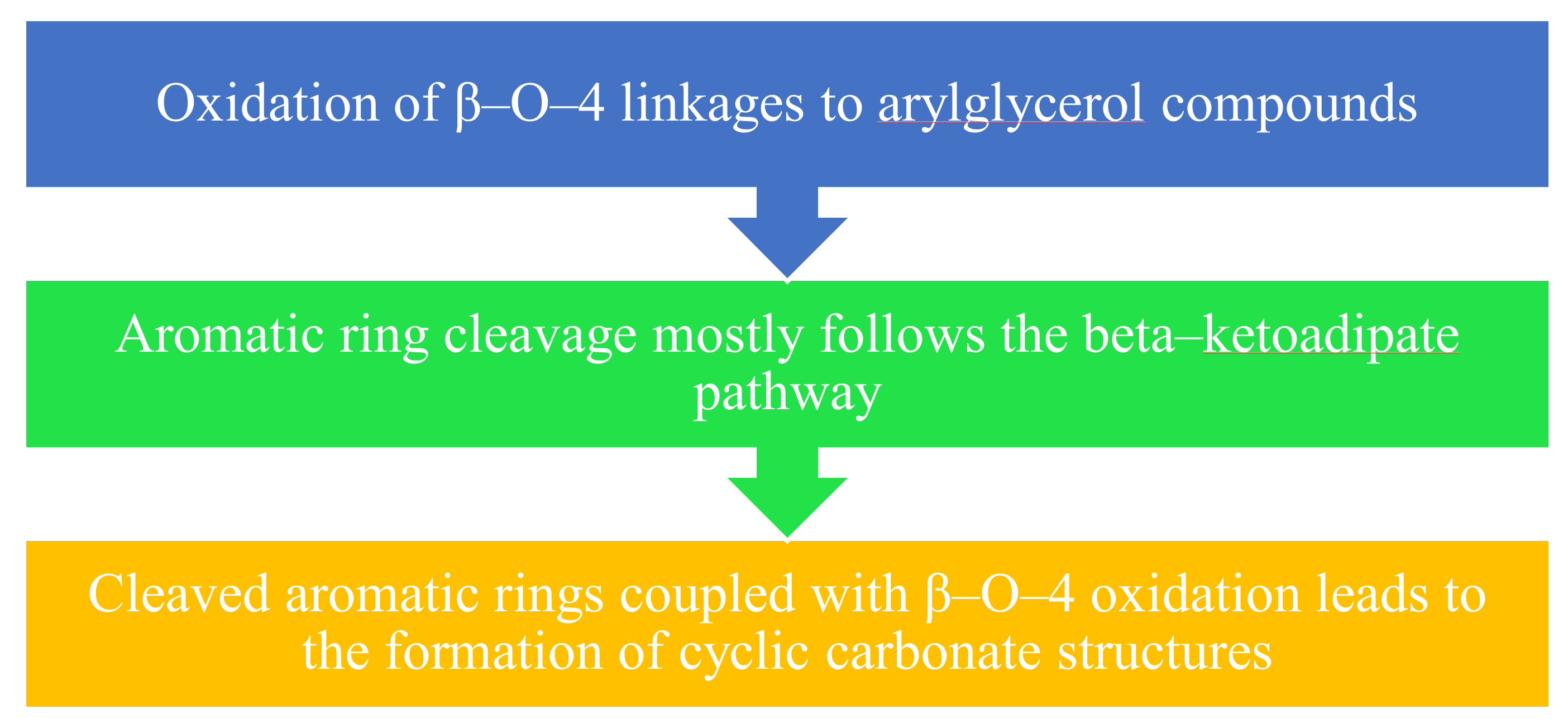
5. Biodegradation of Lignin
6. Lignin Degradation in Soil
7. Lignin-Degrading Enzymes
8. Steps Involved in Lignin Degradation
9. Lignin Degrading Fungi
9.1. White-Rot Fungi
9.2. Brown-Rot Fungi (BRF)
9.3. Soft-Rot Fungi (SRF): Soft Wood
10. Soil Fungi as Lignin Degraders
11. Lignin-Degrading Bacteria
12. Mechanism of Lignin Biodegradation
13. Significance of Lignin Biodegradation
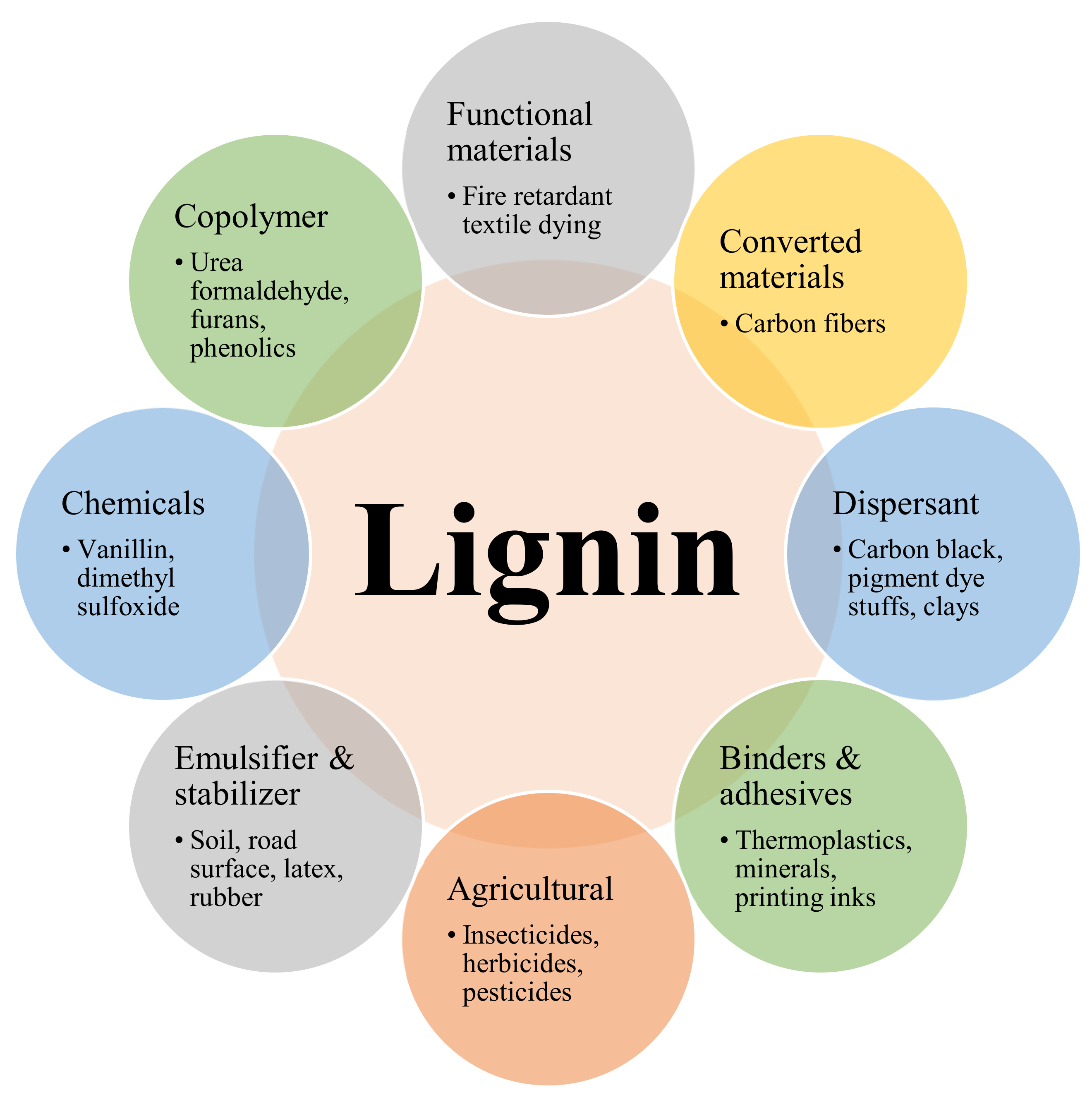
14. Modern Applications of Lignin Micro- and Nanoparticles
Lignin-Based Cement, Phenolic Compounds, Carbon Materials and Hydrocarbon Compounds
15. Lignin-Derived Polymers
16. Applications of Lignin in Medicine
17. Present Challenges
18. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATRP | atom transfer radical polymerization |
| ADMET | acyclic diene metathesis |
| BRF | brown-rot fungi |
| DHP | di hydroxyl phenol |
| FTIR | Fourier transform-infrared spectroscopy |
| LC | lignocellulose |
| LCBM | lingocellulosic biomass |
| Lac | laccase |
| LiP | lignin peroxidases |
| LNPs | lignin nanoparticles |
| DyP | dye-decolorizing peroxidase |
| KL | Kraft lignin |
| LiG | lignin |
| MnP | manganese-dependent peroxidase |
| MLG | monolignols |
| NMP | nitroxide mediated polymerization |
| NMR | nuclear magnetic resonance spectroscopy |
| NPs | nanoparticles |
| OGs | organosolv |
| PEB | poly(ethylene brassylate) |
| RAFT | reversible addition fragmentation chain transfer |
| ROP | ring-opening polymerization |
| ROMP | ring-opening metathesis polymerization |
| SRF | soft-rot fungi |
| THF | tetrahydrofuran |
| UV-Vis | ultraviolet visible spectroscopy |
| VP | versatile peroxidase |
| WRF | white-rot fungi |
References
- Arapova, O.V.; Chistyakov, A.V.; Tsodikov, M.V.; Moiseev, I.I. Lignin as a Renewable Resource of Hydrocarbon Products and Energy Carriers (A Review). Pet. Chem. 2020, 60, 227–243. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef]
- Ruiz-Dueñas, F.J.; Martínez, Á.T. Microbial degradation of lignin: How a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb. Biotechnol. 2009, 2, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Nandal, P.; Singh, J.; Verma, M.L. Nanobiotechnological advancements in lignocellulosic biomass pretreatment. Mater. Sci. Energy Technol. 2020, 3, 308–318. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin Biopolymers in the Age of Controlled Polymerization. Polymers 2019, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Takkellapati, S. The current and emerging sources of technical lignins and their applications. Biofuel Bioprod. Biorefin. 2018, 12, 756–787. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Taha, M.; Foda, M.; Shahsavari, E.; Aburto-Medina, A.; Adetutu, E.; Ball, A. Commercial feasibility of lignocellulose biodegradation: Possibilities and challenges. Curr. Opin. Biotechnol. 2016, 38, 190–197. [Google Scholar] [CrossRef]
- Branco, R.; Serafim, L.; Xavier, A. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2018, 5, 4. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.; Formanek, P. Enzymatic Degradation of Lignin in Soil: A Review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Bonugli-Santos, R.; Durrant, L.; Silva, M.; Sette, L. Production of laccase, manganese peroxidase and lignin peroxidase by Brazilian marine-derived fungi. Enzym. Microb. Technol. 2010, 46, 32–37. [Google Scholar] [CrossRef]
- Su, Y.; Xian, H.; Shi, S.; Zhang, C.; Manik, S.M.N.; Mao, J.; Zhang, G.; Liao, W.; Wang, Q.; Liu, H. Biodegradation of lignin and nicotine with white rot fungi for the delignification and detoxification of tobacco stalk. BMC Biotechnol. 2016, 16, 81. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Dashtban, M.; Schraft, H.; Syed, T.A.; Qin, W. Fungal biodegradation and enzymatic modification of lignin. Int. J. Biochem. Mol. Biol. 2010, 1, 36–50. [Google Scholar]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Naseem, A.; Tabasum, S.; Zuber, M.; Ali, M.; Noreen, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An Overview of Biorefinery Derived Platform Chemicals from a Cellulose and Hemicellulose Biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef]
- Järvinen, J.; Taskila, S.; Isomäki, R.; Ojamo, H. Screening of white-rot fungi manganese peroxidases: A comparison between the specific activities of the enzyme from different native producers. AMB Express 2012, 2, 62. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Bugg, T.D.; Ahmad, M.; Hardiman, E.M.; Rahmanpour, R. Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 2011, 28, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Saxer, S.; Portmann, C.; Tosatti, S.; Gademann, K.; Zürcher, S.; Textor, M. Surface Assembly of Catechol-Functionalized Poly(l-lysine)-graft-poly(ethylene glycol) Copolymer on Titanium Exploiting Combined Electrostatically Driven Self-Organization and Biomimetic Strong Adhesion. Macromolecules 2010, 43, 1050–1060. [Google Scholar] [CrossRef]
- Blondiaux, E.; Bomon, J.; Smoleń, M.; Kaval, N.; Lemière, F.; Sergeyev, S.; Diels, L.; Sels, B.; Maes, B.U.W. Bio-based Aromatic Amines from Lignin-Derived Monomers. ACS Sustain. Chem. Eng. 2019, 7, 6906–6916. [Google Scholar] [CrossRef]
- Bajwa, D.; Pourhashem, G.; Ullah, A.H.; Bajwa, S. A concise review of current lignin production, applications, products and their environment impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Bonilla, A.F.; Bonilla, D.A. Synthesis and Characterization of a Novel Lignin-Based Biopolymer from Ulex europaeus: A Preliminary Study. J 2021, 4, 101–115. [Google Scholar] [CrossRef]
- Larrañeta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Domínguez-Robles, J.; Rodríguez, A.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef]
- An, L.; Yu, Y.H.; Chen, J.; Bae, J.H.; Youn, D.H.; Jeong, H.M.; Kim, Y.S. Synthesis and characterization of tailor-made zwitterionic lignin for resistance to protein adsorption. Ind. Crops Prod. 2021, 167, 113514. [Google Scholar] [CrossRef]
- Spiridon, I.; Dascalu, I.-A.; Coroaba, A.; Apostol, I.; Palamaru, M.N.; Iordan, A.R.; Borhan, A.I. Synthesis and Characterization of New Ferrite-Lignin Hybrids. Polymers 2021, 13, 2495. [Google Scholar] [CrossRef]
- Kumar, R.; Butreddy, A.; Kommineni, N.; Reddy, P.G.; Bunekar, N.; Sarkar, C.; Dutt, S.; Mishra, V.K.; Aadil, K.R.; Mishra, Y.K.; et al. Lignin: Drug/Gene Delivery and Tissue Engineering Applications. Int. J. Nanomed. 2021, 16, 2419–2441. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Hasan, M.A.; Yadav, K.K.; Pinto, M.M.C.; Malik, N.; Yadav, V.K.; Khan, A.H.; Islam, S.; Sharma, G.K. Agro-Nanotechnology as an Emerging Field: A Novel Sustainable Approach for Improving Plant Growth by Reducing Biotic Stress. Appl. Sci. 2021, 11, 2282–2296. [Google Scholar] [CrossRef]
- Yadav, V.K.; Choudhary, N.; Ali, D.; Gnanamoorthy, G.; Inwati, G.K.; Almarzoug, M.H.A.; Kumar, G.; Khan, S.H.; Solanki, M.B. Experimental and Computational Approaches for the Structural Study of Novel Ca-Rich Zeolites from Incense Stick Ash and Their Application for Wastewater Treatment. Adsorpt. Sci. Technol. 2021, 2021, 6066906. [Google Scholar] [CrossRef]
- Yadav, V.K.; Khan, S.H.; Choudhary, N.; Tirth, V.; Kumar, P.; Ravi, R.K.; Modi, S.; Khayal, A.; Shah, M.P.; Sharma, P.; et al. Nanobioremediation: A sustainable approach towards the degradation of sodium dodecyl sulfate in the environment and simulated conditions. J. Basic Microbiol. 2021. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, K.K.; Alam, J.; Cabral-Pinto, M.M.S.; Gnanamoorthy, G.; Alhoshan, M.; Kamyab, H.; Hamid, A.A.; Ahmed, F.A.; Shukla, A.K. Transformation of hazardous sacred incense sticks ash waste into less toxic product by sequential approach prior to their disposal into the water bodies. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Ramar, K.; Padmanaban, A.; Yadav, V.K.; Suresh Babu, K.; Karthikeyan, V.; Narayanan, V. Implementation of ZnSnO3 nanosheets and their RE (Er, Eu, and Pr) materials: Enhanced photocatalytic activity. Adv. Powder Technol. 2020, 31, 1209–1219. [Google Scholar] [CrossRef]
- Modi, S.; Prajapati, R.; Inwati, G.K.; Deepa, N.; Tirth, V.; Yadav, V.K.; Yadav, K.K.; Islam, S.; Gupta, P.; Kim, D.-H.; et al. Recent Trends in Fascinating Applications of Nanotechnology in Allied Health Sciences. Crystals 2022, 12, 39. [Google Scholar] [CrossRef]
- Alam, J.; Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.; Tavker, N.; Choudhary, N.; Shukla, A.K.; Ali, F.A.; Alhoshan, M.; Hamid, A.A. Recent Advances in Methods for the Recovery of Carbon Nanominerals and Polyaromatic Hydrocarbons from Coal Fly Ash and Their Emerging Applications. Crystals 2021, 11, 88. [Google Scholar] [CrossRef]
- Yadav, V.K.; Fulekar, M.H. Advances in Methods for Recovery of Ferrous, Alumina, and Silica Nanoparticles from Fly Ash Waste. Ceramics 2020, 3, 384–420. [Google Scholar] [CrossRef]
- Kumar Yadav, V.; Suriyaprabha, R.; Heena Khan, S.; Singh, B.; Gnanamoorthy, G.; Choudhary, N.; Kumar Yadav, A.; Kalasariya, H. A novel and efficient method for the synthesis of amorphous nanosilica from fly ash tiles. Mater. Today Proc. 2020, 26, 701–705. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, V.K.; Yadav, K.K.; Alwetaishi, M.; Gnanamoorthy, G.; Singh, B.; Jeon, B.-H.; Cabral-Pinto, M.M.S.; Choudhary, N.; Ali, D.; et al. Recovery of iron nanominerals from sacred incense sticks ash waste collected from temples by wet and dry magnetic separation method. Environ. Technol. Innov. 2022, 25, 102150. [Google Scholar] [CrossRef]
- Meng, X.; Poonia, M.; Yoo, C.G.; Ragauskas, A.J. Recent Advances in Synthesis and Application of Lignin Nanoparticles. In Lignin Utilization Strategies: From Processing to Applications; American Chemical Society: Washington, DC, USA, 2021; Volume 1377, pp. 273–293. [Google Scholar]
- Del Cerro, C.; Erickson, E.; Dong, T.; Wong, A.R.; Eder, E.K.; Purvine, S.O.; Mitchell, H.D.; Weitz, K.K.; Markillie, L.M.; Burnet, M.C.; et al. Intracellular pathways for lignin catabolism in white-rot fungi. Proc. Natl. Acad. Sci. USA 2021, 118, e2017381118. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.-C.; Hu, H.-Q.; Xie, F.-J.; Wei, X.-Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef]
- Alzagameem, A.; Khaldi-Hansen, B.E.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic Biomass as Source for Lignin-Based Environmentally Benign Antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef]
- Stark, N.; Yelle, D.; Agarwal, U. Techniques for Characterizing Lignin. In Lignin in Polymer Composites; Omar, F., Sain, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 49–66. [Google Scholar] [CrossRef]
- Sette, M.; Wechselberger, R.; Crestini, C. Elucidation of Lignin Structure by Quantitative 2D NMR. Chem. Eur. J. 2011, 17, 9529–9535. [Google Scholar] [CrossRef]
- Wang, Y.; Chantreau, M.; Sibout, R.; Hawkins, S. Plant cell wall lignification and monolignol metabolism. Front. Plant Sci. 2013, 4, 220. [Google Scholar] [CrossRef]
- Achyuthan, K.E.; Achyuthan, A.M.; Adams, P.D.; Dirk, S.M.; Harper, J.C.; Simmons, B.A.; Singh, A.K. Supramolecular self-assembled chaos: Polyphenolic lignin’s barrier to cost-effective lignocellulosic biofuels. Molecules 2010, 15, 8641–8688. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener synthesis of lignin nanoparticles and their applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from Micro- to Nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; Oliva González, C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef]
- Myint, A.A.; Lee, H.W.; Seo, B.; Son, W.-S.; Yoon, J.; Yoon, T.J.; Park, H.J.; Yu, J.; Yoon, J.; Lee, Y.-W. One pot synthesis of environmentally friendly lignin nanoparticles with compressed liquid carbon dioxide as an antisolvent. Green Chem. 2016, 18, 2129–2146. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mohanty, S.; Nayak, S.K. Synthesis, Characterization and Application of Lignin Nanoparticles (LNPs). Mater. Focus 2014, 3, 444–454. [Google Scholar] [CrossRef]
- Gilca, I.; Popa, V.; Crestini, C. Obtaining lignin nanoparticles by sonication. Ultrason. Sonochem. 2014, 23, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials: 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Matsakas, L.; Gerber, M.; Yu, L.; Rova, U.; Christakopoulos, P. Preparation of low carbon impact lignin nanoparticles with controllable size by using different strategies for particles recovery. Ind. Crops Prod. 2020, 147, 112243. [Google Scholar] [CrossRef]
- Mattinen, M.L.; Valle-Delgado, J.J.; Leskinen, T.; Anttila, T.; Riviere, G.; Sipponen, M.; Paananen, A.; Lintinen, K.; Kostiainen, M.; Osterberg, M. Enzymatically and chemically oxidized lignin nanoparticles for biomaterial applications. Enzym. Microb. Technol. 2018, 111, 48–56. [Google Scholar] [CrossRef]
- Pan, M.; Xie, X.; Liu, K.; Yang, J.; Hong, L.; Wang, S. Fluorescent Carbon Quantum Dots-Synthesis, Functionalization and Sensing Application in FoodAnalysis. Nanomaterials 2020, 10, 930. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef]
- Schneider, W.D.H.; Dillon, A.J.P.; Camassola, M. Lignin nanoparticles enter the scene: A promising versatile green tool for multiple applications. Biotechnol. Adv. 2021, 47, 107685. [Google Scholar] [CrossRef]
- Chen, F.; Hu, X.; Tu, X.; Chen, L.; Liu, X.; Tan, L.; Mao, Y.; Shi, J.; Teng, X.; He, S.; et al. High-Yield Production of Lignin-Derived Functional Carbon Nanosheet for Dye Adsorption. Polymers 2020, 12, 797. [Google Scholar] [CrossRef]
- Henn, A.; Mattinen, M.-L. Chemo-enzymatically prepared lignin nanoparticles for value-added applications. World J. Microbiol. Biotechnol. 2019, 35, 125. [Google Scholar] [CrossRef]
- Nair, S.S.; Sharma, S.; Pu, Y.; Sun, Q.; Pan, S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High Shear Homogenization of Lignin to Nanolignin and Thermal Stability of Nanolignin-Polyvinyl Alcohol Blends. ChemSusChem 2014, 7, 3513–3520. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevičius, M.; Richter, A.; Velev, O.; Stoyanov, S.; Paunov, V. Fabrication of Environmentally Biodegradable Lignin Nanoparticles. Chemphyschem 2012, 13, 4235–4243. [Google Scholar] [CrossRef]
- Cailotto, S.; Gigli, M.; Bonini, M.; Rigoni, F.; Crestini, C. Sustainable Strategies in the Synthesis of Lignin Nanoparticles for the Release of Active Compounds: A Comparison. ChemSusChem 2020, 13, 4759–4767. [Google Scholar] [CrossRef]
- Zhang, Z.; Terrasson, V.; Guenin, E. Lignin Nanoparticles and Their Nanocomposites. Nanomaterials 2021, 11, 1336. [Google Scholar] [CrossRef]
- Agustin, M.B.; Penttilä, P.A.; Lahtinen, M.; Mikkonen, K.S. Rapid and Direct Preparation of Lignin Nanoparticles from Alkaline Pulping Liquor by Mild Ultrasonication. ACS Sustain. Chem. Eng. 2019, 7, 19925–19934. [Google Scholar] [CrossRef]
- Tran, M.H.; Phan, D.-P.; Lee, E.Y. Review on lignin modifications toward natural UV protection ingredient for lignin-based sunscreens. Green Chem. 2021, 23, 4633–4646. [Google Scholar] [CrossRef]
- Salentinig, S.; Schubert, M. Softwood Lignin Self-Assembly for Nanomaterial Design. Biomacromolecules 2017, 18, 2649–2653. [Google Scholar] [CrossRef]
- Yearla, S.R.; Padmasree, K. Preparation and characterisation of lignin nanoparticles: Evaluation of their potential as antioxidants and UV protectants. J. Exp. Nanosci. 2016, 11, 289–302. [Google Scholar] [CrossRef]
- Rangan, A.; Manchiganti, M.V.; Thilaividankan, R.M.; Kestur, S.G.; Menon, R. Novel method for the preparation of lignin-rich nanoparticles from lignocellulosic fibers. Ind. Crops Prod. 2017, 103, 152–160. [Google Scholar] [CrossRef]
- Juikar, S.J.; Nadanathangam, V. Microbial Production of Nanolignin from Cotton Stalks and Its Application onto Cotton and Linen Fabrics for Multifunctional Properties. Waste Biomass Valoriz. 2020, 11, 6073–6083. [Google Scholar] [CrossRef]
- Tao, J.; Chow, S.F.; Zheng, Y. Application of flash nanoprecipitation to fabricate poorly water-soluble drug nanoparticles. Acta Pharm. Sin. B 2019, 9, 4–18. [Google Scholar] [CrossRef]
- Conner, C.G.; Veleva, A.N.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Scalable Formation of Concentrated Monodisperse Lignin Nanoparticles by Recirculation-Enhanced Flash Nanoprecipitation. Part. Part. Syst. Charact. 2020, 37, 2000122. [Google Scholar] [CrossRef]
- Leonowicz, A.; Matuszewska, A.; Luterek, J.; Ziegenhagen, D.; Wojtaś-Wasilewska, M.; Cho, N.-S.; Hofrichter, M.; Rogalski, J. Biodegradation of Lignin by White Rot Fungi. Fungal Genet. Biol. 1999, 27, 175–185. [Google Scholar] [CrossRef]
- Kärkönen, A.; Koutaniemi, S. Lignin Biosynthesis Studies in Plant Tissue Cultures. J. Integr. Plant Biol. 2010, 52, 176–185. [Google Scholar] [CrossRef]
- Goodell, B.; Jellison, J. Fungal Decay of Wood: Soft Rot. In Development of Commercial Wood Preservatives: Efficacy, Environmental, and Health Issues; Schultz, T.P., Militz, H., Freeman, M.H., Goodell, B., Nicholas, D.D., Eds.; American Chemical Society: Washington, DC, USA, 2008; Volume 982, pp. 9–31. [Google Scholar] [CrossRef]
- Goodell, B. Brown-Rot Fungal Degradation of Wood: Our Evolving View. ACS Symp. Ser. 2003, 845, 97–118. [Google Scholar] [CrossRef]
- Purnomo, A.; Mori, T.; Kondo, R. Involvement of Fenton reaction in DDT degradation by brown-rot fungi. Int. Biodeterior. Biodegrad. 2010, 64, 560–565. [Google Scholar] [CrossRef]
- Filley, T.; Cody, G.D.; Goodell, B.; Jellison, J.; Noser, C.; Ostrofsky, A. Lignin demethylation and polysaccharide decomposition in spruce sapwood degraded by brown rot fungi. Org. Geochem. 2002, 33, 111–124. [Google Scholar] [CrossRef]
- Yang, J.M.; Goring, D. The phenolic hydroxyl content of lignin in spruce wood. Can. J. Chem. 2011, 58, 2411–2414. [Google Scholar] [CrossRef]
- Ohashi, Y.; Uno, Y.; Amirta, R.; Watanabe, T.; Honda, Y.; Watanabe, T. Alkoxyl- and carbon-centered radicals as primary agents for degrading non-phenolic lignin-substructure model compounds. Org. Biomol. Chem. 2011, 9, 2481–2491. [Google Scholar] [CrossRef] [PubMed]
- Bjørsvik, H.-R.; Occhipinti, G.; Gambarotti, C.; Cerasino, L.; Jensen, V. Synthesis of Methoxy-Substituted Phenols by Peracid Oxidation of the Aromatic Ring. J. Org. Chem. 2005, 70, 7290–7296. [Google Scholar] [CrossRef]
- Rosini, E.; Allegretti, C.; Melis, R.; Cerioli, L.; Conti, G.; Pollegioni, L.; D’Arrigo, P. Cascade enzymatic cleavage of the β-O-4 linkage in a lignin model compound. Catal. Sci. Technol. 2016, 6, 2195–2205. [Google Scholar] [CrossRef]
- Zhu, Y.; Ouyang, X.; Zhao, Y.; Jiang, L.; Guo, H.; Xueqing, Q. Oxidative depolymerization of lignin improved by enzymolysis pretreatment with laccase. J. Energy Chem. 2017, 27, 801–805. [Google Scholar] [CrossRef]
- Chowdhary, P.; Shukla, G.; Raj, G.; Ferreira, L.F.; Bharagava, R. Microbial manganese peroxidase: A ligninolytic enzyme and its ample opportunities in research. SN Appl. Sci. 2019, 1, 45. [Google Scholar] [CrossRef]
- Linares, N.; Magaña-Ortíz, D.; Guzmán-Ortiz, D.; Fernández, F.; Loske, A.; Gómez Lim, M. Erratum to: High-yield production of manganese peroxidase, lignin peroxidase, and versatile peroxidase in Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2014, 98, 9283–9294. [Google Scholar] [CrossRef]
- Falade, A.O.; Nwodo, U.U.; Iweriebor, B.C.; Green, E.; Mabinya, L.V.; Okoh, A.I. Lignin peroxidase functionalities and prospective applications. Microbiologyopen 2017, 6, e00394. [Google Scholar] [CrossRef]
- De Gonzalo, G.; Colpa, D.I.; Habib, M.H.M.; Fraaije, M.W. Bacterial enzymes involved in lignin degradation. J. Biotechnol. 2016, 236, 110–119. [Google Scholar] [CrossRef]
- Sugano, Y. DyP-type peroxidases comprise a novel heme peroxidase family. Cell. Mol. Life Sci. CMLS 2009, 66, 1387–1403. [Google Scholar] [CrossRef]
- Park, J.-H.; Pavlov, I.N.; Kim, M.-J.; Park, M.S.; Oh, S.-Y.; Park, K.H.; Fong, J.J.; Lim, Y.W. Investigating Wood Decaying Fungi Diversity in Central Siberia, Russia Using ITS Sequence Analysis and Interaction with Host Trees. Sustainability 2020, 12, 2535. [Google Scholar] [CrossRef]
- Daniel, G. Fungal and Bacterial Biodegradation: White Rots, Brown Rots, Soft Rots, and Bacteria. In Deterioration and Protection of Sustainable Biomaterials; American Chemical Society: Washington, DC, USA, 2014; Volume 1158, pp. 23–58. [Google Scholar]
- Savory, J. Breakdown of timber by Ascomycetes and Fungi Imperfecti. Ann. Appl. Biol. 2008, 41, 336–347. [Google Scholar] [CrossRef]
- Lee, Y.S. Observation of Soft-Rot Wood Degradation Caused by Higher Ascomyceteous fungi. Mycobiology 2018, 28, 47–50. [Google Scholar] [CrossRef]
- Martinez, A.T.; Speranza, M.; Ruiz-Dueñas, F.; Ferreira Neila, P.; Camarero, S.; Guillen, F. Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic as pects of the fungal attack of lignin. Int. Microbiol. 2005, 81, 95–204. [Google Scholar]
- Guiraud, P.; Steiman, R.; Seigle-Murandi, F.; Benoit-Guyod, J.L. Comparison of the toxicity of various lignin-related phenolic compounds toward selected fungi perfecti and fungi imperfecti. Ecotoxicol. Environ. Saf. 1995, 32, 29–33. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, Y.; Xiao, Y.; Xu, Z.; Bian, Y. Cloning, expression and phylogenetic analysis of a divergent laccase multigene family in Auricularia auricula-judae. Microbiol. Res. 2014, 169, 453–462. [Google Scholar] [CrossRef]
- Schoemaker, H.E.; Leisola, M.S.A. Degradation of lignin by Phanerochaete chrysosporium. J. Biotechnol. 1990, 13, 101–109. [Google Scholar] [CrossRef]
- Kantelinen, A.; Waldner, R.; Niku-Paavola, M.-L.; Leisola, M.S.A. Comparison of two lignin-degrading fungi:Phlebia radiata andPhanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 1988, 28, 193–198. [Google Scholar] [CrossRef]
- Fackler, K.; Gradinger, C.; Hinterstoisser, B.; Messner, K.; Schwanninger, M. Lignin degradation by white rot fungi on spruce wood shavings during short-time solid-state fermentations monitored by near infrared spectroscopy. Enzym. Microb. Technol. 2006, 39, 1476–1483. [Google Scholar] [CrossRef]
- Hatakka, A.I.; Uusi-Rauva, A.K. Degradation of 14C-labelled poplar wood lignin by selected white-rot fungi. Eur. J. Appl. Microbiol. Biotechnol. 1983, 17, 235–242. [Google Scholar] [CrossRef]
- Bonnarme, P.; Jeffries, T.W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl. Environ. Microbiol. 1990, 56, 210–217. [Google Scholar] [CrossRef]
- Paice, M.G.; Reid, I.D.; Bourbonnais, R.; Archibald, F.S.; Jurasek, L. Manganese Peroxidase, Produced by Trametes versicolor during Pulp Bleaching, Demethylates and Delignifies Kraft Pulp. Appl. Environ. Microbiol. 1993, 59, 260–265. [Google Scholar] [CrossRef]
- Jensen, K.A.; Bao, W.; Kawai, S.; Srebotnik, E.; Hammel, K.E. Manganese-Dependent Cleavage of Nonphenolic Lignin Structures by Ceriporiopsis subvermispora in the Absence of Lignin Peroxidase. Appl. Environ. Microbiol. 1996, 62, 3679–3686. [Google Scholar] [CrossRef]
- Kantelinen, A.; Hatakka, A.; Viikari, L. Production of lignin peroxidase and laccase by Phlebia radiata. Appl. Microbiol. Biotechnol. 1989, 31, 234–239. [Google Scholar] [CrossRef]
- Fernández-Fueyo, E.; Ruiz-Dueñas, F.J.; Miki, Y.; Martínez, M.J.; Hammel, K.E.; Martínez, A.T. Lignin-degrading peroxidases from genome of selective ligninolytic fungus Ceriporiopsis subvermispora. J. Biol. Chem. 2012, 287, 16903–16916. [Google Scholar] [CrossRef] [PubMed]
- Camarero, S.; Martínez, M.J.; Martínez, A.T. Lignin-degrading enzymes produced by Pleurotus species during solid state fermentation of wheat straw. In Advances in Solid State Fermentation; Roussos, S., Lonsane, B.K., Raimbault, M., Viniegra-Gonzalez, G., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 335–345. [Google Scholar] [CrossRef]
- Knežević, A.; Milovanovic, I.; Stajic, M.; Vukojevic, J. Potential of Trametes species to degrade lignin. Int. Biodeterior. Biodegrad. 2013, 85, 52–56. [Google Scholar] [CrossRef]
- Vasina, D.V.; Pavlov, A.R.; Koroleva, O.V. Extracellular proteins of Trametes hirsuta st. 072 induced by copper ions and a lignocellulose substrate. BMC Microbiol. 2016, 16, 106. [Google Scholar] [CrossRef]
- Christopher, L.P.; Yao, B.; Ji, Y. Lignin Biodegradation with Laccase-Mediator Systems. Front. Energy Res. 2014, 2, 12. [Google Scholar] [CrossRef]
- Palma, C.; Lloret, L.; Sepúlveda, L.; Contreras, E. Production of versatile peroxidase from Pleurotus eryngii by solid-state fermentation using agricultural residues and evaluation of its catalytic properties. Prep. Biochem. Biotechnol. 2016, 46, 200–207. [Google Scholar] [CrossRef]
- Fernández-Fueyo, E.; Ruiz-Dueñas, F.J.; Martínez, M.J.; Romero, A.; Hammel, K.E.; Medrano, F.J.; Martínez, A.T. Ligninolytic peroxidase genes in the oyster mushroom genome: Heterologous expression, molecular structure, catalytic and stability properties, and lignin-degrading ability. Biotechnol. Biofuels 2014, 7, 2. [Google Scholar] [CrossRef]
- Grąz, M.; Jarosz-Wilkołazka, A. Oxalic acid, versatile peroxidase secretion and chelating ability of Bjerkandera fumosa in rich and limited culture conditions. World J. Microbiol. Biotechnol. 2011, 27, 1885–1891. [Google Scholar] [CrossRef]
- Ruiz-Dueñas, F.; Lundell, T.; Floudas, D.; Nagy, L.G.; Barrasa, J.; Hibbett, D.; Martinez, A.T. Lignin-degrading peroxidases in Polyporales: An evolutionary survey based on 10 sequenced genomes. Mycologia 2013, 105, 1428–1444. [Google Scholar] [CrossRef]
- Rashid, G.M.M.; Bugg, T.D.H. Enhanced biocatalytic degradation of lignin using combinations of lignin-degrading enzymes and accessory enzymes. Catal. Sci. Technol. 2021, 11, 3568–3577. [Google Scholar] [CrossRef]
- Fisher, A.B.; Fong, S.S. Lignin biodegradation and industrial implications. AIMS Bioeng. 2014, 1, 92–112. [Google Scholar] [CrossRef]
- Ahmad, M.; Roberts, J.N.; Hardiman, E.M.; Singh, R.; Eltis, L.D.; Bugg, T.D.H. Identification of DypB from Rhodococcus jostii RHA1 as a Lignin Peroxidase. Biochemistry 2011, 50, 5096–5107. [Google Scholar] [CrossRef]
- Lee, S.; Kang, M.; Bae, J.-H.; Sohn, J.-H.; Sung, B.H. Bacterial Valorization of Lignin: Strains, Enzymes, Conversion Pathways, Biosensors, and Perspectives. Front. Bioeng. Biotechnol. 2019, 7, 209. [Google Scholar] [CrossRef]
- Majumdar, S.; Lukk, T.; Solbiati, J.O.; Bauer, S.; Nair, S.K.; Cronan, J.E.; Gerlt, J.A. Roles of Small Laccases from Streptomyces in Lignin Degradation. Biochemistry 2014, 53, 4047–4058. [Google Scholar] [CrossRef]
- Rahman Pour, R.; Rea, D.; Jamshidi, S.; Fülöp, V.; Bugg, T. Structure of Thermobifida fusca DyP-type Peroxidase and Activity towards Kraft lignin and Lignin Model Compounds. Arch. Biochem. Biophys. 2016, 594, 54–60. [Google Scholar] [CrossRef]
- Lambertz, C.; Ece, S.; Fischer, R.; Commandeur, U. Progress and obstacles in the production and application of recombinant lignin-degrading peroxidases. Bioengineered 2016, 7, 145–154. [Google Scholar] [CrossRef]
- Yang, C.; Yue, F.; Cui, Y.; Xu, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Biodegradation of lignin by Pseudomonas sp. Q18 and the characterization of a novel bacterial DyP-type peroxidase. J. Ind. Microbiol. Biotechnol. 2018, 45, 913–927. [Google Scholar] [CrossRef]
- Guo, H.; Lin, C.; Wang, S.; Jiang, D.; Zheng, B.; Liu, Y.; Qin, W. Characterization of a Novel Laccase-producing Bacillus sp. A4 and its Application in Miscanthus Degradation. BioResources 2017, 12, 4776–4794. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Swiderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkolazka, A.; Paszczynski, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Reiss, R.; Ihssen, J.; Thöny-Meyer, L. Bacillus pumilus laccase: A heat stable enzyme with a wide substrate spectrum. BMC Biotechnol 2011, 11, 9. [Google Scholar] [CrossRef]
- Buraimoh, O.M.; Amund, O.O.; Ilori, M.O. Kraft lignin degradation by autochtonous streptomyces strains isolated from a tropical lagoon ecosystem. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 248–253. [Google Scholar] [CrossRef][Green Version]
- Ece, S.; Lambertz, C.; Fischer, R.; Commandeur, U. Heterologous expression of a Streptomyces cyaneus laccase for biomass modification applications. AMB Express 2017, 7, 86. [Google Scholar] [CrossRef]
- Jing, D. Improving the simultaneous production of laccase and lignin peroxidase from Streptomyces lavendulae by medium optimization. Bioresour. Technol. 2010, 101, 7592–7597. [Google Scholar] [CrossRef]
- Blánquez, A.; Ball, A.S.; González-Pérez, J.A.; Jiménez-Morillo, N.T.; González-Vila, F.; Arias, M.E.; Hernández, M. Laccase SilA from Streptomyces ipomoeae CECT 3341, a key enzyme for the degradation of lignin from agricultural residues? PLoS ONE 2017, 12, e0187649. [Google Scholar] [CrossRef]
- Miyazaki, K. A hyperthermophilic laccase from Thermus thermophilus HB27. Extremophiles 2005, 9, 415–425. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y. Biotransformation of lignin: Mechanisms, applications and future work. Biotechnol. Prog. 2020, 36, e2922. [Google Scholar] [CrossRef]
- Khan, A.; Nair, V.; Colmenares, J.C.; Gläser, R. Lignin-Based Composite Materials for Photocatalysis and Photovoltaics. Top. Curr. Chem. 2018, 376, 20. [Google Scholar] [CrossRef]
- Kirk, T.K.; Farrell, R.L. Enzymatic “Combustion”: The Microbial Degradation of Lignin. Annu. Rev. Microbiol. 1987, 41, 465–501. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Kuhar, S.; Sharma, K.K.; Shrivastava, B. Microorganisms and Enzymes Involved in Lignin Degradation Vis-à-vis Production of Nutritionally Rich Animal Feed: An Overview. In Biotechnology for Environmental Management and Resource Recovery; Kuhad, R.C., Singh, A., Eds.; Springer: New Delhi, India, 2013; pp. 3–44. [Google Scholar] [CrossRef]
- Hasanov, I.; Raud, M.; Kikas, T. The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass. Energies 2020, 13, 24. [Google Scholar] [CrossRef]
- Pollegioni, L.; Tonin, F.; Rosini, E. Lignin-degrading enzymes. FEBS J. 2015, 282, 1190–1213. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Holowacz, I.; Lukajtis, R.; Glinka, M.; Kaminski, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Fadhel, A.Z.; Pollet, P.; Liotta, C.L.; Eckert, C.A. Combining the Benefits of Homogeneous and Heterogeneous Catalysis with Tunable Solvents and Nearcritical Water. Molecules 2010, 15, 8400–8424. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Du, L.; Wu, W.; Xu, H.; Zhang, G.; Li, S.; Wang, C.; Lu, Z.; Deng, Y. Novel Lignin-Derived Water-Soluble Binder for Micro Silicon Anode in Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 12621–12629. [Google Scholar] [CrossRef]
- Meister, J. Modification of lignin. J. Macromol. Sci.—Polym. Rev. 2007, 42, 235–289. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Meng, X.; Pu, Y.; Ragauskas, A.J. Recent Advances in the Application of Functionalized Lignin in Value-Added Polymeric Materials. Polymers 2020, 12, 2277. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Rita, C.; Morais, A.; Lopes, A.; Lukasik, R.; Anastas, P. Lignin Transformations for High Value Applications: Towards Targeted Modifications Using Green Chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Kamara, J.M.; Heidrich, O.; Tafaro, V.E.; Maltese, S.; Dejaco, M.C.; Re Cecconi, F. Change Factors and the Adaptability of Buildings. Sustainability 2020, 12, 6586. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Liu, Y.; Ruan, X.; Tsang, D.C.W.; Hunt, A.J.; Ok, Y.S.; Song, H.; Zhang, S. Lignin valorization for the production of renewable chemicals: State-of-the-art review and future prospects. Bioresour Technol. 2018, 269, 465–475. [Google Scholar] [CrossRef]
- Akampumuza, O.; Wambua, P.; Ahmed, A.; Wei, l.; Qin, X.-H. A review of the applications of bio composites in the automotive industry. Polym. Compos. 2015, 38, 2553–2569. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Puziy, A.; Poddubnaya, O.; Sevastyanova, O. Carbon Materials from Technical Lignins: Recent Advances. Top. Curr. Chem. 2018, 376, 33. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Tomoeda, S.; Kitayama, Y.; Wakamatsu, J.; Minami, H.; Zetterlund, P.B.; Okubo, M. Nitroxide-Mediated Radical Polymerization in Microemulsion (Microemulsion NMP) of n-Butyl Acrylate. Macromolecules 2011, 44, 5599–5604. [Google Scholar] [CrossRef]
- Gupta, C.; Washburn, N.R. Polymer-Grafted Lignin Surfactants Prepared via Reversible Addition–Fragmentation Chain-Transfer Polymerization. Langmuir 2014, 30, 9303–9312. [Google Scholar] [CrossRef]
- Nomura, K.; Chaijaroen, P.; Abdellatif, M.M. Synthesis of Biobased Long-Chain Polyesters by Acyclic Diene Metathesis Polymerization and Tandem Hydrogenation and Depolymerization with Ethylene. ACS Omega 2020, 5, 18301–18312. [Google Scholar] [CrossRef]
- Bass, G.F.; Epps, T.H. Recent developments towards performance-enhancing lignin-based polymers. Polym. Chem. 2021, 12, 4130–4158. [Google Scholar] [CrossRef]
- Gao, W.; Kong, F.; Chen, J.; Fatehi, P. Chapter 13—Present and future prospective of lignin-based materials in biomedical fields. In Lignin-Based Materials for Biomedical Applications; Santos, H., Figueiredo, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 395–424. [Google Scholar] [CrossRef]
- Garg, J.; Nee Chiu, M.; Krishnan, S.; Kumar Tripathi, L.; Pandit, S.; Farasati Far, B.; Kumar Jha, N.; Kumar Kesari, K.; Tripathi, V.; Pandey, S.; et al. Applications of lignin nanoparticles for cancer drug delivery: An update. Mater. Lett. 2022, 311, 131573. [Google Scholar] [CrossRef]
- Hermosilla, E.; Rubilar, O.; Schalchli, H.; da Silva, A.S.A.; Ferreira-Leitao, V.; Diez, M.C. Sequential white-rot and brown-rot fungal pretreatment of wheat straw as a promising alternative for complementary mild treatments. Waste Manag. 2018, 79, 240–250. [Google Scholar] [CrossRef]
- Abe, M.M.; Branciforti, M.C.; Brienzo, M. Biodegradation of Hemicellulose-Cellulose-Starch-Based Bioplastics and Microbial Polyesters. Recycling 2021, 6, 22. [Google Scholar] [CrossRef]

| Enzyme | Fungi | Reference |
|---|---|---|
| DyP | Auricularia auricular-judae | [97] |
| LiP | P. chrysosporium | [98] |
| Phlebia radiata | [99] | |
| P. tremellosa | [100] | |
| MnP | Phanerochaete sordida | [101] |
| P. chrysosporium | [102] | |
| Trametes versicolor | [103] | |
| Ceriporiopsis subvermispora | [104] | |
| LaC | P. radiata | [105] |
| C. subvermispora | [106] | |
| Pleurotus eryngii | [107] | |
| T. versicolor | [108] | |
| T. hirsuta | [109] | |
| T. ochracea | [110] | |
| VP | P. eryngii | [111,112] |
| Pleurotus ostreatus | [112] | |
| Bjerkandera fumosa | [113] |
| Lignolytic Enzyme | Bacteria | Reference |
|---|---|---|
| DyP A | Amycolatopsis sp. | [12] |
| E. coli | [116] | |
| Rhodococcus jostii | [117] | |
| Streptomyces viridosporus | [117,118] | |
| S. coelicolor | [119] | |
| Thermobifida fusca | [120] | |
| T. fusca YX | [120] | |
| DyP B | Escherichia coli | [121] |
| Pseudomonas sp. | [122] | |
| R. jostii | [117] | |
| S. coelicolor | [89] | |
| Laccase | Bacillus atrophaeus | [123] |
| B. licheniformis | [124] | |
| B. pumilus | [125] | |
| B. subtilis | [12] | |
| S. coelicolor | [12] | |
| S. griseus | [126] | |
| S. ipomoea | [127] | |
| S. lavendulae | [128] | |
| Streptomyces cyaneus | [129] | |
| Thermus thermophilus | [130] | |
| DyP-type peroxidase | Ps. fluorescens, Comamonas testosteroni and Agrobacterium sp. | [115] |
| Category | Type | Products | Applications | References |
|---|---|---|---|---|
| Aromatic macromolecules and fine Chemicals | Klason (Kn), kraft lignin (KL), organosolv (OGs) | Lignin monomers and dimers, aromatic phenols, alkyl phenols, aromatic aldehydes, aromatic alcohols, acids, aryl ketones, antioxidants, dispersants, polyurethanes, phenolic resins, vanillin | Industrial chemicals, bio-based adhesives, multifunctional materials, building blocks for bio-based products | [145] |
| Polymer and nanomaterials | KL, OGs, straw lignin (SL) | 3D printing resin (cationic surfactant), scaffolds, lignin nanotubes, hydrogels, lignin nanotubes | Biomedical applications, tissue engineering, drug delivery | [147] |
| Carbon materials, biofuels | KL, sulfite, soda, OGs | Biochar, bio-oil, syngas, activated carbon, carbon fibers, carbon black | Light-weight polymer composites, adsorbents, electrochemical devices, automotive | [148] |
| Specialized applications | KL, sulfite, soda, OGs | Soil conditioner, controlled release agent in fertilizers and pesticides, sequestering agent, contaminant absorbent, fire retardant | agriculture, textiles, soil reclamation, water purification, fire suppression | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, V.K.; Gupta, N.; Kumar, P.; Dashti, M.G.; Tirth, V.; Khan, S.H.; Yadav, K.K.; Islam, S.; Choudhary, N.; Algahtani, A.; et al. Recent Advances in Synthesis and Degradation of Lignin and Lignin Nanoparticles and Their Emerging Applications in Nanotechnology. Materials 2022, 15, 953. https://doi.org/10.3390/ma15030953
Yadav VK, Gupta N, Kumar P, Dashti MG, Tirth V, Khan SH, Yadav KK, Islam S, Choudhary N, Algahtani A, et al. Recent Advances in Synthesis and Degradation of Lignin and Lignin Nanoparticles and Their Emerging Applications in Nanotechnology. Materials. 2022; 15(3):953. https://doi.org/10.3390/ma15030953
Chicago/Turabian StyleYadav, Virendra Kumar, Nitin Gupta, Pankaj Kumar, Marjan Ganjali Dashti, Vineet Tirth, Samreen Heena Khan, Krishna Kumar Yadav, Saiful Islam, Nisha Choudhary, Ali Algahtani, and et al. 2022. "Recent Advances in Synthesis and Degradation of Lignin and Lignin Nanoparticles and Their Emerging Applications in Nanotechnology" Materials 15, no. 3: 953. https://doi.org/10.3390/ma15030953
APA StyleYadav, V. K., Gupta, N., Kumar, P., Dashti, M. G., Tirth, V., Khan, S. H., Yadav, K. K., Islam, S., Choudhary, N., Algahtani, A., Bera, S. P., Kim, D.-H., & Jeon, B.-H. (2022). Recent Advances in Synthesis and Degradation of Lignin and Lignin Nanoparticles and Their Emerging Applications in Nanotechnology. Materials, 15(3), 953. https://doi.org/10.3390/ma15030953









