Synthesis, Characterization, and Photocatalytic Activity of Ba-Doped BiFeO3 Thin Films
Abstract
:1. Introduction
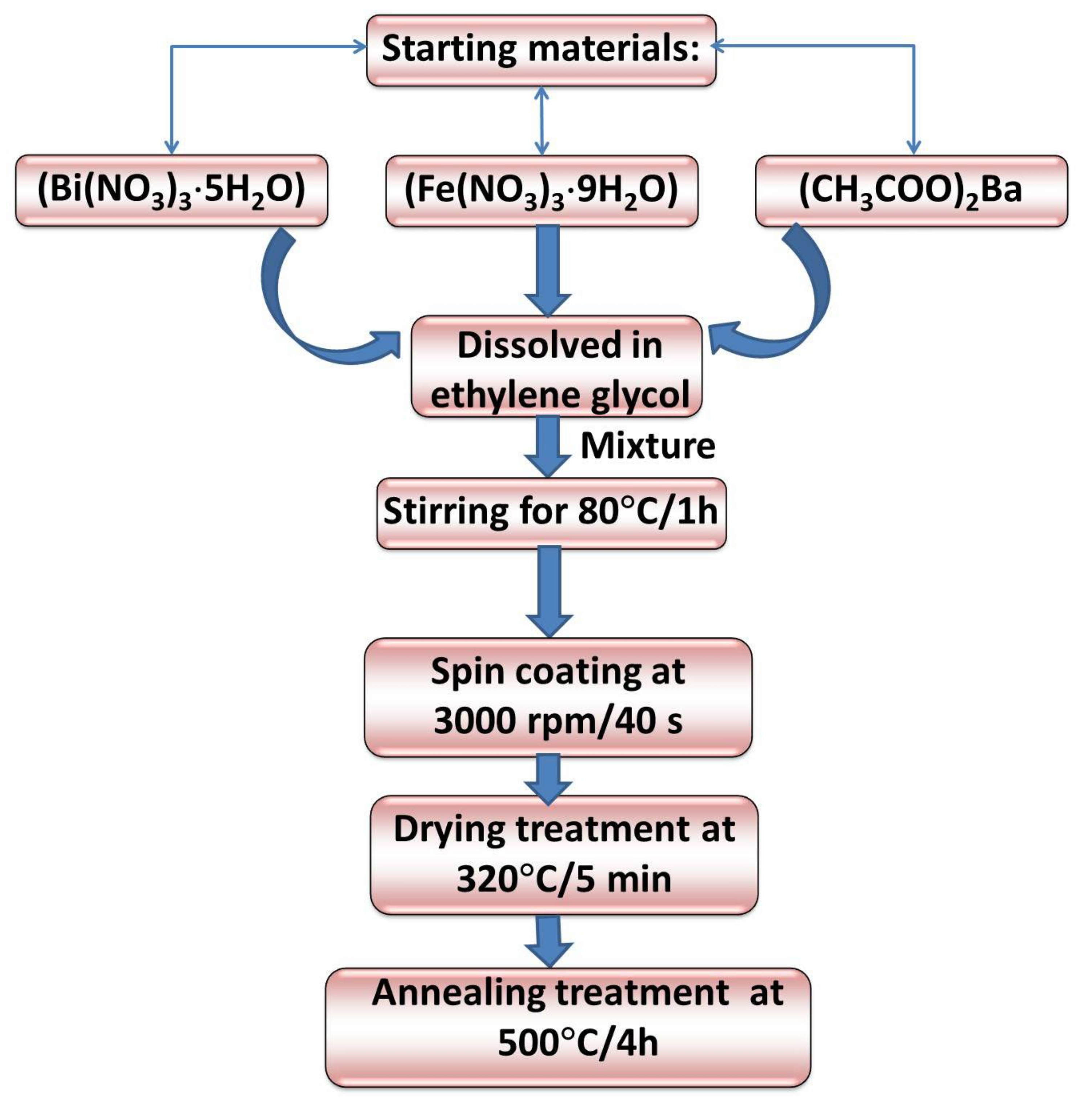
2. Preparation and Experimental Details
3. Results and Discussion
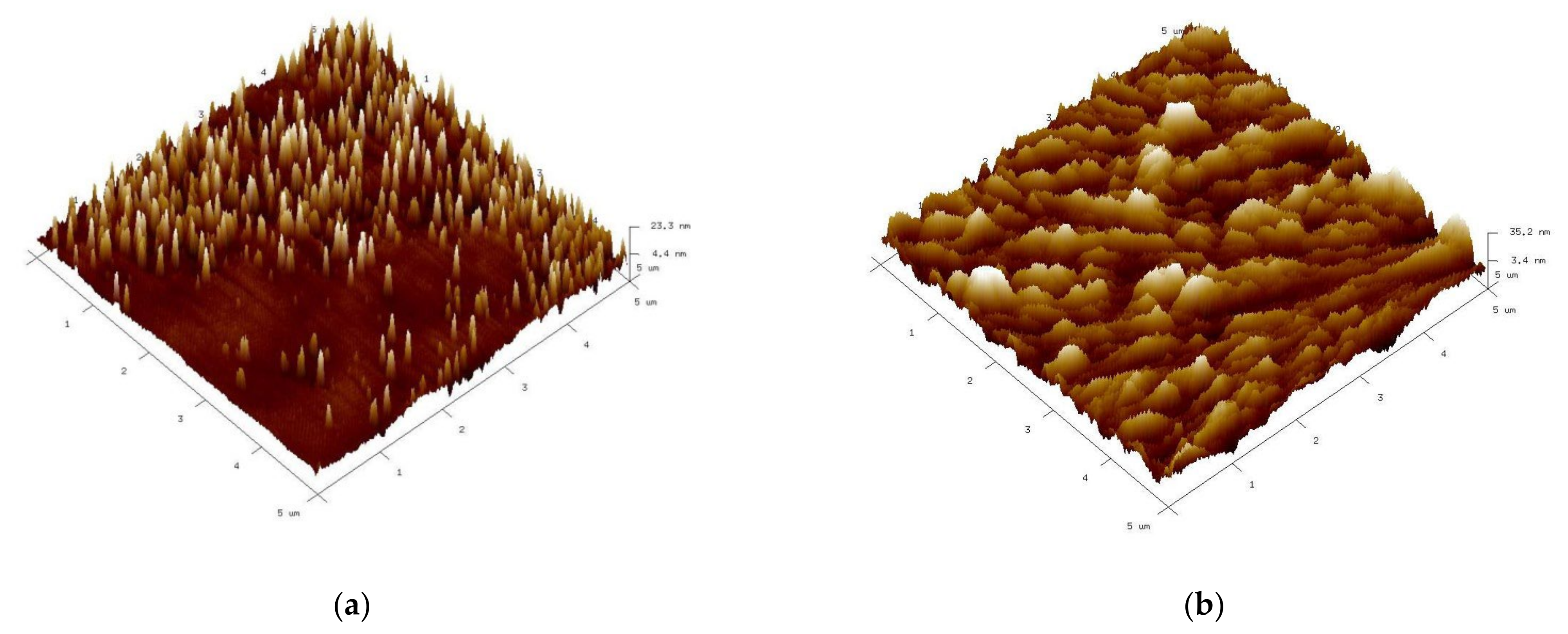
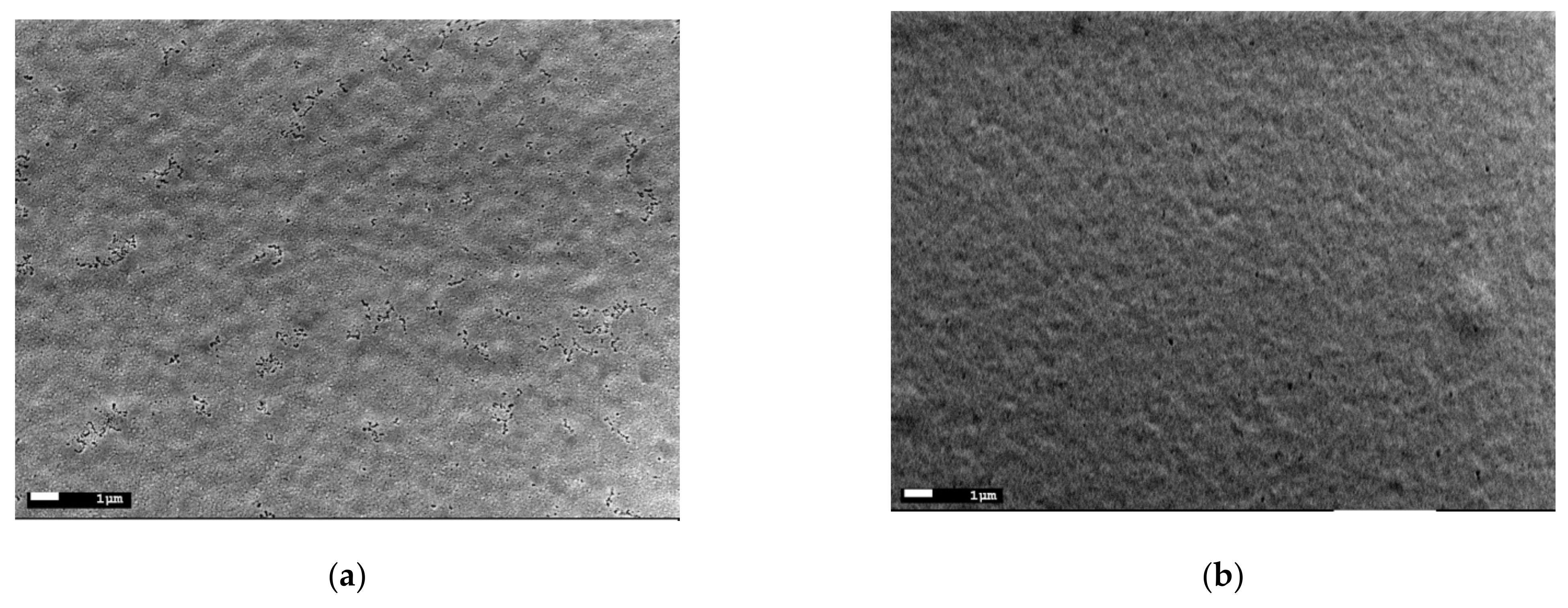
3.1. Phase, Structural, and Microstructural Characterization
3.2. Optical Properties of BBFO Thin Films
3.3. Photocatalytic Degradation of RhB
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ameta, R.; Benjamin, S.; Ameta, A.; Ameta, S.C. Photocatalytic Degradation of Organic Pollutants: A Review. Mater. Sci. Forum 2013, 734, 247–272. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Hua, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for application in photocatalysis: From thin films to nanostructures, Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manage. 2020, 258, 110050. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Lee, G.-J.; Wu, J.J. Recent developments in ZnS photocatalysts from synthesis to photocatalytic applications—A review. Powder Technol. 2017, 318, 8–22. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H.M. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Zoubi, W.A.; Al-Hamdani, A.A.S.; Sunghun, B.; Ko, Y.G. A review on TiO2-based composites for superior photocatalytic activity. Rev. Inorg. Chem. 2021, 41, 213–222. [Google Scholar] [CrossRef]
- Mushtaq, F.; Chen, X.; Torlakcik, H.; Nelson, B.J.; Panè, S. Enhanced catalytic degradation of organic pollutants by multi-stimuli activated multiferroic nanoarchitectures. Nano Res. 2020, 13, 2183–2191. [Google Scholar] [CrossRef]
- Mettout, B.; Toledano, P. Theory of the photovoltaic and light-induced effects in multiferroics. In Emerging Photovoltaic Materials (Silicon & Beyond); ch. 7. part II.; Kurinec, S.K., Ed.; John Wiley&Sons: Hoboken, NJ, USA, 2018; pp. 195–238. [Google Scholar]
- Ianculescu, A.; Prihor, F.; Postolache, P.; Mitoseriu, L.; Dragan, N.; Crisan, D. Preparation, Structural and Magnetic Properties of Mn-Doped La0.1Bi0.9FeO3 Ceramics. Ferroelectrics 2009, 391, 67–75. [Google Scholar] [CrossRef]
- Gheorghiu, F.; Curecheriu, L.; Ianculescu, A.; Calugaru, M.; Mitoseriu, L. Tunable dielectric characteristics of Mn-doped BiFeO3 multiferroic ceramics. Scr. Mater. 2013, 68, 305–308. [Google Scholar] [CrossRef]
- Gheorghiu, F.; Tanasa, R.; Buscaglia, M.T.; Buscaglia, V.; Pastravanu, C.G.; Popovici, E.; Mitoseriu, L. Preparation of Bi2Fe4O9 particles by hydrothermal synthesis and functional properties. Phase Transit. 2013, 86, 726–736. [Google Scholar] [CrossRef]
- Tuluk, A.Y.; Mahon, T.R.; van der Zwaag, S.; Groen, P. BiFeO3 synthesis by conventional solid-state reaction. In Proceedings of the 2019 IEEE International Symposium on Applications of Ferroelectrics (ISAF), Lausanne, Switzerland, 14–19 July 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, K.S.; Kim, H.G.; Lee, H.-G.; Kang, H.-W.; Kim, J.S.; Cheon, C.I. Synthesis and characterization of multiferroic BiFeO3 powders fabricated by hydrothermal method. Ceram. Int. 2010, 36, 1365–1372. [Google Scholar] [CrossRef]
- Majid, F.; Mirza, S.T.; Riaz, S.; Naseem, S. Sol-Gel Synthesis of BiFeO3 Nanoparticles, Mater. Today Proc. 2015, 2, 5293–5297. [Google Scholar]
- Penalva, J.; Lazo, A. Synthesis of Bismuth Ferrite BiFeO3 by solution combustion method. J. Phys. Conf. Ser. 2018, 1143, 12025. [Google Scholar] [CrossRef]
- Ferri, E.A.V.; Santos, I.A.; Radovanovic, E.; Bonzanini, R.; Girotto, E.M. Chemical Characterization of BiFeO3 Obtained by Pechini Method. J. Braz. Chem. Soc. 2008, 19, 1153–1157. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.R.; Kothari, D.; Upadhyay, S.K.; Gupta, A.; Chauhan, N.; Awasthi, A.M. Reduced leakage current of multiferroic BiFeO3 ceramics with microwave synthesis. Ceram. Int. 2014, 40, 4247–4250. [Google Scholar] [CrossRef]
- Gheorghiu, F.P.; Ianculescu, A.; Postolache, P.; Lupu, N.; Dobromir, M.; Luca, D.; Mitoseriu, L. Preparation and properties of (1−x)BiFeO3–xBaTiO3 multiferroic ceramics. J. Alloy. Compd. 2010, 506, 862–867. [Google Scholar] [CrossRef]
- Zargazi, M.; Entezari, M.H. A novel synthesis of forest like BiFeO3 thin film: Photo-electrochemical studies and its application as a photocatalyst for phenol degradation. Appl. Surf. Sci. 2019, 483, 793–802. [Google Scholar] [CrossRef]
- Li, P.; Lin, Y.-H.; Nan, C.W. Effect of nonmagnetic alkaline-earth dopants on magnetic properties of BiFeO3 thin films. J. Appl. Phys. 2011, 110, 033922. [Google Scholar] [CrossRef]
- Gaur, A.; Singh, P.; Choudhary, N.; Kumar, D.; Shariq, M.; Singh, K.; Kaur, N.; Kaur, D. Structural, optical and magnetic properties of Nd-doped BiFeO3 thin films prepared by pulsed laser deposition. Phys. B Condens. Matter 2011, 406, 1877–1882. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Xing, S.; Jian, J.; Liu, J.; Wang, H. Effective doping control in Sm-doped BiFeO3 thin films via deposition temperature. RSC Adv. 2020, 10, 40229–40233. [Google Scholar] [CrossRef]
- Hu, B.L.; Li, Z.Z.; Zhang, H.Q.; Chen, S.Y.; Ye, Q.Y.; Zhao, J.F.; Zhao, Y.; Huang, Z.G. Preparation and Properties of La-Doped BiFeO3 Thin Films. Mater. Sci. Forum. 2016, 848, 645–651. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.-K. Novel and facile synthesis of Ba-doped BiFeO3 nanoparticles and enhancement of their magnetic and photocatalytic activities for complete degradation of benzene in aqueous solution. J. Hazard. Mater. 2016, 316, 122–133. [Google Scholar] [CrossRef]
- Feng, Y.N.; Wang, H.C.; Shen, Y.; Lin, Y.H.; Nan, C.W. Magnetic and Photocatalytic Behaviors of Ba-Doped BiFeO3 Nanofibers. Int. J. Appl. Ceram. Technol. 2014, 11, 676–680. [Google Scholar] [CrossRef]
- Mahbub, R.; Islam, M.F. Sintering Behavior and Microstructure Development of Ba Doped BiFeO3. Int. J. Innov. Technol. Explor. Eng. 2014, 3, 2278. [Google Scholar]
- Shah, S.M.H.; Riaz, S.; Hussain, S.S.; Atiq, S.; Naseem, S. Structural, Magnetic and Dielectric Properties of Ba Doped BiFeO3 Thin Films. Mater. Today Proc. 2015, 2, 5654–5659. [Google Scholar] [CrossRef]
- Pani, T.K.; Sundaray, B.; Sahoo, G.; Rout, D. Influence of Barium Doping on Structural and Magnetic Properties of Bismuth Ferrite Thin Films via Spray Pyrolysis Metyhod. J. Phys. D Appl. Phys. 2020, 53, 325001. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing perovskite structures by tuning tolerance factor: Formation of formamidinium and cesium lead iodide solid-state alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Norton, M.G. X-ray Diffraction: A Practical Approach; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Cebela, M.; Jankovic, B.; Hercigonja, R.; Lukic, M.J. Comprehensive characterization of BiFeO3 powder synthesized by the hydrothermal procedure. Process. Appl. Ceram. 2016, 10, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Anthonyraj, C.; Muneeswaran, M.; Raj, S.G.; Giridharan, N.V.; Sivakumar, V.; Senguttuvan, G. Effect of samarium doping on the structural, optical and magnetic properties of sol-gel processed BiFeO3 thin films. J. Mater. Sci. Mater. Electron. 2015, 26, 49–58. [Google Scholar] [CrossRef]
- Mishra, R.K.; Pradhan, D.K.; Choudhary, R.N.P.; Banerjee, A. Effect of yttrium on improvement of dielectric properties and magnetic switching behavior in BiFeO3. J. Phys. Condens. Matter. 2008, 20, 45218. [Google Scholar] [CrossRef]
- Yongming, H.; Fei, L.; Zhang, Y.; Yuan, J.; Wang, Y.; Gu, H. Synthesis of bismuth ferrite nanoparticles via a wet chemical route at low temperature. J. Nanomater. 2011, 2011, 6. [Google Scholar] [CrossRef]
- Mevada, K.C.; Patel, V.D.; Patel, K.R. FT-IR, XRD and thermal studies of gel-grown barium tartrate crystals. Arch. Phys. Res. 2012, 3, 258–263. [Google Scholar]
- Vijayasundaram, S.V.; Kanagadurai, R. Size dependent magnetic properties of BiFeO3 nanoparticles: A multifunctional material for saving energy. Int. J. ChemTech Res. 2015, 8, 436–440. [Google Scholar]
- Hojamberdiev, M.; Xu, Y.; Wang, F.; Liu, W.; Wang, J. La-modification of multiferroic BiFeO3 by hydrothermal method at low temperature. Inorg. Mater. 2009, 45, 1183–1187. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.M. Enhanced multiferroic characteristics in NaNbO3-modified BiFeO3 ceramics. J. Appl. Phys. 2009, 105, 054107–054115. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Mandal, K. Study of structural, ferromagnetic and ferroelectric properties of nanostructured barium doped bismuth ferrite. J. Magn. Magn. Mater. 2014, 353, 57–64. [Google Scholar] [CrossRef]
- Xu, X.; Lin, Y.H.; Li, P.; Shu, L.; Nan, C.W. Synthesis and photocatalytic behaviors of high surface area BiFeO3 thin films. J. Am. Ceram. Soc. 2011, 94, 2296–2299. [Google Scholar] [CrossRef]
- Soltani, T.; Entezari, M.H. Sono-synthesis of bismuth ferrite nanoparticles with high photocatalytic activity in degradation of Rhodamine B under solar light irradiation. Chem. Eng. J. 2013, 223, 145–154. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, M. Band gap tuning and optical properties of BiFeO3 nanoparticles. Mater. Today Proc. 2020, 28, 168–171. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Dong, Z.; Shen, J.; Song, Q.; Wang, X.; Mao, W.; Pu, Y.; Li, X. Enhanced photocatalytic activity of Ba doped BiFeO3 by turning morphologis and band gap. J. Mater. Sci. Mater. Electron. 2020, 31, 15007–15012. [Google Scholar] [CrossRef]
- Haruna, A.; Abdulkadir, I.; Idris, S.O. Photocatalytic activity and doping effects of BiFeO3 nanoparticles in model organic dyes. Heliyon 2020, 6, e03237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
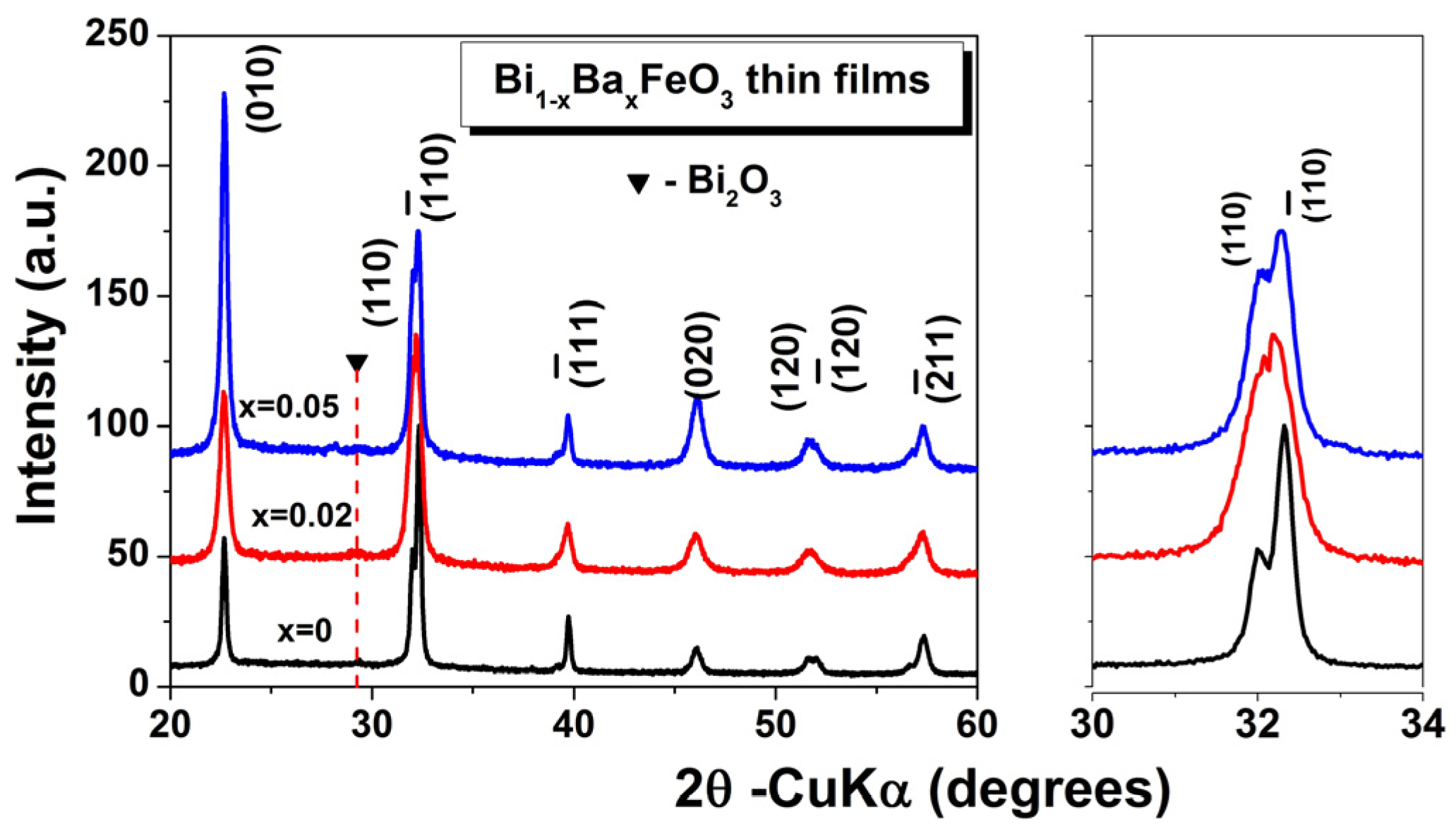


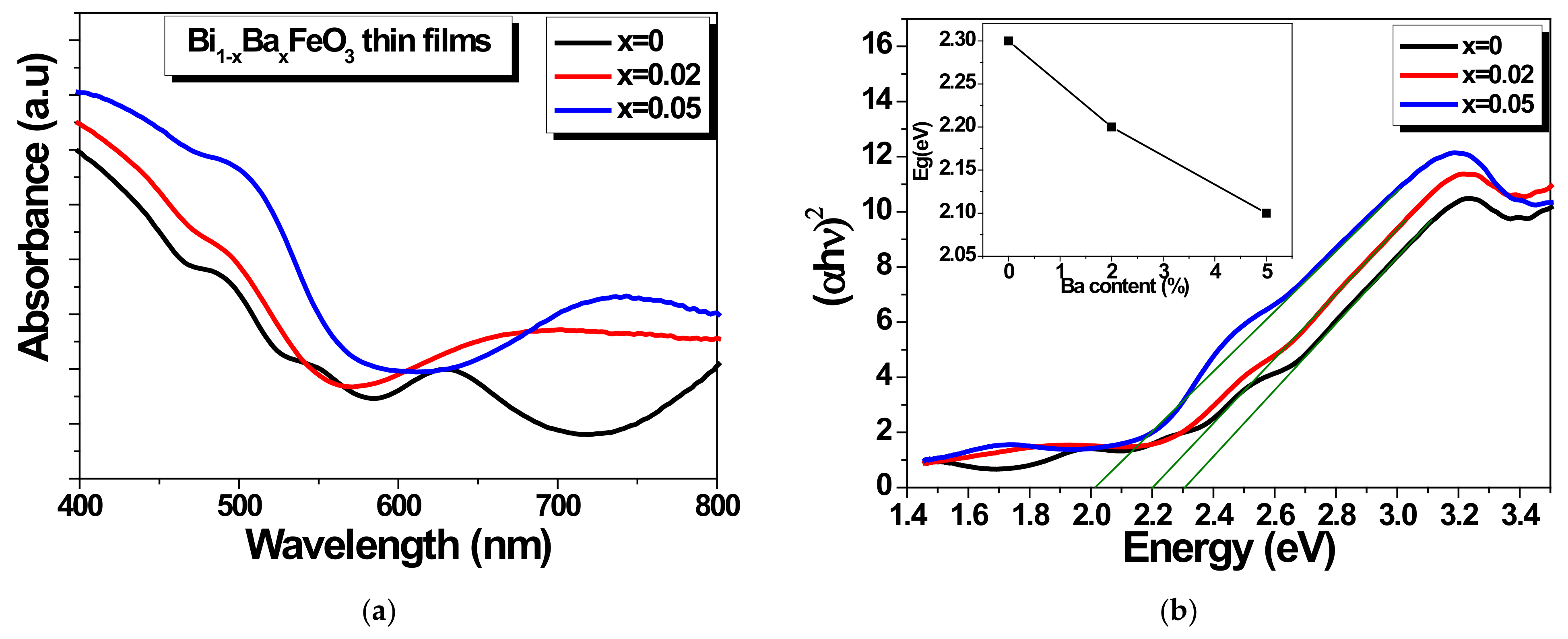
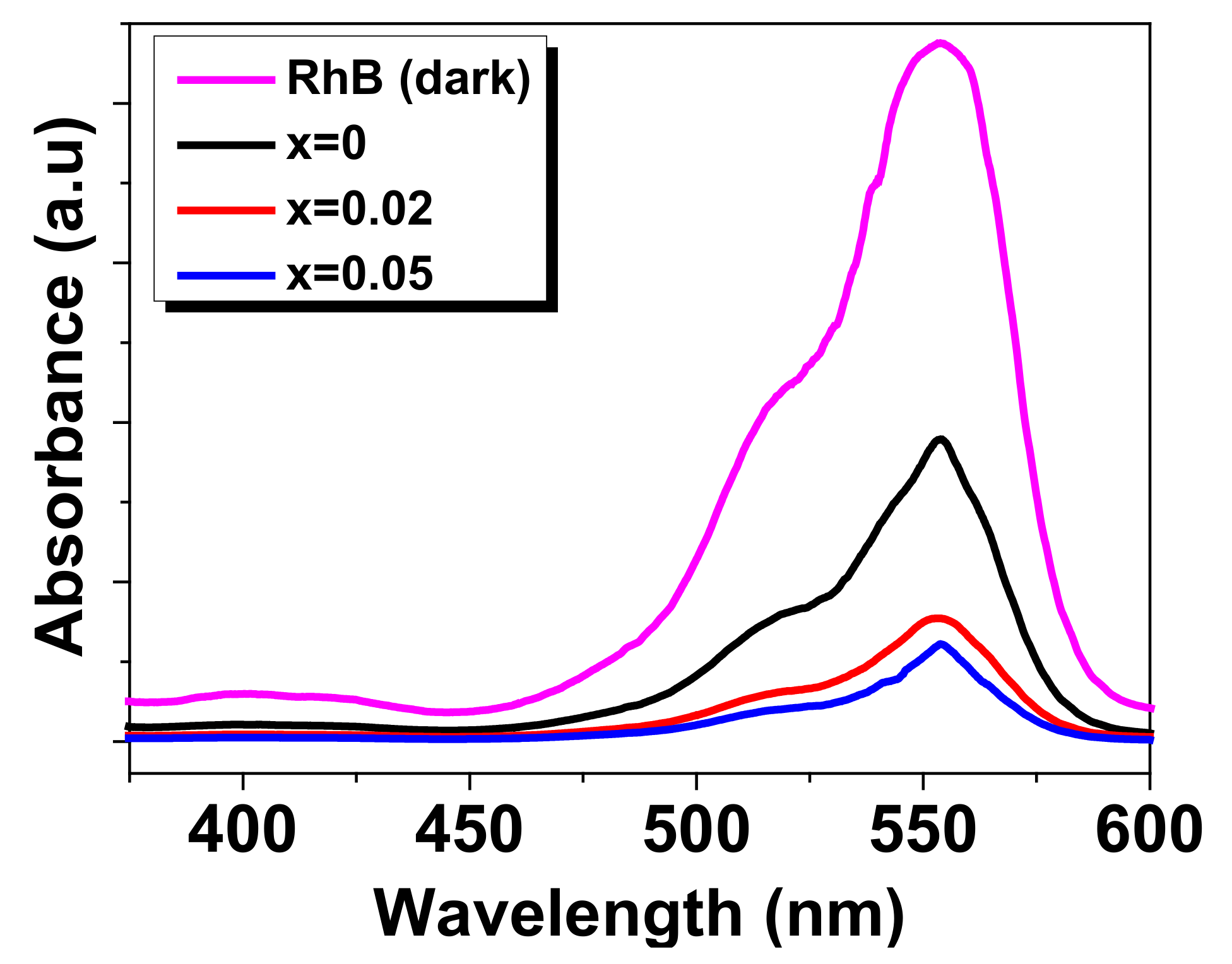
| Samples | Crystal Symmetry | a(Å) | Cell Volume V (Å3) | Crystallite Size D (nm) | Tolerance Factor (t) |
|---|---|---|---|---|---|
| x = 0 | Rhombohedral | 3.9262 | 60.57 | 41.22 | 0.890 |
| x = 0.02 | Rhombohedral | 3.9441 | 61.34 | 14.55 | 0.892 |
| x = 0.05 | Rhombohedral | 3.9322 | 60.79 | 18.67 | 0.893 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmadjid, K.; Gheorghiu, F.; Abderrahmane, B. Synthesis, Characterization, and Photocatalytic Activity of Ba-Doped BiFeO3 Thin Films. Materials 2022, 15, 961. https://doi.org/10.3390/ma15030961
Abdelmadjid K, Gheorghiu F, Abderrahmane B. Synthesis, Characterization, and Photocatalytic Activity of Ba-Doped BiFeO3 Thin Films. Materials. 2022; 15(3):961. https://doi.org/10.3390/ma15030961
Chicago/Turabian StyleAbdelmadjid, Khiat, Felicia Gheorghiu, and Boughelout Abderrahmane. 2022. "Synthesis, Characterization, and Photocatalytic Activity of Ba-Doped BiFeO3 Thin Films" Materials 15, no. 3: 961. https://doi.org/10.3390/ma15030961
APA StyleAbdelmadjid, K., Gheorghiu, F., & Abderrahmane, B. (2022). Synthesis, Characterization, and Photocatalytic Activity of Ba-Doped BiFeO3 Thin Films. Materials, 15(3), 961. https://doi.org/10.3390/ma15030961







