Promoters for Improved Adhesion Strength between Addition-Cured Liquid Silicone Rubber and Low-Melting-Point Thermoplastic Polyurethanes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
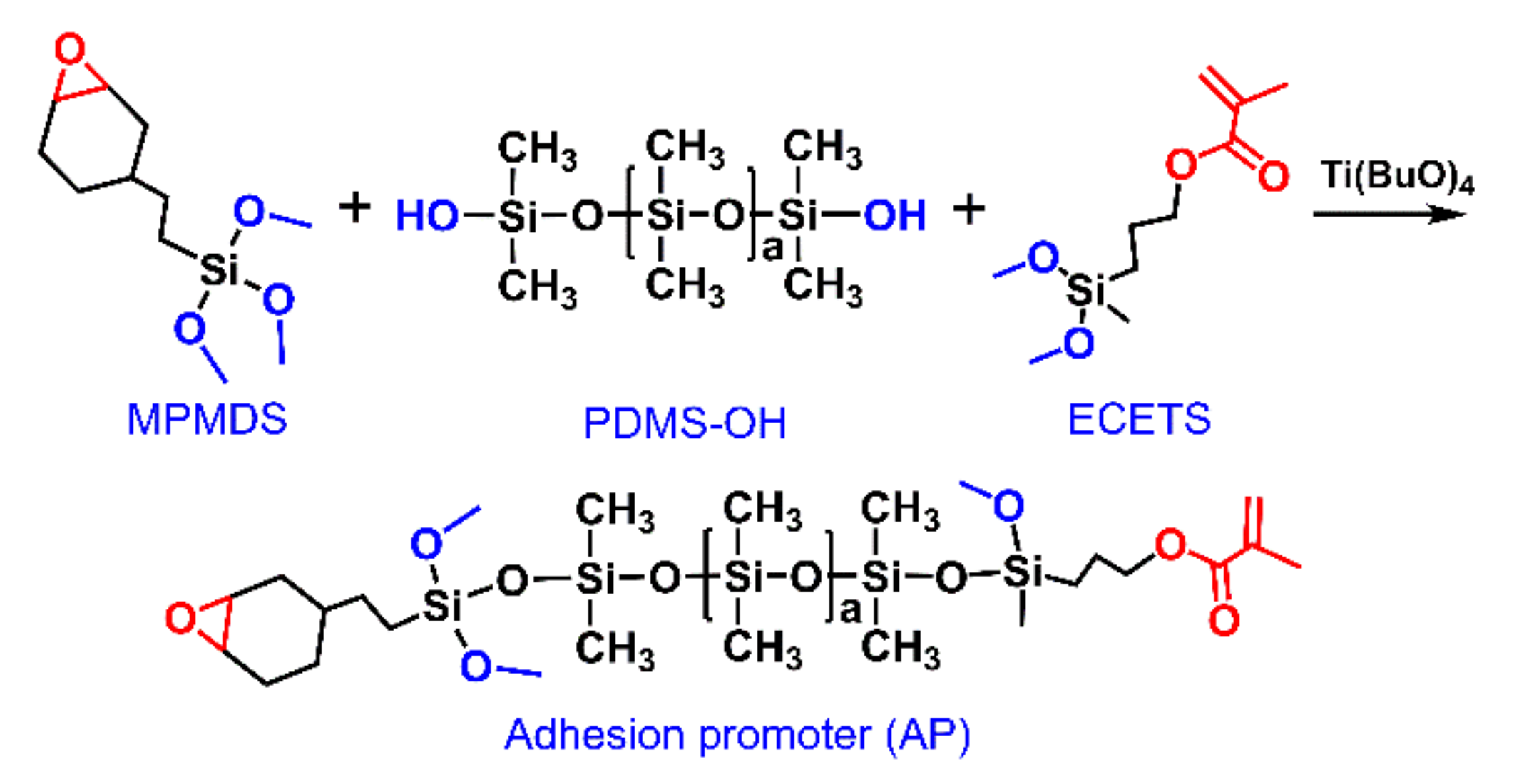
2.2. Synthesis of the Epoxy, Alkoxy and Acrylate Groups Modified Polydimethylsiloxane
2.3. Preparation of SA-LSR Samples
2.4. Characterization
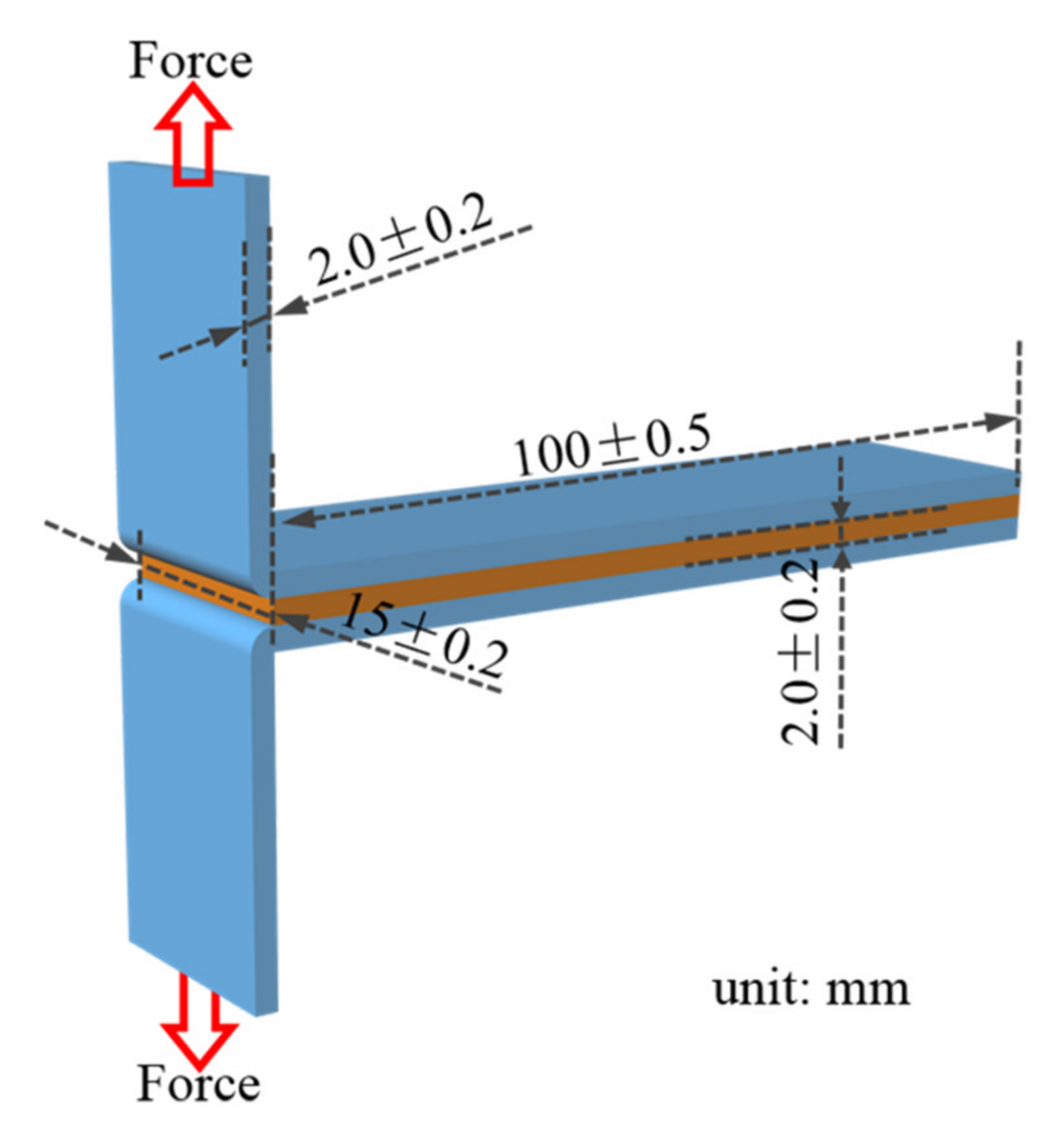
2.5. Adhesion Strength Test
3. Results
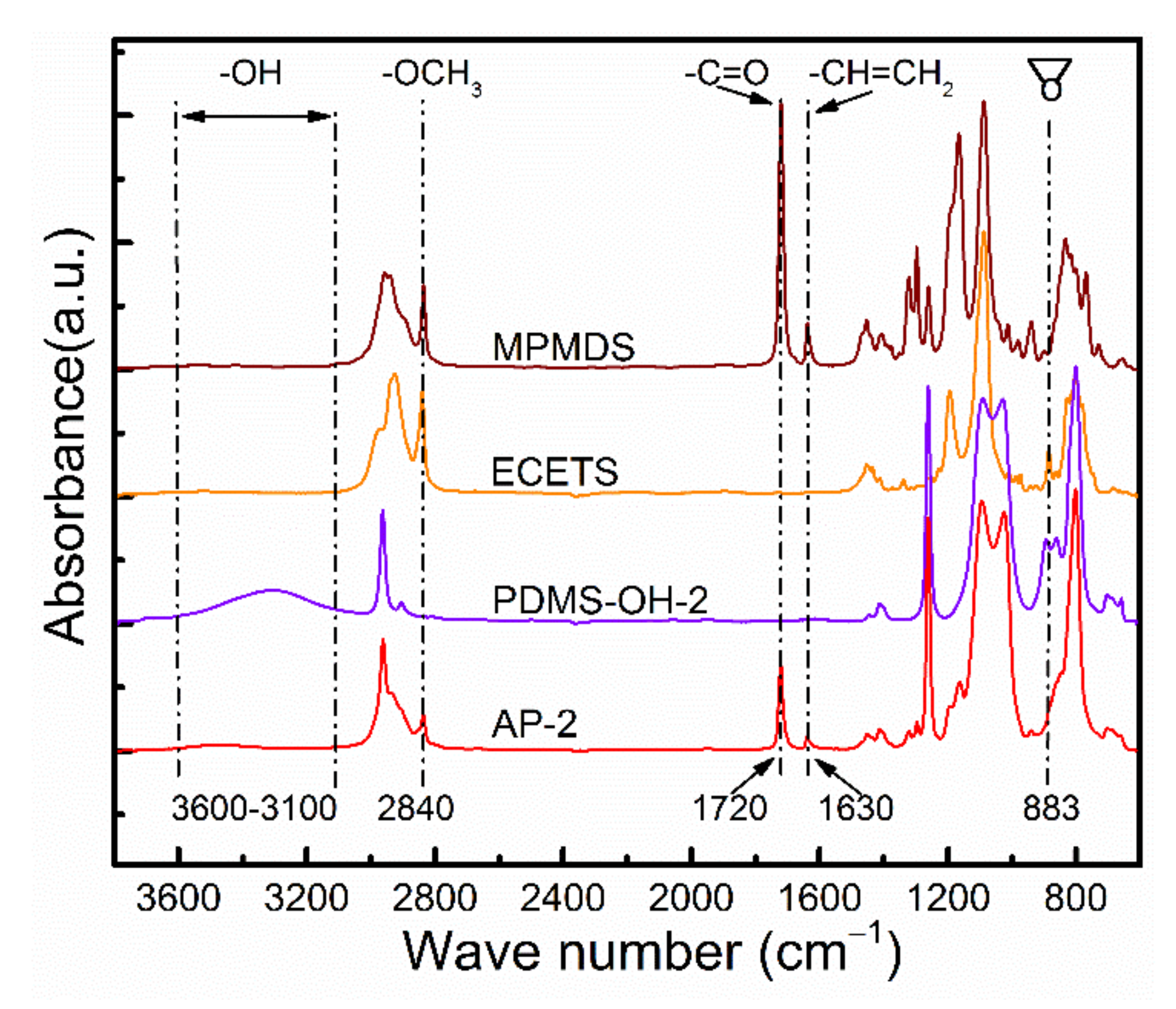
3.1. Characterizations of AP
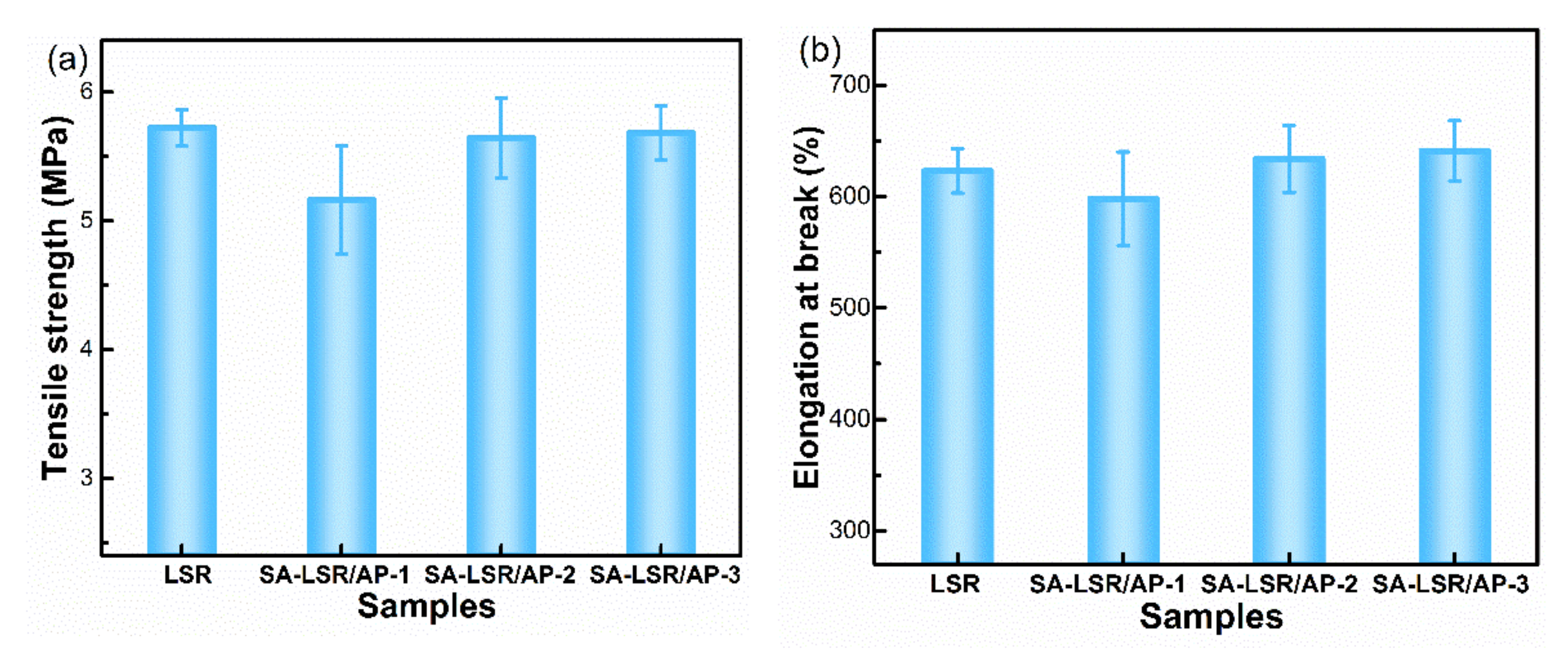
3.2. Mechanical Properties of SA-LSR
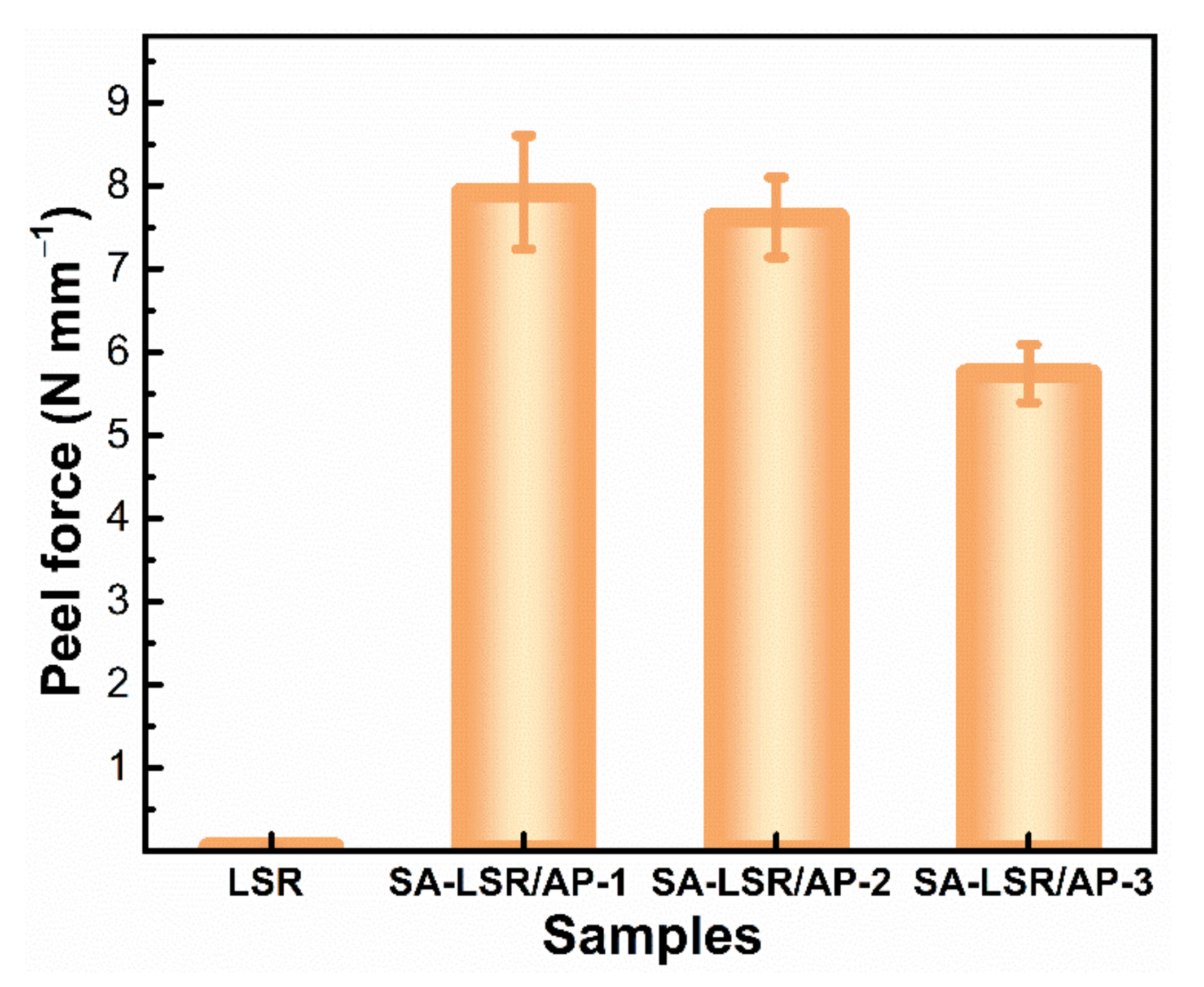
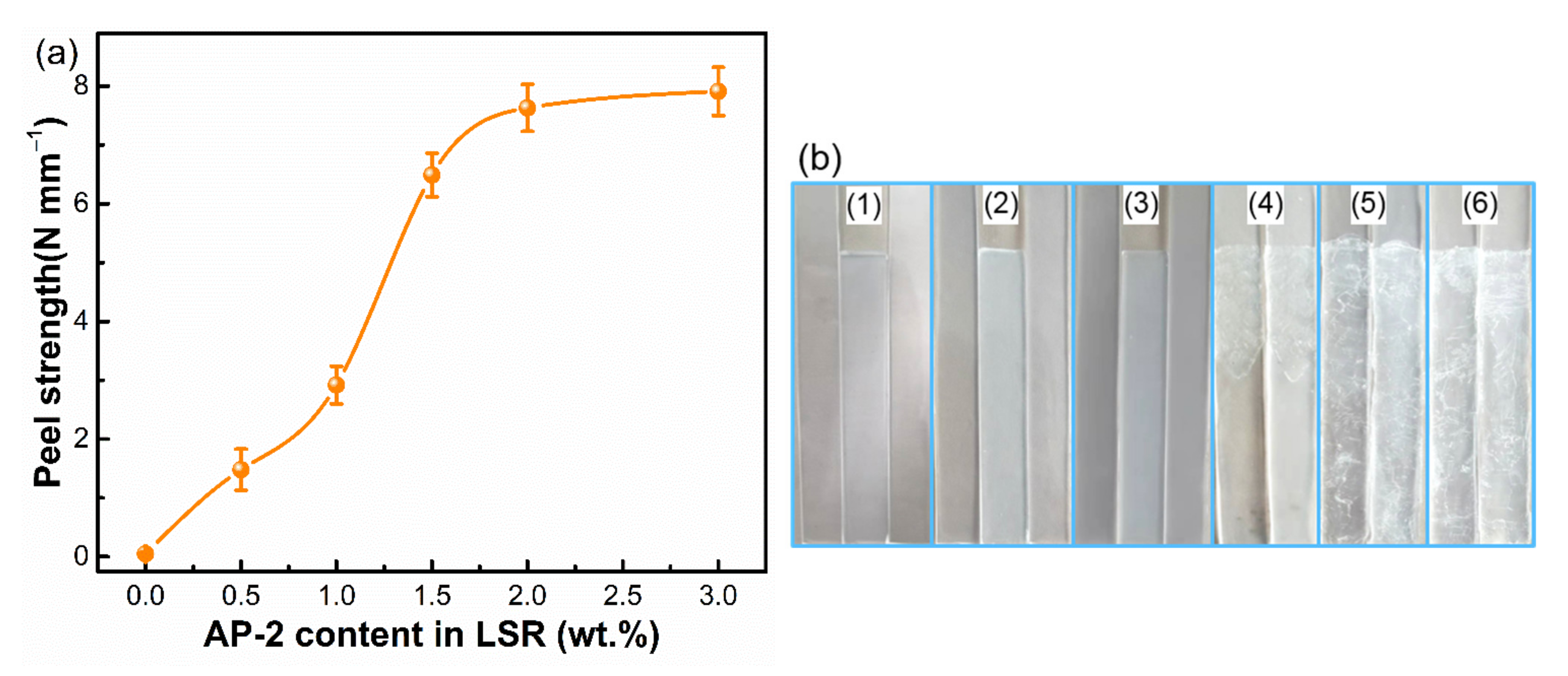
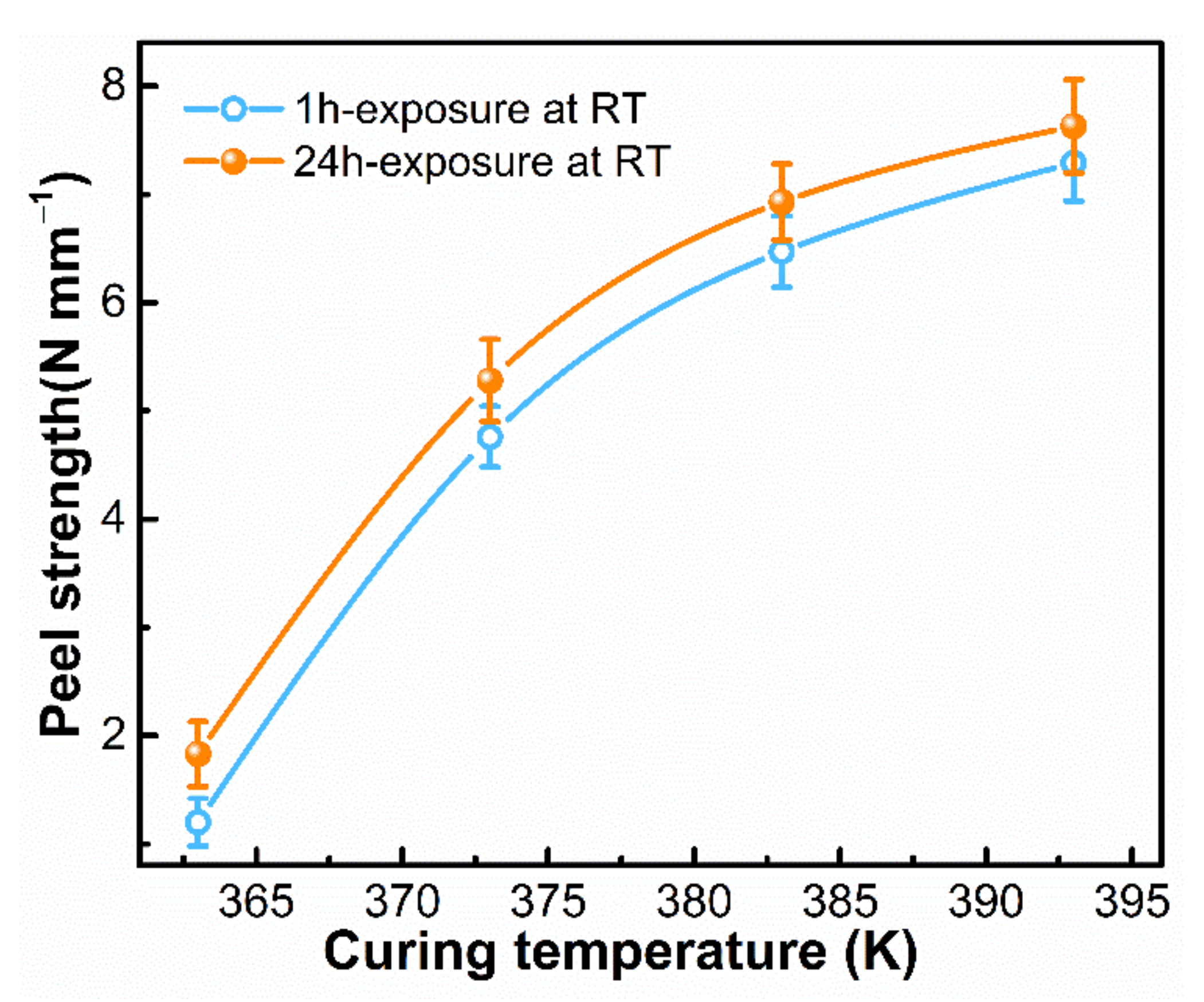
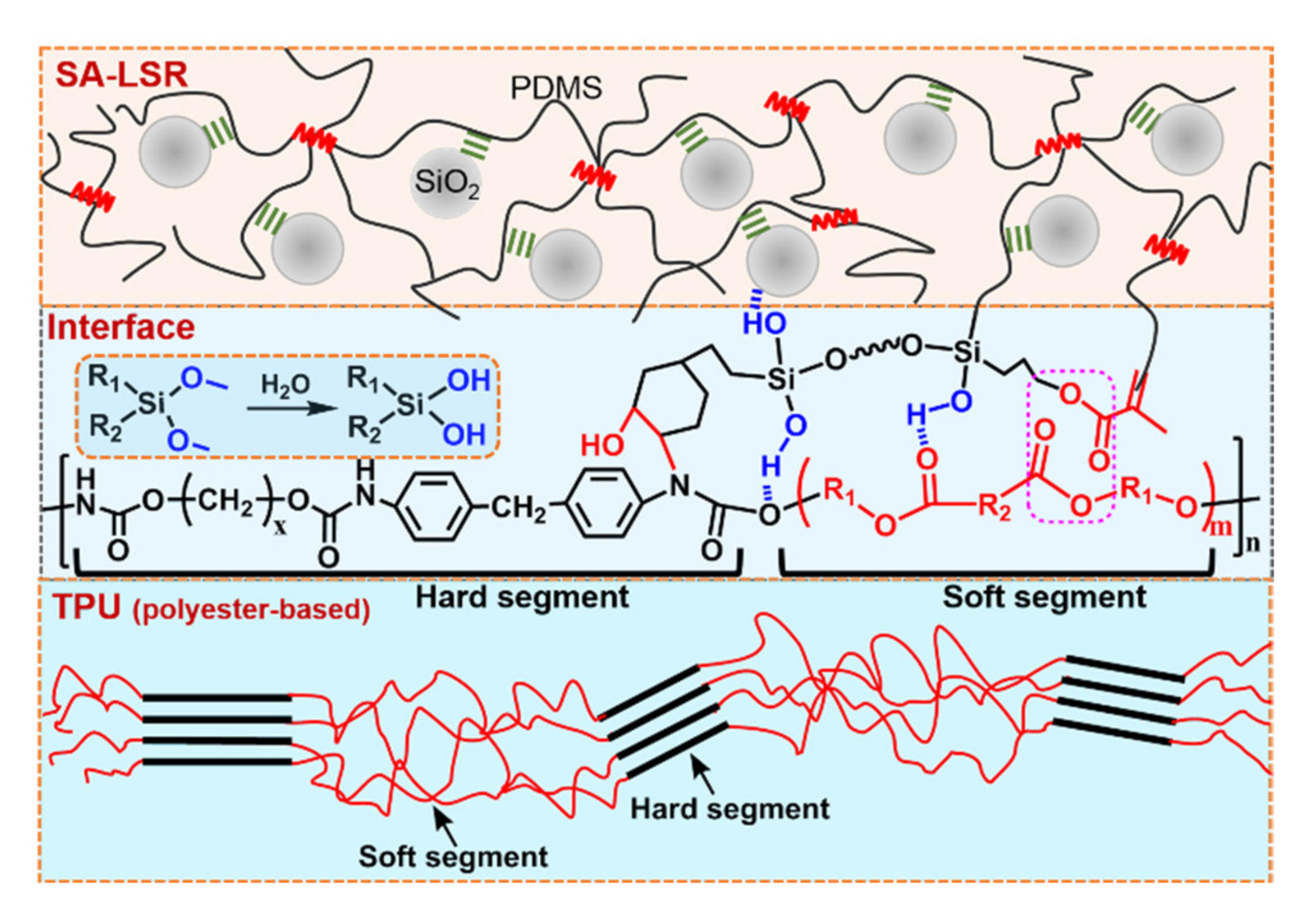
3.3. Adhesion Performance of SA-LSR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shit, S.C.; Shah, P. A review on silicone rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Shang, N.Q.; Chen, Q.G.; Wei, X.Z. Preparation and dielectric properties of SiC/LSR nanocomposites for insulation of high voltage direct current cable accessories. Materials 2018, 11, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Zhao, J.; Ren, J.; Rong, L.; Cao, P.F.; Advincula, R.C. 3D printed multifunctional, hyperelastic silicone rubber foam. Adv. Funct. Mater. 2019, 29, 1900469. [Google Scholar] [CrossRef]
- Zou, Z.; Wu, W.; Wang, Y.; Wang, L. Enhancement of thermal conductivity and tensile strength of liquid silicone rubber by three-dimensional alumina network. Soft Mater. 2019, 17, 297–307. [Google Scholar] [CrossRef]
- Rajan, K.P.; Al-Ghamdi, A.; Ramesh, P.; Nando, G.B. Blends of thermoplastic polyurethane (TPU) and polydimethyl siloxane rubber (PDMS), part-I: Assessment of compatibility from torque rheometry and mechanical properties. J. Polym. Res. 2012, 19, 9872. [Google Scholar] [CrossRef]
- Moiz, A.; Padhye, R.; Wang, X. Coating of TPU-PDMS-TMS on Polycotton Fabrics for Versatile Protection. Polymers 2017, 9, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zhang, X.; Cao, T.; Wang, T.; Sun, L.; Wang, K.; Fan, X. Antiliquid-Interfering, antibacteria, and adhesive wearable strain sensor based on superhydrophobic and conductive composite hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 46022–46032. [Google Scholar] [CrossRef]
- Lee, B.K.; Ryu, J.H.; Baek, I.; Kim, Y.; Jang, W.I.; Kim, S.; Yoon, Y.S.; Kim, S.H.; Hong, S.; Byun, S.; et al. Silicone-Based adhesives with highly tunable adhesion force for Skin-Contact applications. Adv. Healthc. Mater. 2017, 6, 1700621. [Google Scholar] [CrossRef]
- Cornejo-Bravo, J.M.; Palomino, K.; Palomino-Vizcaino, G.; Pérez-Landeros, O.M.; Curiel-Alvarez, M.; Valdez-Salas, B.; Bucio, E.; Magaña, H. Poly(N-vinylcaprolactam) and salicylic acid polymeric prodrug grafted onto medical silicone to obtain a novel thermo- and pH-Responsive drug delivery system for potential medical devices. Materials 2021, 14, 1065. [Google Scholar] [CrossRef]
- Haryńska, A.; Gubanska, I.; Kucinska-Lipka, J.; Janik, H. Fabrication and characterization of flexible Medical-Grade TPU filament for fused deposition modeling 3DP technology. Polymers 2018, 10, 1304. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Mi, H.; Huang, H.; Turng, L. Shape memory thermoplastic polyurethane (TPU)/poly(ε-caprolactone) (PCL) blends as self-knotting sutures. J. Mech. Behav. Biomed. 2016, 64, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.; Turng, L. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drupitha, M.P.; Bankoti, K.; Pal, P.; Das, B.; Parameswar, R.; Dhara, S.; Nando, G.B.; Naskar, K. Morphology-induced physico-mechanical and biological characteristics of TPU–PDMS blend scaffolds for skin tissue engineering applications. J. Biomed. Mater. Res. B 2019, 107, 1634–1644. [Google Scholar] [CrossRef]
- Drupitha, M.P.; Das, B.; Parameswaran, R.; Dhara, S.; Nando, G.B.; Naskar, K. Hybrid electrospun fibers based on TPU-PDMS and spherical nanohydroxyapatite for bone tissue engineering. Mater. Today Commun. 2018, 16, 264–273. [Google Scholar]
- Basak, S. Thermoplastic elastomers in biomedical industry—Evolution and current trends. J. Macromol. Sci. A 2021, 58, 579–593. [Google Scholar] [CrossRef]
- Fink, J.K. Liquid Silicone Rubber: Chemistry, Materials, and Processing; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Zhao, X.; Zang, C.; Wen, Y.; Jiao, Q. Studies on adhesion performance of addition-curable liquid silicone rubber on epoxy networks treated with different structures borosiloxane oligomers. J. Adhes. Sci. Technol. 2019, 33, 861–870. [Google Scholar] [CrossRef]
- Wang, J.; Xia, W.; Liu, K.; Tuo, X. Improved adhesion of silicone rubber to polyurethane by surface grafting. J. Appl. Polym. Sci. 2011, 121, 1245–1253. [Google Scholar] [CrossRef]
- Tsai, M.F.; Lee, Y.D.; Ling, Y.C. Improved adhesion of silicone rubber to polyurethane by induced surface reconstruction. J. Appl. Polym. Sci. 1998, 70, 1669–1675. [Google Scholar] [CrossRef]
- Bloomfield, L.A. Primer system for bonding conventional adhesives and coatings to silicone rubber. Int. J. Adhes. Adhes. 2016, 68, 239–247. [Google Scholar] [CrossRef]
- Seitz, V.; Arzt, K.; Mahnel, S.; Rapp, C.; Schwaminger, S.; Hoffstetter, M.; Wintermantel, E. Improvement of adhesion strength of self-adhesive silicone rubber on thermoplastic substrates—Comparison of an atmospheric pressure plasma jet (APPJ) and a Pyrosil® flame. Int. J. Adhes. Adhes. 2016, 66, 65–72. [Google Scholar] [CrossRef]
- Zhao, X.; Zang, C.; Sun, Y.; Liu, K.; Wen, Y.; Jiao, Q. Borosiloxane oligomers for improving adhesion of addition-curable liquid silicone rubber with epoxy resin by surface treatment. J. Mater. Sci. 2018, 53, 1167–1177. [Google Scholar] [CrossRef]
- Roth, J.; Albrecht, V.; Nitschke, M.; Bellmann, C.; Simon, F.; Zschoche, S.; Michel, S.; Luhmann, C.; Grundke, K.; Voit, B. Surface functionalization of silicone rubber for permanent adhesion improvement. Langmuir 2008, 24, 12603–12611. [Google Scholar] [CrossRef]
- Chang, P.P.; Hansen, N.A.; Phoenix, R.D.; Schneid, T.R. The effects of primers and surface bonding characteristics on the adhesion of polyurethane to two commonly used silicone elastomers. J. Prosthodont. 2009, 18, 23–31. [Google Scholar] [CrossRef]
- Grard, A.; Belec, L.; Perrin, F.X. Characterization and evaluation of primer formulations for bonding silicone rubber to metal. Prog. Org. Coat. 2020, 140, 105513. [Google Scholar] [CrossRef]
- Picard, L.; Phalip, P.; Fleury, E.; Ganachaud, F. Chemical adhesion of silicone elastomers on primed metal surfaces: A comprehensive survey of open and patent literatures. Prog. Org. Coat. 2015, 80, 120–141. [Google Scholar] [CrossRef]
- Pan, Z.; Huang, B.; Zhu, L.; Zeng, K. Synthesis of siloxane oligomers containing boron and epoxy groups for promoting the adhesion of addition-curable silicone rubber to PPA and copper plate. J. Adhes. Sci. Technol. 2021, 35, 626–640. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Wang, D. Adhesion enhancement for liquid silicone rubber and different surface by organosilane and Pt catalyst at room temperature. Bull. Mater. Sci. 2013, 36, 1013–1017. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Sun, R.; Zhu, S.; Kang, Y.; Huang, B.; Zhu, L. The synthesis, characterization and properties of silicone adhesion promoters for addition-cure silicone rubber. J. Adhes. Sci. Technol. 2018, 32, 1517–1530. [Google Scholar] [CrossRef]
- Li, C.; Su, S.; Wang, B.; Zhou, J. Preparation of one-component addition-cure liquid silicone rubber coating with enhanced storage stability and bond strength. J. Mater. Sci.-Mater. Electron. 2021, 32, 21052–21064. [Google Scholar] [CrossRef]
- Tsai, M.F.; Lee, Y.D.; Long, Y.C. Synthesis of a polydimethylsiloxane-block-hydroxyl grafted acrylate prepolymer copolymer to improve the adhesion between silicone rubber and polyurethane by induced surface reconstruction. J. Polym. Res. 2000, 7, 73–79. [Google Scholar] [CrossRef]
- Ana, U.J.; Critchlow, G.W.; Ford, K.M.; Godfrey, N.R.; Grandy, D.B.; Spence, M.A. A preliminary investigation into the apparent abhesive effect of stearic acid on cured silicone elastomer. Int. J. Adhes. Adhes. 2010, 30, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.H.; Shen, M.Y.; Kuan, C.F.; Kuan, H.C.; Ke, C.Y.; Chiang, C.L. Improving thermal stability of polyurethane through the addition of hyperbranched polysiloxane. Polymers 2019, 11, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medda, S.K.; Kundu, D.; De, G. Inorganic–organic hybrid coatings on polycarbonate.: Spectroscopic studies on the simultaneous polymerizations of methacrylate and silica networks. J. Non-Cryst. Solids 2003, 318, 149–156. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kurosawa, A.; Nagao, D.; Konno, M. Fabrication of barium titanate nanoparticles-epoxy resin composite films and their dielectric properties. Polym. Compos. 2010, 31, 1179–1183. [Google Scholar] [CrossRef]
- Pan, K.X.; Zeng, X.R.; Li, H.Q.; Lai, X.J. Synthesis of silane oligomers containing vinyl and epoxy group for improving the adhesion of addition-cure silicone encapsulant. J. Adhes. Sci. Technol. 2016, 30, 1131–1142. [Google Scholar] [CrossRef]
- Myers, J.N.; Zhang, X.; Xiu, Y.; Wei, Y.; Williamson, J.M.; Lee, K.; Chen, Z. Nondestructive characterization of molecular structures at buried Copper/Epoxy interfaces and their relationship to locus of failure analysis. IEEE Trans. Compon. Packag. Manuf. Technol. 2015, 5, 1432–1440. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]

| Compositions | Content (g) |
|---|---|
| PDMS-Vi-1 | 50.0 |
| PDMS-Vi-2 | 90.0 |
| PHMS | 3.8 |
| Hydrophobic fumed silica | 20.0 |
| Platinum catalyst | 0.2 |
| 3,5-dimethyl-1-hexyn-3-ol | 0.3 |
| AP-2 | 0–5.0 |
| Samples | Mn | Mw | Mw/Mn | OH (wt.%) a |
|---|---|---|---|---|
| PDMS-OH-1 | 565 | 898 | 1.59 | 6.02 |
| PDMS-OH-2 | 1028 | 1452 | 1.41 | 3.31 |
| PDMS-OH-3 | 2205 | 3321 | 1.51 | 1.54 |
| AP-1 | 1205 | 1962 | 1.63 | / |
| AP-2 | 1605 | 2392 | 1.49 | / |
| AP-3 | 2698 | 4187 | 1.55 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-K.; Zheng, K.-W.; Nie, X.-C.; Ge, H.-R.; Wang, Q.-Y.; Xu, J.-T. Promoters for Improved Adhesion Strength between Addition-Cured Liquid Silicone Rubber and Low-Melting-Point Thermoplastic Polyurethanes. Materials 2022, 15, 991. https://doi.org/10.3390/ma15030991
Wu J-K, Zheng K-W, Nie X-C, Ge H-R, Wang Q-Y, Xu J-T. Promoters for Improved Adhesion Strength between Addition-Cured Liquid Silicone Rubber and Low-Melting-Point Thermoplastic Polyurethanes. Materials. 2022; 15(3):991. https://doi.org/10.3390/ma15030991
Chicago/Turabian StyleWu, Jia-Kai, Kai-Wen Zheng, Xing-Cheng Nie, Huang-Rong Ge, Qiong-Yan Wang, and Jun-Ting Xu. 2022. "Promoters for Improved Adhesion Strength between Addition-Cured Liquid Silicone Rubber and Low-Melting-Point Thermoplastic Polyurethanes" Materials 15, no. 3: 991. https://doi.org/10.3390/ma15030991






