1. Introduction
Aerogels have become one of the most interesting materials. They have outstanding properties, such as large specific surface area, high porosity, low density and thermal conductivity, which are attributed mostly to their mesoporous nanostructure [
1,
2]. These properties have made aerogels intriguing as thermal insulators, dielectrics for electronics [
1,
3,
4], molecular sieves, catalysis supports, sensors and as energy storage media [
5]. Kistler was the first to introduce aerogels in 1931 [
6]. A basic aerogel synthesis involves three distinct steps: gelation, aging, and drying. Given the desired chemistry of the aerogels (e.g., SiO
2, SiOC), the selected precursors are dispersed in a solvent and allowed to gel, thus forming a continuous network of solid particles throughout the liquid, called wet gel.
The most critical step in preparation of the aerogel is supercritical drying. It is a tricky process that eliminates the liquid that lies in the pores of the gel by taking it to the supercritical state, thus avoiding collapse of the solid network. Ideally, the microstructure of a dry aerogel is identical to that of the wet gel before drying. On the other hand, conventional drying is responsible for large deformations as capillary stresses arise at the liquid-vapour interface. This capillary pressure may reach as high as 100–200 MPa [
5,
7] due to the presence of micro- and mesopores (it is inversely proportional to pore radius), causing the wet gel to collapse, eventually cracking and changing the size and shape of the mesoporous network. In general, the main benefits of the supercritical drying over ambient pressure drying are (1) extremely porous monolithic crack-free aerogels are prepared and (2) the relatively very fast process. However, drying with supercritical CO
2 has certain limitations, such as high processing costs for commercial applications, since drying is carried out at high pressures, low moisture stability and low mechanical resistance [
8].
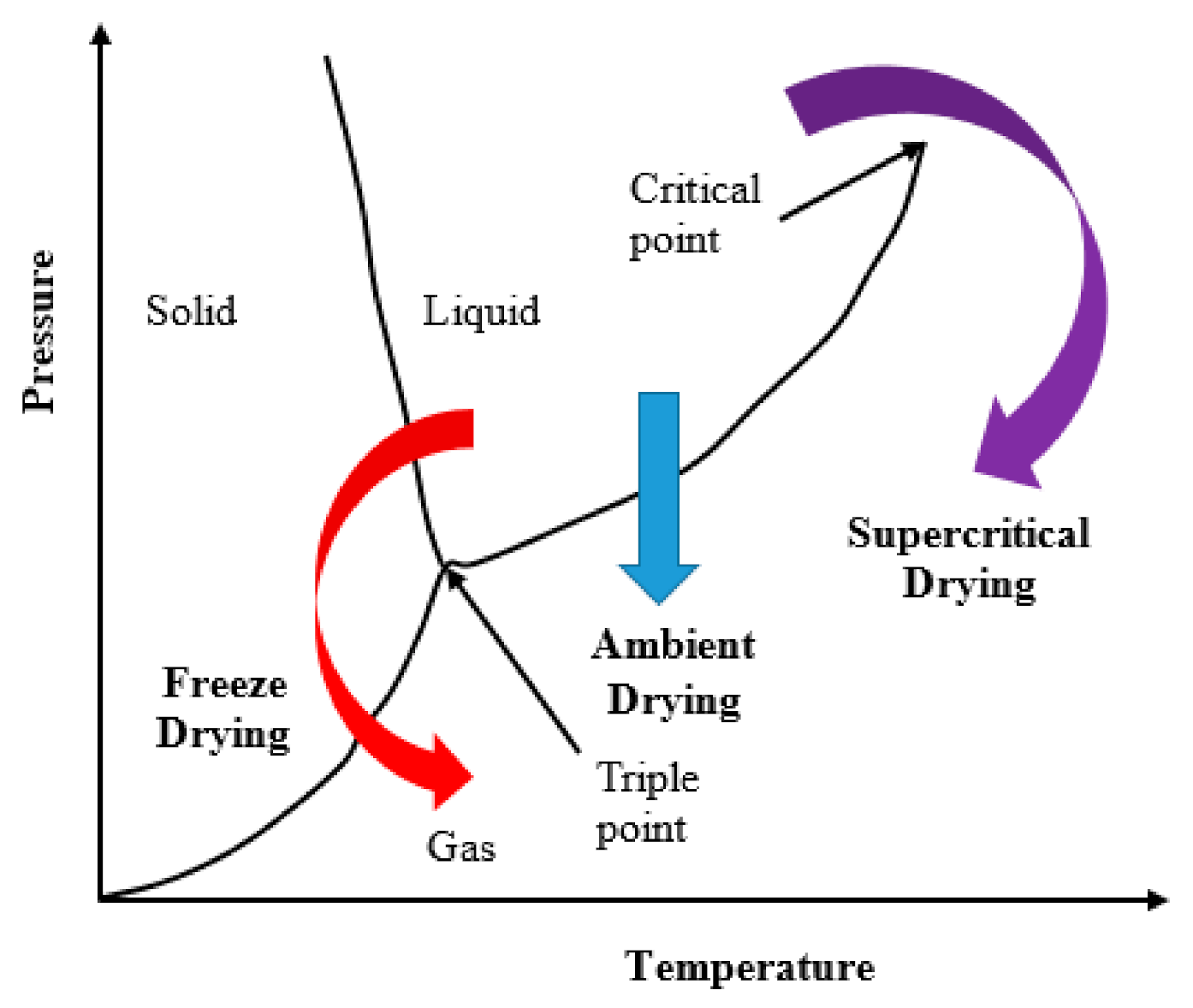
In general, three basic drying routes are usually utilized (shown in
Figure 1); those are: (a) freeze-drying, where the solvent within the pores must pass the liquid–solid and solid–gas equilibrium curves; (b) evaporation or ambient pressure (it is the pressure of the airtight space in which a given wet gel is placed) drying, which involves crossing of the liquid–gas equilibrium curve; and (c) supercritical fluid drying, in which the system is brought into a supercritical state where there is no liquid-gas interface. The fluid is then slowly vented to the atmosphere. Since this last drying takes place in the absence of liquid–vapor interfaces, no capillary pressure is present. Therefore, the solid skeleton is maintained in a highly porous state after drying.
Silica aerogels are the most researched aerogel materials, which are often synthesized by the sol-gel processes using an alkoxysilane (typically tetraethoxysilane, TEOS) polymer precursor. The inclusion of carbon atoms into the silica network can provide improved mechanical, thermal, optical, and electrical properties [
9,
10] due to the formation of rigid carbidic carbon (CSi
4) units with a denser atomic network than pure silica. This results in SiOC aerogels, which are synthesized from hybrid silicon alkoxides. Silicon oxycarbide networks contain Si, O, and C, sometimes named “black glasses”. The black color derives from excess C in the amorphous network [
9,
11]. Transparent SiOC glasses can be obtained when a dried gel is pyrolysized in the presence of hydrogen gas, where H
2 reacts with the organic groups to form methane gas, thus removing the excess carbon [
9,
11]. Synthesis of highly porous SiOC glasses may have potential applications in dielectrics for integrated photonics [
12], gas sensors [
13,
14], and materials for lithium-ion batteries [
15], biosensors, and photovoltaic cells. Moreover, SiOC glasses have high temperature resistances up to 1200 °C either in oxidizing or in inert atmospheres [
10].
This article deals with the synthesis of porous silicon oxycarbide aerogels and xerogels derived from a sol-gel procedure involving bis(triethoxysiyl)methane as SiOC precursor. Ceramization was achieved using H2 as pyrolysis gas to remove the excess carbon and produce transparent SiOC. Furthermore, the result of CO2 supercritical drying is compared to conventional drying to assess the effect of porosity and pore size distribution on the microstructural features of these materials.
2. Materials and Methods
2.1. Sol-Gel Process
Bis(triethoxysilyl)methane (C2H5O)3Si-CH2-Si(OC2H5) (BTEM) CAS 18418-72-9 was received from Fluorochem chemicals, Hadfield, England. Isopropanol alcohol (C3H7OH) CAS 6763-0 was received from J.T. Baker chemicals, Deventer, Netherlands; and ammonia (NH3) CAS 7664-41-7 was purchased from Deselab Line chemicals, Piombino Dese, Italy. BTEM (340.56 g/mol) and isopropanol (60.10 g/mol) chemicals are used as received without any further purification. The 12 M HCl and 16.5 M NH3 chemicals were diluted to 3 M and 13.4 M, respectively, for further use.
Considering silicon oxycarbide gels, the sol-gel process is based on a three reaction mechanism according to
Figure 2. In hydrolysis, Si-alkoxide reacts with water to produce silanol groups and alcohol. During the following step, condensation reactions generate Si-O-Si bridges, water, and alcohol. These reactions take place in the presence of a solvent and catalysts. At the functional group level, the reaction of alkosilanes to form the silica network can be written as in
Figure 2 [
13,
14,
15,
16,
17,
18]:
The first step to prepare a precursor gel is to choose the appropriate molar ratio of silicon alkoxide:solvent; silicon-alkoxide:catalyst(s); and hydrolysis-condensation reaction conditions such as time and temperature. Varying these processing parameters strongly affects the materials microstructure and the chemistry of the solid parts. In the synthesis of BTEM gels, the acid-base two-steps process was applied. In the present case, both acidic and basic solutions controlled the hydrolysis and condensation reactions. The molar ratios between Si-alkoxides/isopropanol/HCl(aq)/NH
3(aq) were set to 1/5/8/0.5 [
10,
11].
The main synthesis procedure in producing a BTEM gel can be described in detail as follows. One mole of BTEM silicon alkoxide was dissolved in 4 moles of isopropanol solvent and then hydrolyzed with 8 moles of acidic water (3 mol/L HCl(aq)). After hydrolysis, condensation was induced by drop-wise addition of 0.5 moles of a solution 13.36 mol/L NH
3(aq) and 1 mole of isopropanol. To activate the condensation reactions, 20% by mole of isopropanol alcohol was used to dilute the ammonia solution to control the reaction and to achieve a homogeneous system (shown in
Figure 3). This method was adopted to control the condensation process by diluting the ammonia aqueous solution with isopropanol. The sols were then stirred for a period of time and then transferred to a plastic mold. The cover of the plastic mold was closed in order to avoid the evaporation of isopropanol, which is volatile at room temperature. The surfaces of the obtained gels were kept wet with the drop-wise addition of isopropanol. The gels, covered by a thin layer of isopropanol and sealed in plastic mold, were aged for one week at 50 °C in a controlled atmosphere before swapping the synthesis solvent with neat isopropanol. The purpose of washing at this stage is mainly to remove the excess water present in the catalyst solutions and the one produced during condensation reactions. Finally, drying was accomplished via supercritical drying and ambient pressure drying.
Capillary pressure is responsible for the shrinkage of the SiOC gels during ambient pressure drying, and it is proportional to the surface tension of the utilized solvent. Isopropanol was selected for the SiOC gel synthesis, as it has a reasonably lower surface tension at 50 °C compared to other solvents [
16]. After three weeks of complete gelation, a small pinhole was made on the plastic mold cap for the isopropanol to slowly vent out.
Similarly, in supercritical drying, the isopropanol was used to wash out excess water exploiting the complete miscibility between the two liquids. Then, the wet gel was placed into a customized supercritical CO
2 reactor, where the extraction of the isopropanol, its substitution with liquid CO
2 and the final drying of the gel took place. The extraction through solvent exchange was performed two times per day for about a week. Once the amount of isopropanol reached a negligible amount, the temperature of the reactor was increased to 45 °C, which is above the critical point of carbon dioxide. As a matter of fact, at this condition there is no distinction between liquid and vapor CO
2. Finally, after maintaining the whole system for about an hour at 45 °C (at a pressure of 100 bar), carbon dioxide was slowly vented out, and the dried-gel was obtained.
Table 1 shows the different designations for ambient and supercritical dried gels.
2.2. Pyrolysis Process
The dried SiOC gels were placed in an alumina boat and pyrolyzed in H
2 atmosphere up to 800 or 1100 °C for 1 h at the maximum temperature. This heat treatment had been done in Japan by Prof. M. Narisawa at the Osaka Prefecture University. Based on previous studies [
11], the samples were heated at 5 °C/min until the temperature reached 600 °C, the sample was kept for 1 h and then heated at 3.33 °C/min up to the desired temperature. Finally, a 600 °C/h cooling rate was employed to bring the system to room temperature.
2.3. Characterization
The skeletal density of all the gels was calculated by he-Pycnometry using Micromeritics AccuPyc 1330 equipment (Micromeritics, Norcross, GA, USA).
Fourier transform infrared (FT-IR) spectra were recorded with a Varian Excalibur Series 4100 (Lake Forest, CA, USA) at room temperature using the ATR mode with a diamond crystal in the 4000–500 cm−1 range, acquiring 64 scans with 4 cm−1 resolution. For comparison, the infrared spectra were also recorded in transmission mode using KBr pellets with a Thermo Optics Avatar 330 instrument (Thermo Fischer Scientific, Waltham, MA, USA) in the 4000–400 cm−1 range, acquiring similar number scans and resolution.
Nitrogen sorption isotherms were recorded at 77 K using an ASAP 2010 Micromeritics apparatus (Micromeritics, Norcross, GA, USA). Each sample was degassed for several hours at 50 °C under secondary vacuum. The specific surface area, total pore volume, mean pore size and pore size distribution are determined from the N2 adsorption-desorption measurements.
XRD diffraction (XRD, Rigaku, Tokyo, Japan) was collected from samples pyrolyzed at 800 & 1100 °C using Cu Kα radiation at 40 kV and 30 mA. The powder diffraction pattern was recorded at 2θ = 10–80° with a 0.02°/s scanning rate.
The thermal evolution of SiOC samples was monitored by means of a thermogravimetric analysis (TGA) (Netzsch-Geratebau GmbH, Selb, Germany) with a Netzsch STA 409 thermobalance. The analysis was performed up to 1100 °C with a heating rate of 3.33 °C/min. To avoid any damage to the instrument, hydrogen could not be used as pyrolysis gas, so an inert argon flux of 50 cm3/min was employed. In this way it was possible to assess the ceramic yield of BTEM-derived SiOC without extracting the free carbon content.
UV-Vis-NIR spectra of bulk transparent ceramic aerogels were acquired with an Alignet Cary 5000 UV-Vis-NIR spectrophotometer (Alignet, Santa Clara, CA, USA) in the 400–800 nm wavelength range with a 600 nm/min acquisition rate. Transmittance data were normalized on a sample thickness of 3.1 mm, this resulting from the linear shrinkage of the two samples being different upon drying.
The morphological features of the SiOC aerogels were analyzed from fresh fracture surfaces using a Supra 40 Zeiss Field Emission Scanning Electron Microscope (FE-SEM) (JEOL JSM 6300F, Tokyo, Japan) after gold film deposition by sputtering.
4. Discussion
The peaks in infrared spectra in the ATR experiment are distorted at high wavenumbers, especially the ones which are pyrolyzed at high temperatures. In the ATR experiment, the depth of penetration
dp is a function of wavelength (
λ), incident angle of the beam (
θ), the reflective indices of the ATR crystal (
n1), and the sample (
n2), and all are correlated by the following equation [
21,
28].
Assuming that the
ratio is constant, then the path of penetration is linearly related with the wavelength of IR radiation [
21], and this results in the change in intensity in ATR. However, in transmission mode, the value
dp is related to the thickness of the sample, which is constant.
For comparison, the FT-IR measurements in both ATR and transmission modes at room temperature and 800 °C are revealed in
Figure 14. Some of the peaks in
Figure 14a at higher wavenumbers are not visible. This is particularly true for the sample pyrolyzed at high temperatures (800 °C), and the relative intensity of the peaks at higher wavenumbers seems to disappear. However, when the transmission experiment is done for the BTEM sample at 800 °C (shown
Figure 14b), the broad band appeared from 3200–3600 cm
−1 which can be allotted to the H
2O water or Si-OH groups. The peaks at 1280, 1470 and 1645 cm
−1 belong to Si-CH
3 vibrations, C-H bending and adsorbed moisture, respectively. Furthermore, the bands at 2980 cm
−1 could be assigned to C-H vibrations in the –CH
3 group and at 2855–2940 cm
−1 are related to C-H vibration in the –CH
2 group.
The transmission spectrum of the BTEM sample decarbonized at 800 °C shows intense peaks at 1060, 882 and 800 cm−1. In the ATR spectrum, these bands are shifted by 15, 0 and 0 cm−1 toward lower frequency, respectively. These large frequency shifts and peak intensity changes will reduce the performance of any computer search routine.
The BET equation is used to calculate the specific surface area (SSA) from 0.05 to 0.3 relative pressure. The high SSA of BTEMx and BTEM gels are 1107 and 1140 m
2/g, respectively. The high surface area of the BTEM aerogel is due to its high TPV of 2.522 cm
3/g. The presence of high total pore volume in the BTEM sample is associated with the use of supercritical CO
2 drying, which maintained the integrity of the structural network. This type of drying method results in high porosity and TPV. However, high SSA of BTEMx xerogel comes with a smaller pore size. During the ambient drying, the integrity of the SiOC network shrinks too much and some parts of the xerogel sample showed cracks on the surface (see
Figure 4) and possessed a smaller pore size. The average pore size of BTEMx xerogel is 3.4 nm, which is 2.6 fold smaller than that of the BTEM aerogel. In general, the surface areas (SA
BET) are directly related to TPV or V and inversely related to the mean pore size according to Equation (4). As the pyrolysis temperatures increased to 800 and 1100 °C, the SSA and TPV of the samples decreased.
The BTEMx xerogel samples are more transparent than the BTEM aerogels. The transparency of the samples at room temperature correlate well with the mean pore size. This transparency can be seen up to 800 °C. However, the further increasing of the pyrolysis temperature to 1100 °C makes the BTEMx samples become dark, but the transparency is maintained in some parts, whereas BTEM samples become brown and opaque.
5. Conclusions
FT-IR and nitrogen sorption results revealed the successful synthesis of SiOC gels and the formation of mesoporous materials, respectively. From measurements, it is evident that the drying steps significantly affect the mean pore size and shape. Ambient dried samples resulted in smaller average pore sizes compared to the supercritical CO2 dried SiOC samples and therefore they appear totally transparent. It was also shown that the SiOC aerogels can be synthesized in crack-free monoliths and exhibit homogeneity and good transparency, being less than that of the xerogel. The synthesized SiOC samples present extremely porous structures, possessing 1140 m2/g SSA and 2.522 m2/g TPV. Generally, all the samples at room temperature show high surface areas (>1100 m2/g), that derive from a narrow distribution of small mesopores in the case of the xerogel, whereas those of the aerogel samples are associated with their high total pore volume. As pyrolysis temperature is raised to 800 and 1100 °C, the SSA and TPV for both samples are slightly reduced. The SSA of BTEM aerogels are 890 and 366 m2/g at 800 and 1100 °C, respectively. These SiOC ceramics have potential applications in photonics, gas sensors, and for Li-ion batteries, biosensors and photovoltaic cells.