The Synergistic Effect of Dibenzyldithiocarbamate Based Accelerator on the Vulcanization and Performance of the Silica-Filled Styrene–Butadiene Elastomer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of SBR Composites
3. Results and Discussion
3.1. Influence of the Dibenzyldithiocarbamate Accelerator on the Curing Characteristics and the Crosslink Density of the SBR Composites
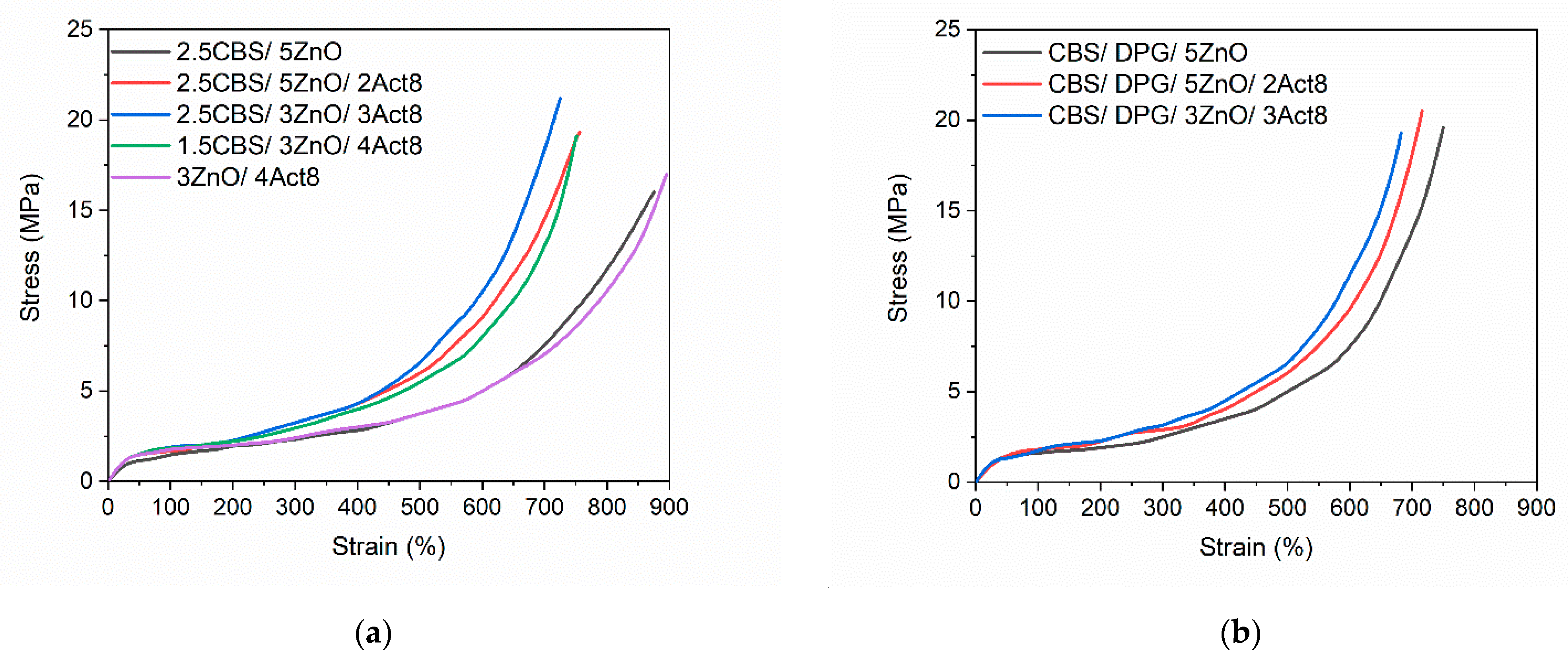
3.2. Influence of the Dibenzyldithiocarbamate Accelerator on the Tensile Properties and Hardness of the SBR Composites
3.3. Influence of the Dibenzyldithiocarbamate Accelerator on the Dynamic Mechanical Properties of the SBR Composites
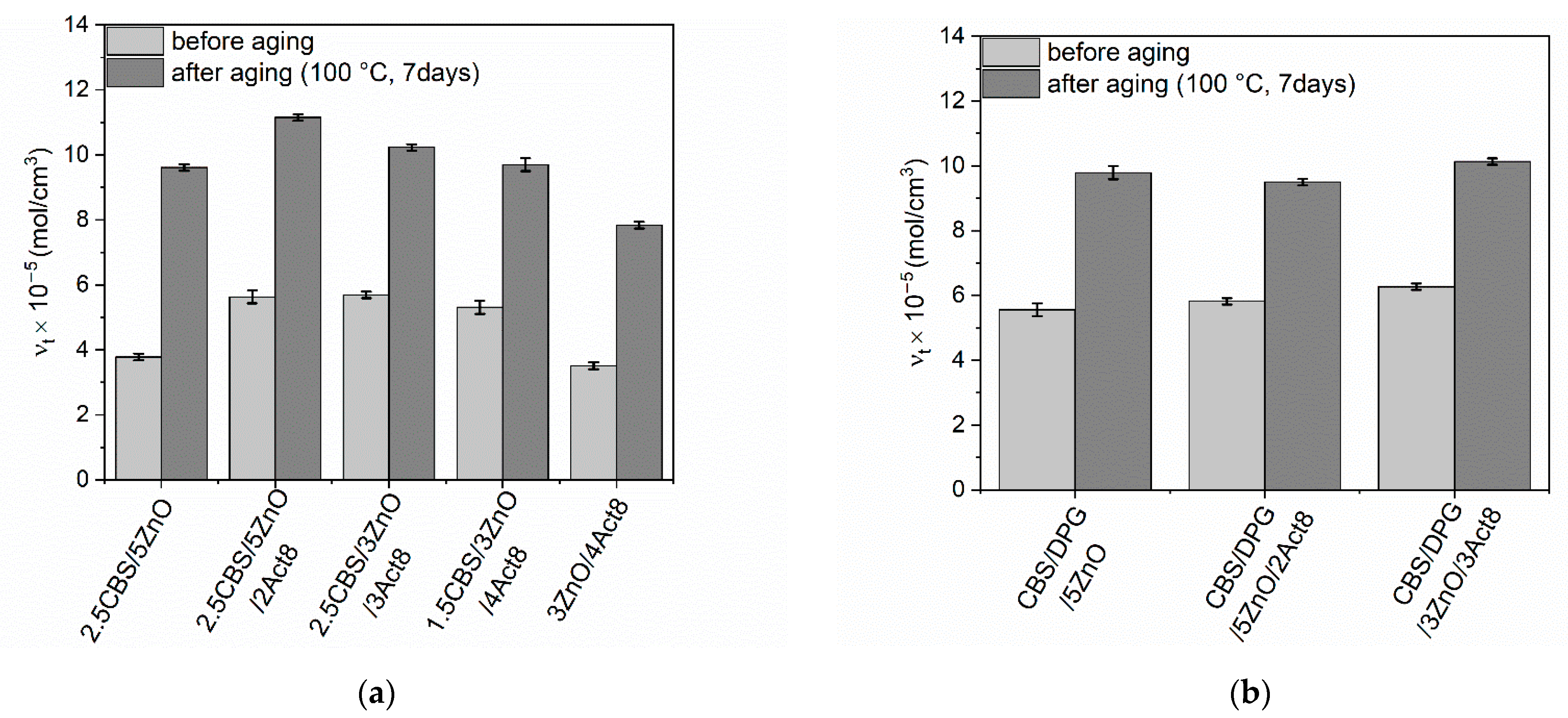
3.4. Influence of the Dibenzyldithiocarbamate Accelerator on the Thermo-Oxidative Aging Resistance of the SBR Composites
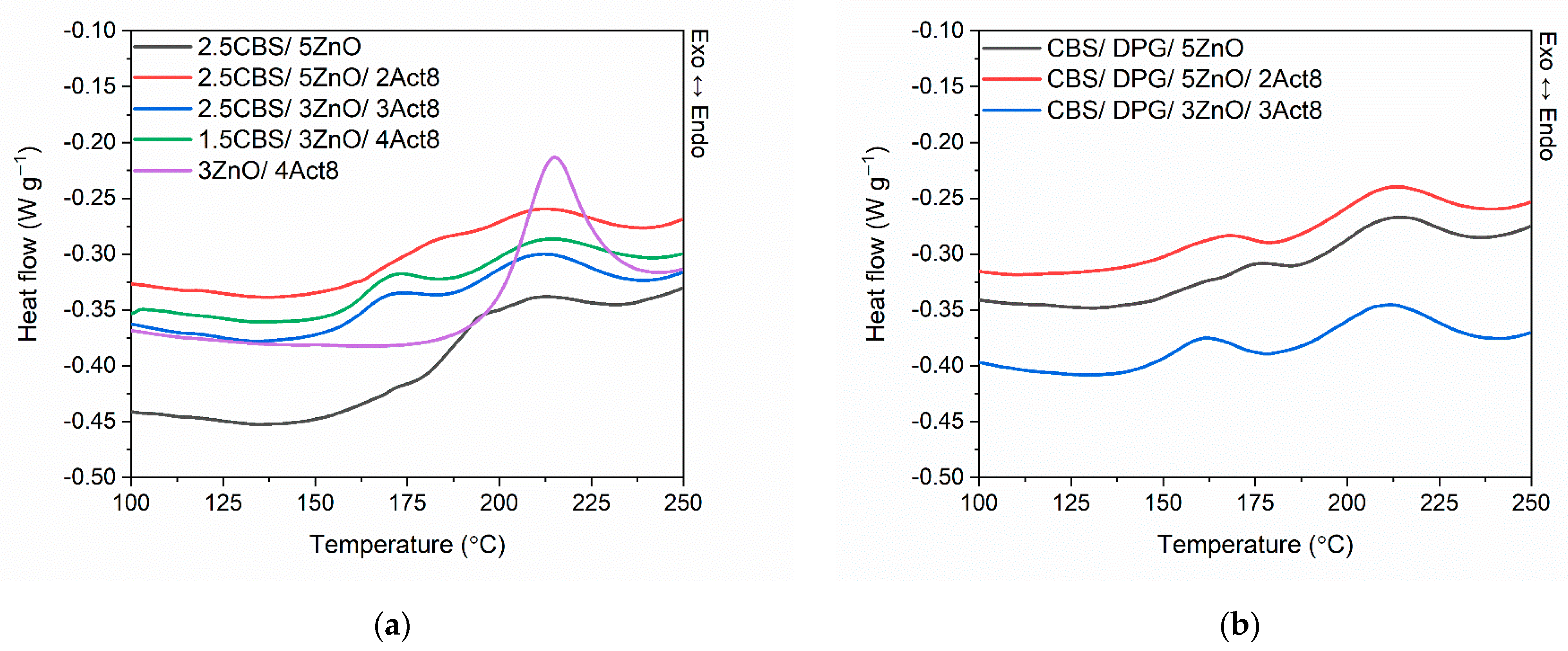
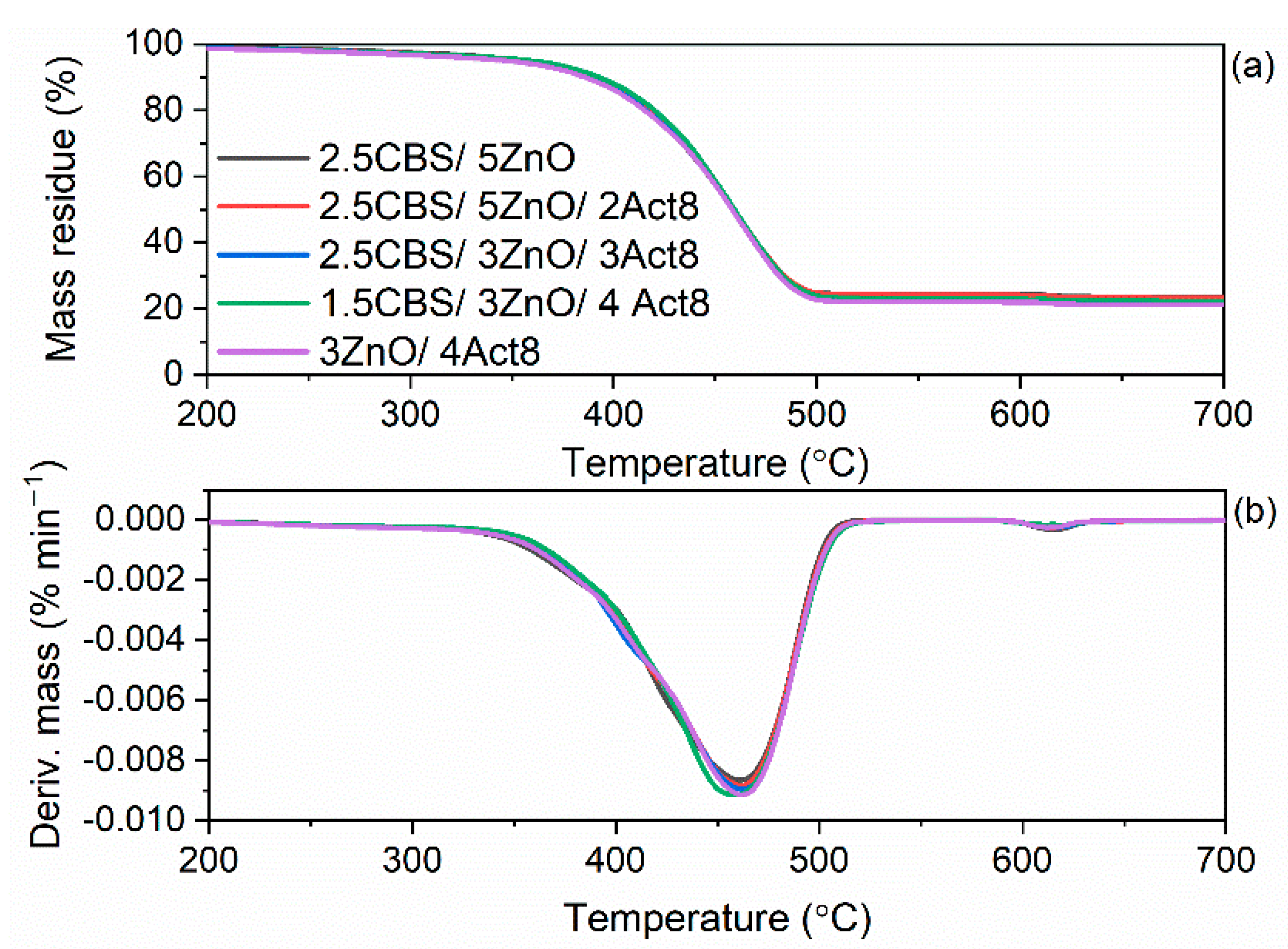
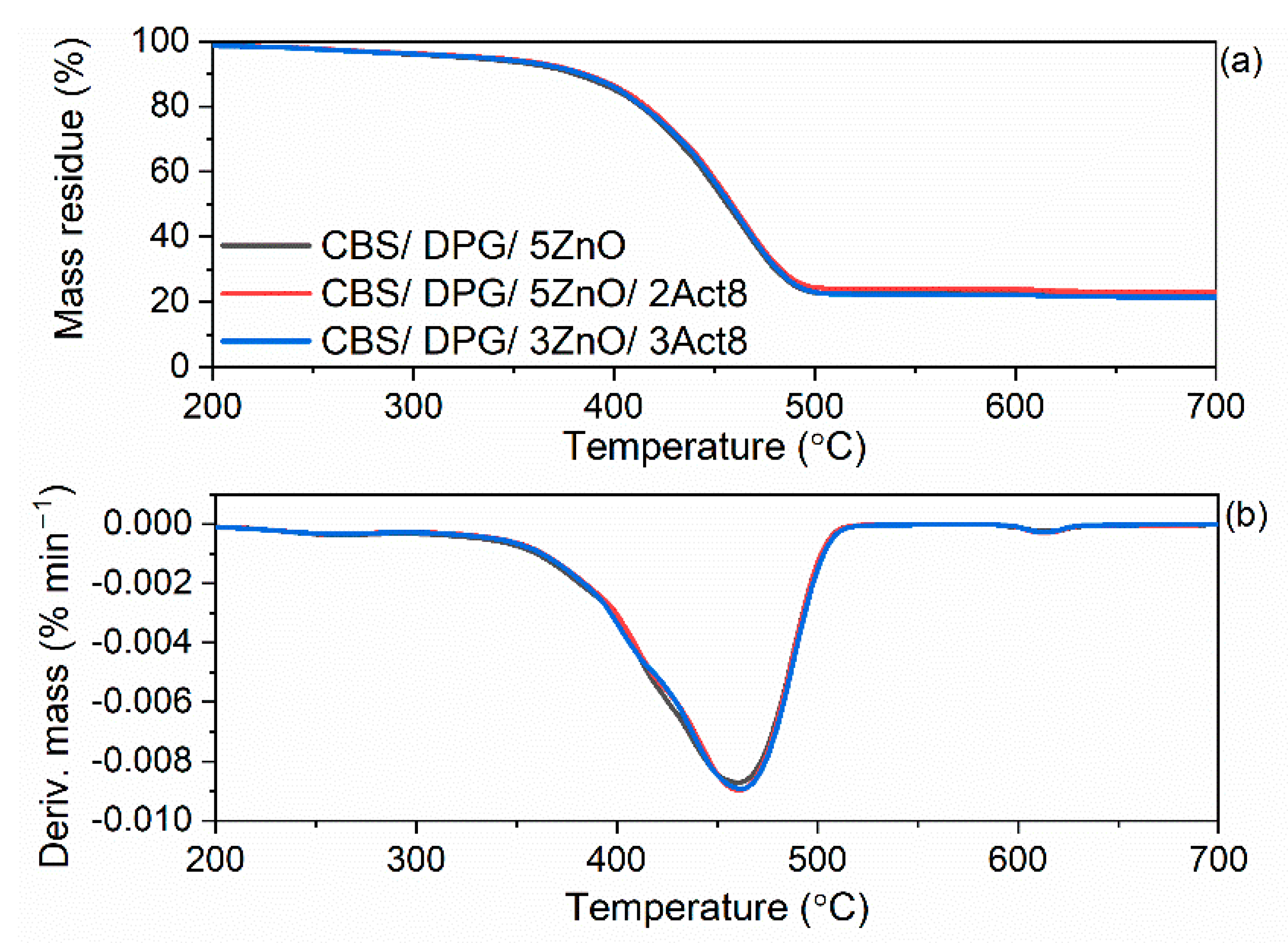
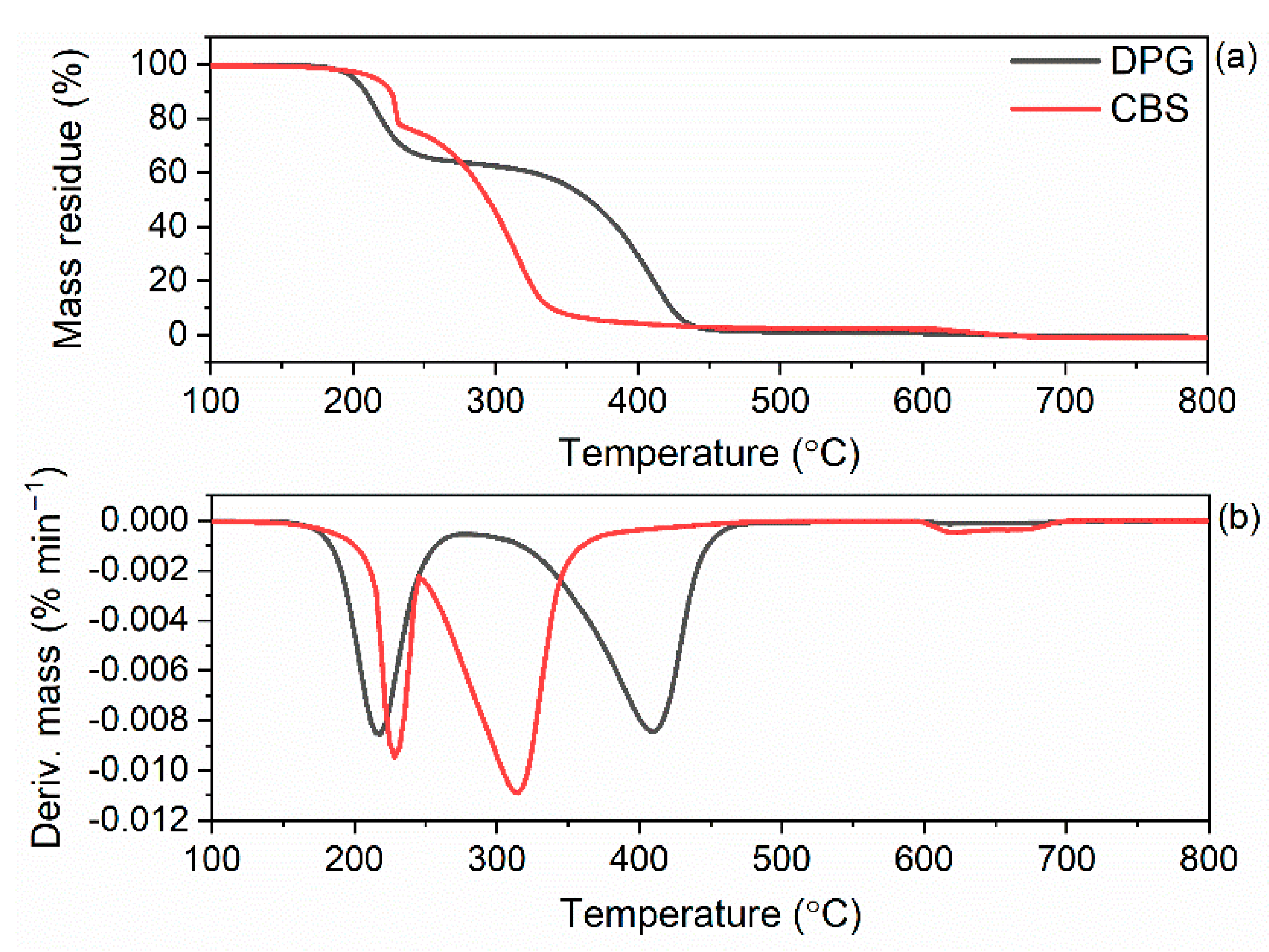
3.5. Influence of the Dibenzyldithiocarbamate Accelerator on the Thermal Stability of the SBR Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coran, A.Y. Chemistry of the vulcanization and protection of elastomers: A review of the achievements. J. Appl. Polym. Sci. 2003, 87, 24–30. [Google Scholar] [CrossRef]
- Akiba, M.; Hashim, A.S. Vulcanization and crosslinking in elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Nabil, H.; Ismail, H.; Azura, A.R. Optimisation of accelerators and vulcanising systems on thermal stability of natural rubber/recycled ethylene-propylene-diene-monomer blends. Mater. Des. 2014, 53, 651–661. [Google Scholar] [CrossRef]
- Ignatz-Hoover, R. Review of vulcanization chemistry. Rubber World 1999, 220, 24–32. [Google Scholar]
- Heinrich, G.; Klüppel, M.; Vilgis, T.A. Reinforcement of Elastomers. Curr. Opin. Solid State Mater. Sci. 2002, 6, 195–203. [Google Scholar] [CrossRef]
- Zhong, B.; Dong, H.; Lin, J.; Jia, Z.; Luo, Y.; Jia, D.; Liu, F. Preparation of halloysite nanotubes–silica hybrid supported vulcanization accelerator for enhancing interfacial and mechanical strength of rubber composites. Ind. Eng. Chem. Res. 2017, 56, 9135–9142. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, J.C.; Lee, S.G.; Joo, Y.L. Influence of the cure systems on long time thermal aging behaviors of NR composites. Macromol. Res. 2008, 16, 561–566. [Google Scholar] [CrossRef]
- Rattanasom, N.; Poonsuk, A.; Makmoon, T. Effect of curing system on the mechanical properties and heat aging resistance of natural rubber/tire tread reclaimed rubber blends. Polym. Test. 2005, 24, 728–732. [Google Scholar] [CrossRef]
- Marković, G.; Radovanović, B.; Marinović-Cincović, M.; Budinski-Simendić, J. The effect of accelerators on curing characteristics and properties of natural rubber/chlorosulphonated polyethylene rubber blend. Mater. Manuf. Process. 2009, 24, 1224–1228. [Google Scholar] [CrossRef]
- Formela, K.; Wąsowicz, D.; Formela, M.; Hejna, A.; Haponiuk, J. Curing characteristics, mechanical and thermal properties of reclaimed ground tire rubber cured with various vulcanizing systems. Iran. Polym. J. 2015, 24, 289–297. [Google Scholar] [CrossRef]
- Gobbi, V.G.; da Silva, T.G.; Cunha, M.S.; dos Santos Mendonça, T.; Gobbi, V.G.; da Silva Moreira Thiré, R.M.; Mendonça, R.H. Comparative study among TMTD, TBzTD, and ZBEC accelerators in isobutylene-isoprene elastomer vulcanization. J. Appl. Polym. Sci. 2020, 137, 48965. [Google Scholar] [CrossRef]
- Ghosh, J.; Ghorai, S.; Jalan, A.K.; Roy, M.; De, D. Manifestation of accelerator type and vulcanization system on the properties of silica-reinforced SBR/devulcanize SBR blend vulcanizates. Adv. Polym. Technol. 2018, 37, 2636–2650. [Google Scholar] [CrossRef]
- Susamma, A.P.; Kurien, M.; Kuriakose, A.P. New binary accelerator systems for sulphur vulcanisation of styrene butadiene rubber. Plast. Rubber Compos. 2004, 33, 63–70. [Google Scholar] [CrossRef]
- Alam, M.N.; Kumar, V.; Potiyaraj, P.; Lee, D.-J.; Choi, J. Synergistic activities of binary accelerators in presence of magnesium oxide as a cure activator in the vulcanization of natural rubber. J. Elastomers Plast. 2021, 54, 123–144. [Google Scholar] [CrossRef]
- Alam, M.N.; Mandal, S.K.; Roy, K.; Debnath, S.C. Synergism of novel thiuram disulfide and dibenzothiazyl disulfide in the vulcanization of natural rubber: Curing, mechanical and aging resistance properties. Int. J. Ind. Chem. 2014, 5, 8. [Google Scholar] [CrossRef]
- Mayasari, H.E.; Setyorini, I.; Yuniari, A. The blending of EPDM/NR with maleic anhydride as compatibilizer: Comparing the effect of accelerators on cure characteristic and mechanical properties. Indones. J. Chem. 2019, 1, 106–114. [Google Scholar] [CrossRef]
- Samarasinghe, I.; Walpalage, S.; Edirisinghe, D.; Egodage, S. Study on sulfur vulcanized natural rubber formulated with nitrosamine safe diisopropyl xanthogen polysulfide/tertiary butyl benzothiazole sulphenamide binary accelerator system. Prog. Rubber Plast. Recycl. Technol. 2021, 37, 190–202. [Google Scholar] [CrossRef]
- Ahsan, Q.; Mohamad, N.; Soh, T.C. Effects of accelerators on the cure characteristics and mechanical properties of natural rubber compounds. Int. J. Automot. Mech. Eng. 2015, 12, 2954–2966. [Google Scholar] [CrossRef]
- Alam, M.N.; Mandal, S.K.; Debnath, S.C. Bis(N-benzyl piperazino) thiuram disulfide and dibenzothiazyl disulfide as synergistic safe accelerators in the vulcanization of natural rubber. J. Appl. Polym. Sci. 2012, 126, 1830–1836. [Google Scholar] [CrossRef]
- Debnath, S.C.; Basu, D.K. Studies on cure synergism. I. Effect of safe zinc dithiocarbamates on NR vulcanization accelerated by thiazole-based accelerators. J. Appl. Polym. Sci. 1994, 52, 597–603. [Google Scholar] [CrossRef]
- Palaty, S.; Joseph, R. Studies on xanthate–zinc diethyl dithiocarbamate accelerator combination in natural rubber. Plast. Rubber Compos. 2001, 30, 270–274. [Google Scholar] [CrossRef]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N.; van Baarle, B. Various ways to reduce zinc oxide levels in S-SBR rubber Compounds. Macromol. Symp. 2006, 245–246, 657–667. [Google Scholar] [CrossRef]
- Azar, F.A.N.; Sen, M. Effects of accelerator type on stress relaxation behavior and network structure of aged natural rubber/chloroprene rubber vulcanizates. J. Elastomers Plast. 2017, 49, 381–396. [Google Scholar] [CrossRef]
- Alam, N.; Mandal, S.K.; Debnath, S.C. Effect of zinc dithiocarbamates and thiazole-based accelerators on the vulcanization of natural rubber. Rubber Chem. Technol. 2012, 85, 120–131. [Google Scholar] [CrossRef]
- Datta, S. Special-purpose elastomers. In Rubber Compounding Chemistry and Applications; Rodgers, B., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 105–132. [Google Scholar]
- Ciullo, P.A.; Hewitt, N. The Rubber Formulatory; Noyes Publications/William Andrew Publishing, LLC: New York, NY, USA, 1999; pp. 122–182. [Google Scholar]
- Yang, J.-K.; Park, W.; Ryu, C.; Kim, S.J.; Kim, D.; Seo, G.; Kim, J.-H.; Chung, C.-B. Roles of sulfur and accelerators in the vulcanization of SBR compounds deduced through simulation. Rubber Chem. Technol. 2018, 91, 595–608. [Google Scholar] [CrossRef]
- Bahadar, A.; Zwawi, M. Development of SWCNTs-reinforced EPDM/SBR matrices for shock absorbing applications. Mater. Res. Express 2020, 7, 025310. [Google Scholar] [CrossRef]
- Kim, I.J.; Kim, D.; Ahn, B.; Lee, H.J.; Kim, H.J.; Kim, W. Vulcanizate structures of SBR compounds with silica and carbon black binary filler systems at different curing temperatures. Polymers 2020, 12, 2343. [Google Scholar] [CrossRef]
- Bosch, R. Novel activator for DPG replacement and property improvement. Elastomers 2020, 24, 3–14. [Google Scholar]
- Miedzianowska, J.; Masłowski, M.; Rybiński, P.; Strzelec, K. Modified nanoclays/straw fillers as functional additives of natural rubber biocomposites. Polymers 2021, 13, 799. [Google Scholar] [CrossRef]
- Sowińska-Baranowska, A.; Maciejewska, M. Influence of the silica specific surface area and ionic liquids on the curing characteristics and performance of styrene–butadiene rubber composites. Materials 2021, 14, 5302. [Google Scholar] [CrossRef]
- Zedler, Ł.; Burger, P.; Wang, S.; Formela, K. Ground tire rubber modified by ethylene-vinyl acetate copolymer: Processing, physico-mechanical properties, volatile organic compounds emission and recycling possibility. Materials 2020, 13, 4669. [Google Scholar] [CrossRef] [PubMed]
- ISO 6502-3:2018; Rubber—Measurement of Vulcanization Characteristics Using Curemeters—Part 3: Rotorless Rheometer. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 11357-1:2016; Plastics—Differential Scanning Calorimetry (DSC)—Part 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 1817:2015; Rubber, Vulcanized or Thermoplastic—Determination of Effect of Liquids. International Organization for Standardization: Geneva, Switzerland, 2017.
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-linked Polymer Networks.II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Furuta, I.; Kimura, S.; Iwama, M. Physical Constants of Rubbery Polymers. In Polymer Handbook, 4th ed.; Brandrup, J., Immergut, E.H., Grulke, E.A., Eds.; John Wiley and Sons Inc.: New York, NY, USA, 1999; pp. 1–7. [Google Scholar]
- ISO 37:2017; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 868:2003; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). International Organization for Standardization: Geneva, Switzerland, 2003.
- ISO 188:2011; Rubber, Vulcanized or Thermoplastic—Accelerated Ageing and Heat Resistance Tests. International Organization for Standardization: Geneva, Switzerland, 2011.
- Maslowski, M.; Miedzianowska, J.; Strzelec, K. Natural rubber composites filled with crop residues as an alternative to vulcanizates with common fillers. Polymers 2019, 11, 972. [Google Scholar] [CrossRef]
- Sae-oui, P.; Sirisinha, C.; Thepsuwan, U.; Hatthapanit, K. Dependence of mechanical and aging properties of chloroprene rubber on silica and ethylene thiourea loadings. Eur. Polym. J. 2007, 43, 185–193. [Google Scholar] [CrossRef]
- Datta, R.N.; Ingham, F.A.A. Rubber additives—Compounding ingredients. In Rubber Technologist’s Handbook, 1st ed.; De, S.K., White, J.K., Eds.; Rapra Technology Limited: Shawbury, UK, 2001; pp. 167–181. [Google Scholar]
- Jin, J.; van Swaaij, A.P.J.; Noordermeer, J.W.M.; Blume, A.; Dierkes, W.K. On the various roles of 1,3-DIPHENYL guanidine in silica/silane reinforced SBR/BR blends. Polym. Test. 2021, 93, 106858. [Google Scholar] [CrossRef]
- Zaborski, M.; Donnet, J.B. Activity of fillers in elastomer networks of different structure. Macromol. Symp. 2003, 194, 87–100. [Google Scholar] [CrossRef]
- Lim, S.-H.; Lee, S.; Lee, N.; Ahn, B.K.; Park, N.; Kim, W. Effect of 1,3-Diphenyl-guanidine (DPG) mixing step on the properties of SSBR-silica compounds. Elastomers Compos. 2016, 51, 81–92. [Google Scholar] [CrossRef][Green Version]
- Kosmalska, A.; Zaborski, M.; Slusarski, L. Adsorption of curatives and activity of silica toward elastomers. Macromol. Symp. 2003, 194, 269–275. [Google Scholar] [CrossRef]
- Rattanasom, N.; Saowapark, T.; Deeprasertkul, C. Reinforcement of natural rubber with silica/carbon black hybrid filler. Polym. Test. 2007, 26, 369–377. [Google Scholar] [CrossRef]
- Prochon, M.; Janowska, G.; Przepiorkowska, A.; Kucharska-Jastrzabek, A. Thermal properties and combustibility of elastomer-protein composites Part I. Composites SBR-keratin. J. Therm. Anal. Calorim. 2012, 109, 1563–1570. [Google Scholar] [CrossRef]
- Skinner, T.D.; Watson, A.A. EV systems for NR. I. The purpose of efficient vulcanization and development of curing system. Rubber Chem. Technol. 1969, 42, 404–417. [Google Scholar] [CrossRef]
- Debnath, S.C.; Basu, D.K. Studies on the effect of thiuram disulfide on NR vulcanization accelerated by thiazole-based accelerator systems. J. Appl. Polym. Sci. 1996, 60, 845–855. [Google Scholar] [CrossRef]
- Alam, M.; Debnath, S.C.; Choi, J. Nitrosamine-safe thiuram disulfide and benzothiazole sulfenamide as a synergistic pair of accelerators for the vulcanization of rubber. J. Polym. Res. 2021, 28, 317. [Google Scholar] [CrossRef]
- Zhao, F.; Weina, B.; Zhao, S. Influence of crosslink density on mechanical properties of natural rubber vulcanizates. J. Macromol. Sci. B. 2011, 50, 1460–1469. [Google Scholar] [CrossRef]
- Coran, A.Y. Vulcanization. In Science and Technology of Rubber, 4th ed.; Mark, J.E., Erman, B., Roland, M., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2013; pp. 337–381. [Google Scholar] [CrossRef]
- Bouaziz, R.; Truffault, L.; Borisov, R.; Ovalle, C.; Laiarinandrasana, L.; Miquelard-Garnier, G.; Fayolle, B. Elastic properties of polychloroprene rubbers in tension and compression during ageing. Polymers 2020, 12, 2354. [Google Scholar] [CrossRef]
- Patti, A.; Acierno, D.; Latteri, A.; Tosto, C.; Pergolizzi, E.; Recca, G.; Cristaudo, M.; Cicala, G. Influence of the processing conditions on the mechanical performance of sustainable bio-based PLA compounds. Polymers 2020, 12, 2197. [Google Scholar] [CrossRef]
- Iijima, T.; Yoshioka, N.; Tomoi, M. Effect of cross-link density on modification of epoxy resins with reactive acrylic elastomers. Eur. Polym. J. 1992, 28, 573–581. [Google Scholar] [CrossRef]
- Menard, K.P.; Menard, N.R. Dynamic mechanical analysis in the analysis of polymers and rubbers. In Encyclopedia of Polymer Science and Technology, 4th ed.; Matyjaszewski, K., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Ahankari, S.S.; Kar, K.K. Processing of styrene butadiene rubber–carbon black nanocomposites with gradation of crosslink density: Static and dynamic mechanical characterization. Mater. Sci. Eng. A 2008, 491, 454–460. [Google Scholar] [CrossRef]
- Sombatsompop, N. Analysis of cure characteristics on cross-link density and type, and viscoelastic properties of natural rubber. Polym. Plast. Technol. Eng. 1998, 37, 333–349. [Google Scholar] [CrossRef]
- Nabil, H.; Ismail, H.; Azura, A.R. Comparison of thermo-oxidative ageing and thermal analysis of carbon black-filled NR/Virgin EPDM and NR/Recycled EPDM blends. Polym. Test. 2013, 32, 631–639. [Google Scholar] [CrossRef]
- Kruželák, J.; Dosoudil, R.; Hudec, I. Thermooxidative aging of rubber composites based on NR and NBR with incorporated strontium ferrite. J. Elastomers Plast. 2018, 50, 71–91. [Google Scholar] [CrossRef]
- Rezig, N.; Bellahcene, T.; Aberkane, M.; Abdelaziz, M.N. Thermo-oxidative ageing of a SBR rubber: Effects on mechanical and chemical properties. J. Polym. Res. 2020, 27, 339. [Google Scholar] [CrossRef]
- Varatharajan, K.; Cheralathan, M. Effect of aromatic amine antioxidants on NOx emissions from a soybean biodiesel powered DI diesel engine. Fuel Process. Technol. 2013, 106, 526–532. [Google Scholar] [CrossRef]
- Debnath, S.C.; Basu, D.K. Studies on cure synergism: Use of safe thiocarbamyl sulfenamide-dibenzothiazyl disulfide accelerator system in the vulcanization of natural rubber. Polym. Plast. Technol. Eng. 1995, 34, 539–550. [Google Scholar] [CrossRef]
- Madathingal, R.R.; Wunder, S.L. Thermal degradation of poly(methyl methacrylate) on SiO2 nanoparticles as a function of SiO2 size and silanol density. Thermochim. Acta 2011, 526, 83–89. [Google Scholar] [CrossRef]
- Travas-Sejdic, J.; Jelencic, J.; Bravar, M.; Fröbe, Z. Characterization of the natural rubber vulcanizates obtained by different accelerators. Eur. Polym. J. 1996, 32, 1395–4401. [Google Scholar] [CrossRef]
- Ghosh, J.; Ghorai, S.; Bhunia, S.; Roy, M.; De, D. The role of devulcanizing agent for mechanochemical devulcanization of styrene butadiene rubber vulcanizate. Polym. Eng. Sci. 2018, 58, 74–85. [Google Scholar] [CrossRef]
- Mostafa, A.; Abouel-Kasem, A.; Bayoumi, M.R.; El-Sebaie, M.G. The influence of CB loading on thermal aging resistance of SBR and NBR rubber compounds under different aging temperature. Mater. Des. 2009, 30, 791–795. [Google Scholar] [CrossRef]
- Hu, Q.; Jin, H.L.; Chen, X.A.; Wang, S. Thermal and FTIR spectral studies of N,N’-diphenylguanidine. J. Therm. Anal. Calorim. 2012, 110, 593–599. [Google Scholar] [CrossRef]
- Samide, A.; Rotaru, P.; Ionescu, C.; Tutunaru, B.; Moantă, A.; Barragan-Montero, V. Thermal behaviour and adsorption properties of some benzothiazole derivatives. J. Therm. Anal. Calorim. 2014, 118, 651–659. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Liu, Q.; Cheng, H.; Frost, R.L. Thermal stability of styrene butadiene rubber (SBR) composites filled with kaolinite/silica hybrid filler. J. Therm. Anal. Calorim. 2014, 115, 1013–1020. [Google Scholar] [CrossRef]
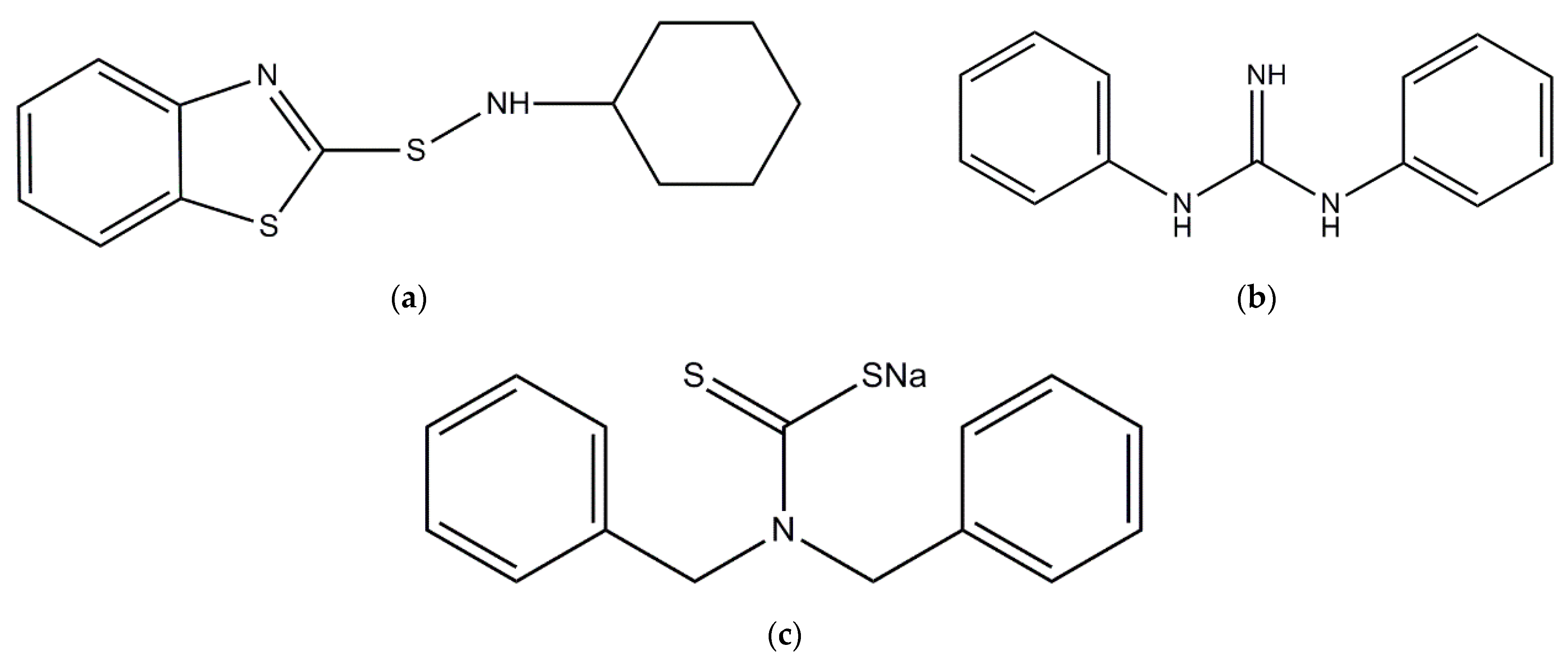





| Ingredient (phr) | Reference Samples | Rubber Compounds with Act8 | ||||||
|---|---|---|---|---|---|---|---|---|
| REF 1 | REF 2 | SBR1 | SBR2 | SBR3 | SBR4 | SBR5 | SBR6 | |
| SBR | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Sulfur | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| CBS | 2.5 | 1.5 | 2.5 | 2.5 | 1.5 | ₋ | 1.5 | 1.5 |
| DPG | - | 2 | - | - | - | - | 2 | 2 |
| ZnO | 5 | 5 | 5 | 3 | 3 | 3 | 5 | 3 |
| St. A. | 1 | 1 | ₋ | ₋ | ₋ | ₋ | - | - |
| A380 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Act 8 | ₋ | - | 2 | 3 | 4 | 4 | 2 | 3 |
| Composites | Smin (dNm) | ΔS (dNm) | t02 (min) | t90 (min) | (mol/) |
|---|---|---|---|---|---|
| Rubber compounds with CBS | |||||
| 2.5CBS/5ZnO | 6.6 ± 0.6 | 19.4 ± 0.6 | 3.5 ± 0.2 | 25.6 ± 1.1 | 3.8 ± 0.4 |
| 2.5CBS/5ZnO/2Act8 | 7.0 ± 0.5 | 20.8 ± 0.5 | 3.4 ± 0.2 | 10.4 ± 0.9 | 5.6 ± 0.6 |
| 2.5CBS/3ZnO/3Act8 | 6.7 ± 0.5 | 22.5 ± 0.5 | 3.0 ± 0.2 | 7.8 ± 0.8 | 5.7 ± 0.4 |
| 1.5CBS/3ZnO/4Act8 | 6.9 ± 0.4 | 21.7 ± 0.4 | 1.0 ± 0.1 | 7.1 ± 0.8 | 5.3 ± 0.3 |
| 3ZnO/4Act8 | 7.3± 0.6 | 17.1 ± 0.6 | 0.8 ± 0.1 | 38.5 ± 1.1 | 3.5 ± 0.4 |
| Rubber compounds with CBS and DPG | |||||
| CBS/DPG/5ZnO | 6.9 ± 0.4 | 22.0 ± 0.4 | 4.7 ± 0.3 | 8.2 ± 0.9 | 5.6 ± 0.4 |
| CBS/DPG/5ZnO/2Act8 | 6.2 ± 0.6 | 24.5 ± 0.6 | 2.9 ± 0.2 | 6.9 ± 0.6 | 5.8 ± 0.6 |
| CBS/DPG/3ZnO/3Act8 | 7.0 ± 0.5 | 23.0 ± 0.5 | 1.9 ± 0.2 | 5.2 ± 0.6 | 6.3 ± 0.5 |
| Composites | Temperature of Crosslinking | ∆Hcross (J/g) | ||
|---|---|---|---|---|
| Tonset (°C) | Tendset (°C) | Tpeak (°C) | ||
| Rubber compounds with CBS | ||||
| 2.5CBS/5ZnO | 178 ± 1 | 232 ± 1 | 196 ± 1 | 8.4 ± 1.2 |
| 2.5CBS/5ZnO/2Act8 | 163 ± 2 | 236 ± 2 | 209 ± 2 | 9.8 ± 1.0 |
| 2.5CBS/3ZnO/3Act8 | 160 ± 2 | 235 ± 2 | 209 ± 2 | 11.0 ± 1.0 |
| 1.5CBS/3ZnO/4Act8 | 158 ± 1 | 237 ± 1 | 210 ± 1 | 10.2 ± 1.1 |
| 3ZnO/4Act8 | 199± 2 | 230 ± 2 | 214 ± 2 | 14.7 ± 0.9 |
| Rubber compounds with CBS and DPG | ||||
| CBS/DPG/5ZnO | 147 ± 1 | 233 ± 1 | 211 ± 1 | 8.0 ± 1.2 |
| CBS/DPG/5ZnO/2Act8 | 147 ± 1 | 234 ± 1 | 211 ± 1 | 8.6 ± 1.0 |
| CBS/DPG/3ZnO/3Act8 | 145 ± 2 | 234 ± 2 | 210 ± 2 | 9.8 ± 0.9 |
| Composites | E (MPa) | Se300 (MPa) | TS (MPa) | Eb (%) | H (Shore A) |
|---|---|---|---|---|---|
| Vulcanizates with CBS | |||||
| 2.5CBS/5ZnO | 1.9 ± 0.1 | 2.4 ± 0.1 | 16.0 ± 0.4 | 875 ± 14 | 61 ± 1 |
| 2.5CBS/5ZnO/2Act8 | 2.2 ± 0.1 | 3.1 ± 0.1 | 19.3 ± 0.1 | 756 ± 8 | 63 ± 1 |
| 2.5CBS/3ZnO/3Act8 | 2.4 ± 0.1 | 3.4 ± 0.1 | 21.2 ± 0.8 | 725 ± 9 | 63 ± 1 |
| 1.5CBS/3ZnO/4Act8 | 2.2 ± 0.1 | 2.9 ± 0.1 | 19.9 ± 1.4 | 751 ± 15 | 62 ± 1 |
| 3ZnO/4Act8 | 1.9 ± 0.1 | 2.2 ± 0.1 | 17.0 ± 0.4 | 895 ± 6 | 60 ± 1 |
| Vulcanizates with CBS and DPG | |||||
| CBS/DPG/5ZnO | 2.1 ± 0.1 | 3.1 ± 0.1 | 19.6 ± 1.6 | 750 ± 20 | 62 ± 1 |
| CBS/DPG/5ZnO/2Act8 | 2.3 ± 0.1 | 3.2 ± 0.1 | 20.5 ± 1.8 | 716 ± 15 | 63 ± 1 |
| CBS/DPG/3ZnO/3Act8 | 2.6 ± 0.1 | 3.4± 0.1 | 19.3 ± 1.1 | 682 ± 16 | 64 ± 1 |
| Composites | Tg (°C) | Tan δ at Tg (-) | Tan δ at 25 °C (-) | Tan δ at 60 °C (-) |
|---|---|---|---|---|
| Vulcanizates with CBS | ||||
| 2.5CBS/5ZnO | −51 ± 1 | 0.94 ± 0.08 | 0.09 ± 0.01 | 0.07 ± 0.01 |
| 2.5CBS/5ZnO/2Act8 | −47 ± 1 | 0.80 ± 0.06 | 0.08 ± 0.02 | 0.06 ± 0.02 |
| 2.5CBS/3ZnO/3Act8 | −47 ± 1 | 0.82 ± 0.05 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| 1.5CBS/3ZnO/4Act8 | −47 ± 1 | 0.74 ± 0.08 | 0.09 ± 0.01 | 0.07 ± 0.01 |
| 3ZnO/4Act8 | −50 ± 1 | 0.88 ± 0.07 | 0.08 ± 0.02 | 0.08 ± 0.02 |
| Vulcanizates with CBS and DPG | ||||
| CBS/DPG/5ZnO | −49 ± 1 | 0.83 ± 0.08 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| CBS/DPG/5ZnO/2Act8 | −48 ± 1 | 0.76 ± 0.06 | 0.08 ± 0.02 | 0.06 ± 0.02 |
| CBS/DPG/3ZnO/3Act8 | −47 ± 1 | 0.81 ± 0.05 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| Composites | Af (-) |
|---|---|
| Vulcanizates with CBS | |
| 2.5CBS/5ZnO | 0.55 ± 0.08 |
| 2.5CBS/5ZnO/2Act8 | 0.19 ± 0.05 |
| 2.5CBS/3ZnO/3Act8 | 0.20 ± 0.06 |
| 1.5CBS/3ZnO/4Act8 | 0.28 ± 0.08 |
| 3ZnO/4Act8 | 0.77 ± 0.09 |
| Vulcanizates with CBS and DPG | |
| CBS/DPG/5ZnO | 0.49 ± 0.05 |
| CBS/DPG/5ZnO/2Act8 | 0.56 ± 0.08 |
| CBS/DPG/3ZnO/3Act8 | 0.54 ± 0.08 |
| Composites | T5% (°C) | TDTG (°C) | ∆m25–600 °C (%) | ∆m600–700 °C (%) | Residue at 700 °C (%) |
|---|---|---|---|---|---|
| Vulcanizates with CBS | |||||
| 2.5CBS/5ZnO | 355 | 478 | 75.3 | 1.1 | 23.6 |
| 2.5CBS/5ZnO/2Act8 | 352 | 478 | 76.0 | 1.1 | 22.9 |
| 2.5CBS/3ZnO/3Act8 | 351 | 479 | 76.7 | 1.0 | 22.3 |
| 1.5CBS/3ZnO/4Act8 | 350 | 479 | 77.4 | 0.9 | 21.7 |
| 3ZnO/4Act8 | 358 | 477 | 76.4 | 0.9 | 22.7 |
| Vulcanizates with CBS and DPG | |||||
| CBS/DPG/5ZnO | 326 | 477 | 77.0 | 1.0 | 22.0 |
| CBS/DPG/5ZnO/2Act8 | 333 | 477 | 76.8 | 1.0 | 22.2 |
| CBS/DPG/3ZnO/3Act8 | 331 | 478 | 77.9 | 0.9 | 21.2 |
| Accelerator | T5% (°C) | TDTG (1st Step) (°C) | ∆m (1st Step) (%) | TDTG (2nd Step) (°C) | ∆m (2nd Step) (%) |
|---|---|---|---|---|---|
| CBS | 219 | 240 | 27.3 | 329 | 71.6 |
| DPG | 198 | 226 | 36.4 | 427 | 62.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejewska, M.; Sowińska-Baranowska, A. The Synergistic Effect of Dibenzyldithiocarbamate Based Accelerator on the Vulcanization and Performance of the Silica-Filled Styrene–Butadiene Elastomer. Materials 2022, 15, 1450. https://doi.org/10.3390/ma15041450
Maciejewska M, Sowińska-Baranowska A. The Synergistic Effect of Dibenzyldithiocarbamate Based Accelerator on the Vulcanization and Performance of the Silica-Filled Styrene–Butadiene Elastomer. Materials. 2022; 15(4):1450. https://doi.org/10.3390/ma15041450
Chicago/Turabian StyleMaciejewska, Magdalena, and Anna Sowińska-Baranowska. 2022. "The Synergistic Effect of Dibenzyldithiocarbamate Based Accelerator on the Vulcanization and Performance of the Silica-Filled Styrene–Butadiene Elastomer" Materials 15, no. 4: 1450. https://doi.org/10.3390/ma15041450
APA StyleMaciejewska, M., & Sowińska-Baranowska, A. (2022). The Synergistic Effect of Dibenzyldithiocarbamate Based Accelerator on the Vulcanization and Performance of the Silica-Filled Styrene–Butadiene Elastomer. Materials, 15(4), 1450. https://doi.org/10.3390/ma15041450









