Abstract
It has been shown by a set of corrosion, electrochemical and physical methods that a chamber corrosion inhibitor that consists of a mixture of octadecylamine (ODA) and benzotriazole (BTA) efficiently protects copper and brass from atmospheric corrosion and can be used for the temporary protection of metal items. The optimum temperatures of treatment with the ODA + BTA mixed inhibitor is 120 °C for brass and 100 °C for copper. One-hour treatment in ODA + BTA vapors at these temperatures results in the formation of nanosized adsorption films on the surface of these metals. These films stabilize the passive state and provide efficient temporary protection of metal items. The ODA + BTA inhibitor is superior to its components in terms of protective aftereffect. Our analysis of the mutual effect of BTA and ODA indicated that they show an antagonism of protective action on copper, but there is also a synergistic enhancement in the case of brass. Electrochemical impedance spectroscopy studies demonstrate that the inhibitors in question mainly act by using a blocking mechanism on copper and brass. Chamber treatment of the metals studied in vapors of the ODA + BTA mixture resulted in a noticeable hydrophobization of the copper surface and an insignificant effect on the brass surface. Chamber treatment of copper samples with artificially created polymodal roughness made it possible to obtain a superhydrophobic surface.
1. Introduction
The vapor-phase protection of metals with inhibitors [1,2,3,4,5,6,7,8,9,10,11] is a reliable way for the preservation of metal items. Volatile corrosion inhibitors (VCIs) are widely used in practical applications. Agents of this type were developed in the 1940s, and studies aimed at their improvement continue to date [1,2,3,4,5,6]. Chamber inhibitors (CINs) are not so well-known. The theory of action of CINs was founded by the authors of this article and has been developed in the past five years [7,8,9,10,11]. The pros and cons of VCIs and CINs are described in detail in Reference [12]. Commercial CINs are now only beginning to appear on the market as means for temporary protection. Moreover, CINs that protect a few metals at once have the best prospects of industrial application. A mixture of octadecylamine (ODA) and 1,2,3-benzotriazole (BTA) belongs to formulations of this kind.
The protective properties of this CIN on steel was studied previously [12]. In the same work, the following is noteworthy:
- -
- It was shown that a CIN comprising an ODA + BTA mixture efficiently protects steel from atmospheric corrosion and can be used for the temporary protection of metal items;
- -
- The optimal temperature of chamber treatment (TCT) and its duration (τCT) required to form nanoscale adsorption films on the steel surface in order to stabilize the metal passive state were determined;
- -
- It was found that the ODA + BTA mixture is superior to ODA or BTA alone in the protective aftereffect (PAE), but estimation of the mutual effect of the mixture components indicates their antagonism;
- -
- The protection of steel with ODA, BTA and their mixtures was found to occur by a mixed (blocking-activation) mechanism;
- -
- It was shown that chamber treatment (CT) of steel with an ODA + BTA mixture resulted in surface hydrophobization or superhydrophobization if a polymodal surface was created on steel before the CT.
The materials presented in this article continue the previous publication [12], are closely related to it, rely on the theoretical concepts put forward in it and deal with a study of the protective properties of the ODA + BTA combination and its components toward copper and brass.
2. Materials and Methods
Samples and electrodes of M1 copper and L62 brass were used in the experiments. Their composition is defined in the corresponding standards [13,14]. The experimental techniques used were identical to those reported previously [12]. In addition, the protective properties of the CIN were studied in a salt fog chamber (SFC). These experiments were carried out at room temperature in an WEISS SC/KWT 450 chamber (WEISS Technik, Germany). The number of cycles until the appearance of corrosion damage on the samples was recorded in the tests. Each cycle included a 15-min spraying of 3% sodium chloride solution, followed by 45-min exposure of the specimens in the resulting salt fog. The samples were examined after each cycle.
3. Results
3.1. Copper
3.1.1. Accelerated Tests
The optimal conditions for the chamber treatment of copper (TCT and τCT) were determined in two series of accelerated tests, namely in a KTV-0.1-002 heat-and-moisture chamber (HMC) (OJSC “KlimatSpetsMash”, Volgograd, Russian Federation), in which 100% relative air humidity was supplemented by daily moisture condensation on the samples, and in a salt fog chamber. Thermal treatment (TT) of copper samples without inhibitors (blank test) did not affect the protection time (τprot) under recurrent condensation conditions (Table 1). After 4 h of exposure in a corrosive environment, dark spots appeared on the copper. It is essential to note that one-hour exposure of copper samples at a temperature of 120 °C or higher changed the color of samples, due to metal oxidation.

Table 1.
Protective aftereffect (PAE) of adsorption films formed upon treatment of copper in CIN vapors at different t measured in a heat and moisture chamber. τCT = 60 min.
The adsorption films of ODA and BTA significantly inhibited the beginning of corrosion, even at TCT = 80 °C. The first signs of corrosion damage on copper appeared after 288 h (ODA) or after 456 h (BTA). A longer protective period (3576 h) under these conditions is provided by films obtained in vapors of the ODA + BTA mixture.
As one can see from Table 1, a TCT increase to 100 °C improved the protective effect of the adsorption films. The value of τprot increased twofold after treatment in BTA vapors, or threefold after treatment in ODA vapors. In the case of the ODA + BTA mixture, the PAE increased 1.3-fold. The first traces of corrosion damage appeared on copper samples in 960 h after the start of the experiment with BTA, after 1080 h with ODA or after 4800 h with ODA + BTA.
If TCT was increased to 120 °C or higher, undesirable darkening of the copper surface occurred, both after treatment with inhibitors and without them. Therefore, the subsequent study was limited to CT in the temperature range of 80–100 °C.
Thus, TCT = 100 °C is the optimal temperature for the protection of copper with the CIN mixture. As in the case of steel 3 [12], the CIN mixture provided more efficient copper protection compared to its components. However, the calculation of the coefficient of mutual effect of the mixture components α introduced in the previous publication [12] indicates an antagonism of BTA and ODA at 100 °C:
where the “meas” and “calc” indices refer to the protection times determined experimentally and are calculated while assuming that no mutual effects of components exist:
α = τprot, ODA + BTAmeas/τprot, ODA + BTAcalc = 0.02
τprot, ODA + BTAcalc = τprot, ODAmeas τprot, BTA meas/τprot, 0
The protection of copper by the CIN were studied by using TCT = 100 °C and τCT = 60 min. The first corrosion damage on copper samples not treated in CIN vapors appeared in the SFC after 2 h of testing (Table 2), and local corrosion was observed.

Table 2.
Protective aftereffect of adsorption films formed on copper in CIN vapors at 100 °C and at various τCT in the salt fog chamber.
Even CT for 20 min at 100 °C resulted in significant (7–7.5 fold) inhibition of copper depassivation. All the CIN studied showed approximately the same efficiency. The corrosion pattern was the same after chamber treatment with any of them.
An increase in CT duration from 20 to 60 min in BTA or ODA + BTA vapors almost did not affect the PAE of the adsorption films. Only treatment with ODA vapor resulted in a monotonic PAE increase with an increase in the CT time. Table 2 shows that, with a one-hour treatment of copper in ODA vapors, the PAE reached 24 cycles; that is, it increased by 70%.
3.1.2. Ellipsometry
It should be noted that CT with ODA vapors demonstrated a better protective effect in SFC tests of copper compared to BTA or ODA + BTA vapors. Based on the corrosion tests described above, this CIN by itself can be used for efficient protection of copper. However, ODA used alone is not so versatile, since it is inferior to the ODA + BTA mixture in the protection of steel [12] and brass, as shown below. As concerns the mixed CIN, the data given in Table 2 indicate that one-hour CT is certainly sufficient for the formation of protective layers with the maximum PAE for copper.
The ellipsometric estimates of the thickness of films formed on copper upon treatment in CIN vapors are given in Table 3.

Table 3.
Effect of copper chamber treatment on the thickness of surface films. TCT = 100 °C, and τCT = 60 min.
In the original state of copper before heat treatment, the air-formed oxide was 1 nm thick. Heat treatment in the absence of a CIN the increased thickness of the oxide film to 4–5 nm. Treatment of copper in CIN vapors decreased the thickness of the oxide film two- to three-fold and, at the same time, resulted in the formation of rather thick surface adsorption films of the CIN. As one can see from Table 3, the thickness of these films increases in the series BTA < ODA < ODA + BTA. These CIN films noticeably inhibit the growth of the air-formed copper oxide during the chamber heat treatment and directly affect the increase in the protective aftereffect of inhibitor-treated copper.
3.1.3. Voltametric Experiments
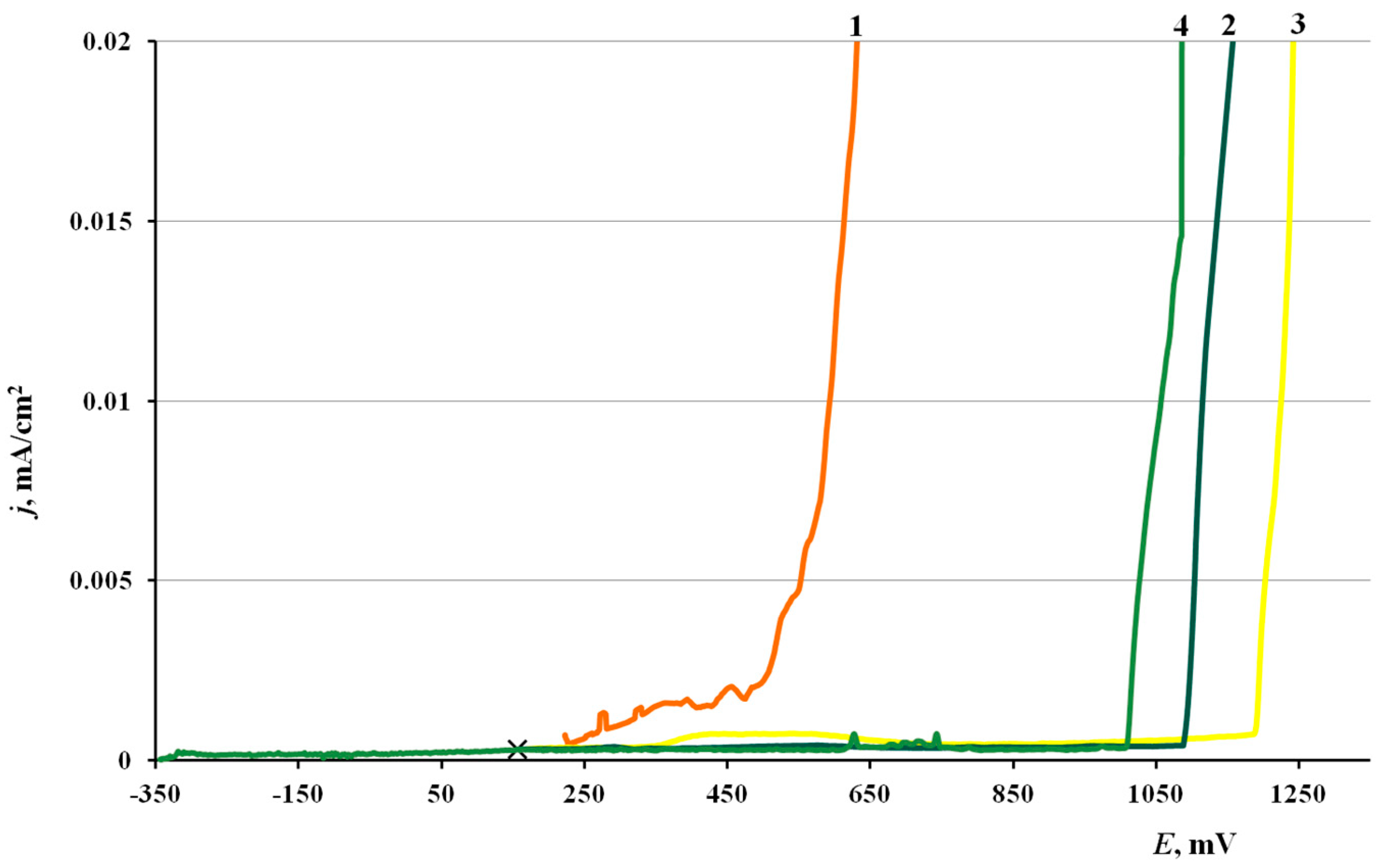
Figure 1 presents the anodic polarization curves on copper samples with various methods of surface modification with the CIN or without treatment. The characteristic potentials obtained in these experiments are summarized in Table 4. The shape of all the polarization curves is characteristic of the passive metal in a certain potential range before reaching Epit and/or the passive film breakdown potential Ebr under considerable anodic polarization. The corrosion potential, Ecor, of copper samples shifts cathodically after treatment with inhibitor vapors. The individual components (BTA and ODA) give nearly the same shift in Ecor (about −70 mV), while the mixed inhibitor shifts the open-circuit potential by −560 mV.
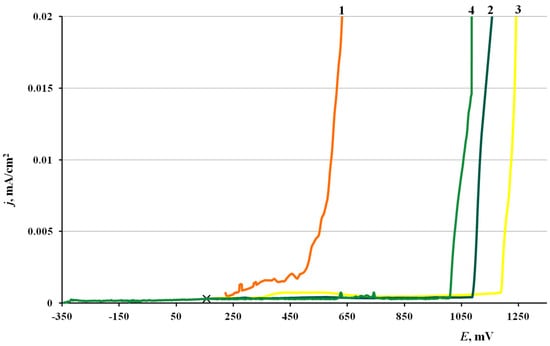
Figure 1.
Polarization curves of copper in borate buffer (pH 7.36) +0.001 M NaCl. (1) No treatment with a CIN, (2) BTA, (3) ODA and (4) ODA + BTA.

Table 4.
Effect of copper CT on the characteristics of anodic potentiodynamic curves. TCT = 100 °C, and τCT = 60 min.
The anodic polarization of samples that were not treated with a CIN resulted in a gradual current increase. Minor current oscillations were observed above Epit = 0.270 V, due to the beginning of surface oxide degradation, and film breakdown occurred at E = 0.510 V. Local dissolution pits were visible on the surface after removal of electrodes from the cell in the region of current oscillations.
Copper samples that underwent CT with inhibitors showed nearly no noticeable current oscillations during the anodic polarization until the film breakdown occurs. Compared to the “blank sample”, treatment with the CIN increased the breakdown potential considerably (by 500–700 mV). The greatest effect was obtained for the sample treated in ODA vapors. This effect of a sharp increase in Ebr unambiguously indicates that the protective properties of the surface oxide on copper increase significantly after modification in CIN vapors.
The conclusions obtained by analysis of polarization curves match the results of testing the corrosion resistance of copper in a salt fog, where the highest protection efficiency was also achieved after treatment in ODA vapors. Though the Epr of copper was lower after treatment with ODA + BTA vapors than in the case of ODA, the anti-pitting basis of the mixed inhibitor was larger.
3.1.4. Electrochemical Impedance Spectroscopy (EIS)
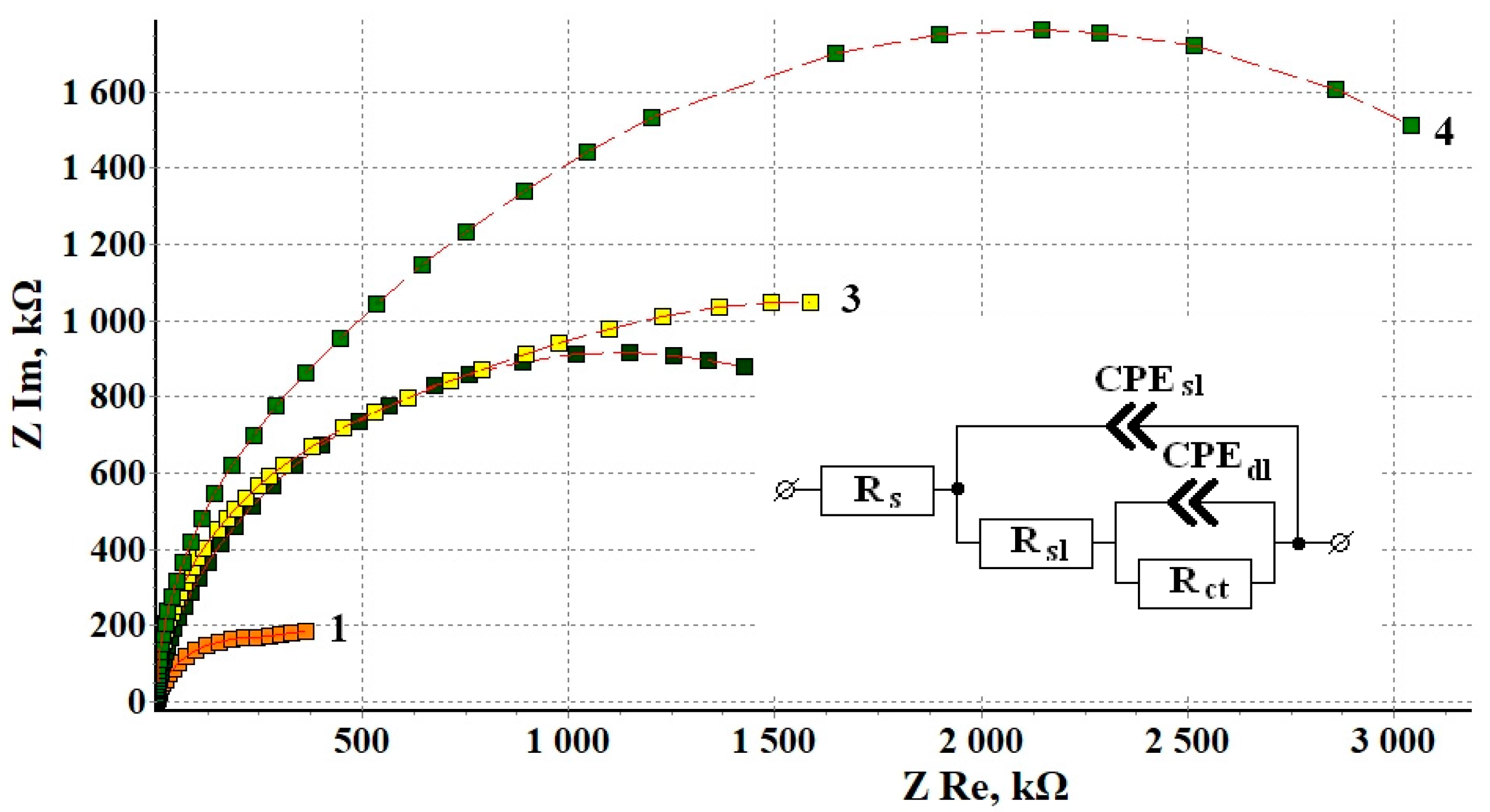
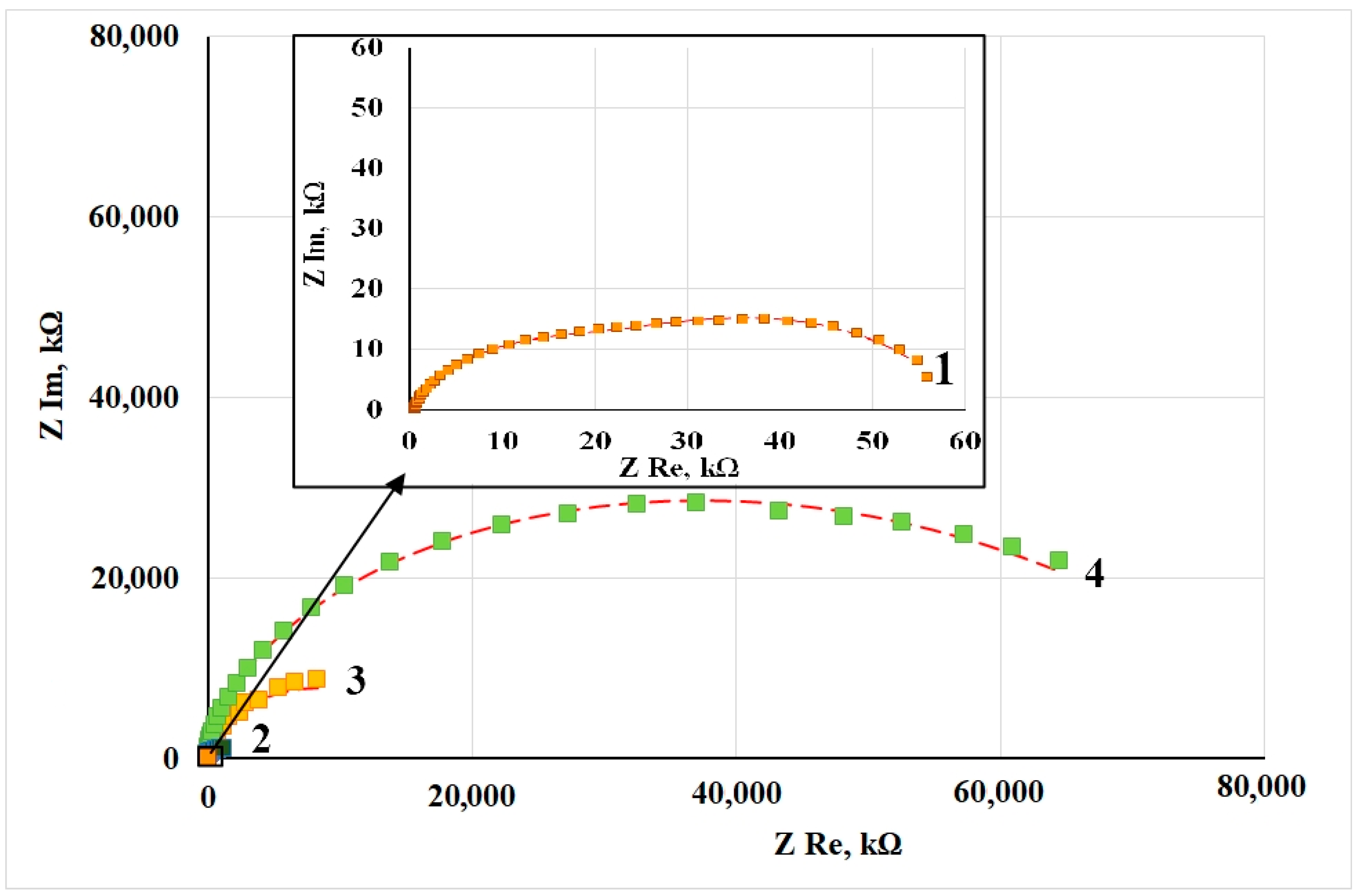
Figure 2 shows the Nyquist plots of copper samples after CT without and in the presence of CIN. Impedance measurements were performed in potentiostatic mode at open circuit potentials for each sample. All the hodographs are slightly deformed semicircles with varying curvature depending on frequency. The presence of at least two time constants in the frequency spectra allows this system to be simulated by using an equivalent circuit similar to the one we used before [12]. The mentioned article provides a detailed discussion of the interpretation of its elements, which is traditional for corrosion studies of metals with film layers.
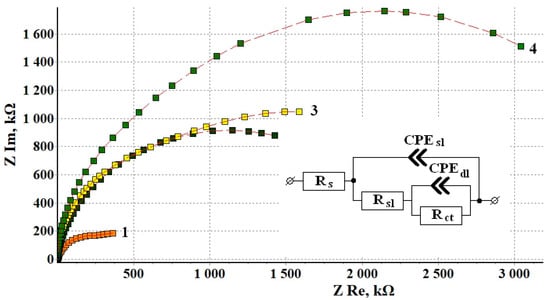
Figure 2.
Nyquist plots of copper samples after thermal treatment without a CIN (1) and after CT with the vapors of: BTA (2), ODA (3) and ODA + BTA (4).
The values of the equivalent circuit elements calculated by simulation of the experimental results are presented in Table 5. The deviation S between the experimental impedance points and those calculated by the model did not exceed 4%. Turning to the analysis of the results obtained, it should be noted that the Rs parameter refers to the properties of the electrolyte and the cell, and its value is the same in all the cases.

Table 5.
Values of equivalent circuit elements based on the results of impedance spectroscopy, as calculated for different variants of copper CT. TCT = 100 °C, and τCT = 60 min.
In this model, the Rsl parameter characterizes the resistance that appears in the surface layer to the transfer of ions from the near-electrode solution layer to the metal surface (and back). This layer is a thermal air-formed oxide in the “blank experiment” and the oxide modified with CIN adsorption films. In these experiments, we failed to separate the two sublayers (in contrast to in ellipsometry). However, one can see that, in comparison to a sample that did not undergo treatment with an inhibitor, the use of a CIN leads to a sharp increase in Rsl.
If we estimate the coefficient of corrosion inhibition because of surface blocking (γsl) as the ratio of Rsl resistances of a sample after CT and after thermal treatment without a CIN, we get the following:
where one can see that the protective properties of the surface layer increase almost 6-fold in the following series: BTA < ODA < ODA + BTA. Inhibited samples show a nearly linear relationship between the thickness of the adsorption film and the resistance of the surface layer.
γsl = RslCT/RslTT,
The Rct parameter (The Rct values, similar to the other parameters in the table, are given with respect to the geometric surface area of the sample) refers to the charge transfer resistance in the Faraday corrosion process on the active areas of the metal surface (in this case, copper). This factor also increases sharply if a CIN is used, but in contrast to Rsl, no correlation with the film thickness derived from the ellipsometric data is observed. The inhibition coefficient of the charge transfer Faraday process is shown in Table 6:
where the increase is as follows: ODA < ODA + BTA < BTA.
γct = RctCT/RctTT

Table 6.
Inhibition coefficients of chamber inhibitors by the blocking and activation mechanisms after different options of copper CT. TCT = 100 °C, and τCT = 60 min.
The capacitive characteristics of the system can be estimated from the modulus and phase factor of the constant phase elements in our model. Here, CPEsl is responsible for the geometric capacitance of the sample whose value is determined by a combination of the thickness and dielectric properties of the surface layer, the roughness and/or homogeneity factor, and the diffusivity of the liquid part of the capacitor plate. For all the samples, this element can be considered a fairly perfect capacitor whose specific capacitance consistently decreases in the series “Without CIN” > BTA > ODA > ODA + BTA, i.e., antibately to the surface film thickness. This confirms the strong surface blocking effect of the CIN found in the analysis of Rsl values.
The CPEdl element characterizes the properties of the double-layer capacitance of the electrochemical reaction itself on the active surface areas. A decrease in the CPEdl modulus is apparent. Its value decreased more than tenfold for samples treated with a CIN compared to the samples that underwent heat treatment without inhibitors. It may indicate that inhibition of the reaction and blocking of the active surface occur. This effect is most noticeable in the case of BTA, where the phase factor n = 0.79 indicates a noticeable heterogeneity of the active sites.
Thus, the results of experimental data simulation by the equivalent circuit for copper samples treated with CIN allow one to assume that the CIN studied predominantly act on copper by using a blocking mechanism. As one can see from Table 5, the overall protective efficiency, Z, determined from electrochemical impedance data increases as follows: BTA < ODA < ODA + BTA, and it is about 90% for all the inhibitors.
3.1.5. Contact Angle Measurement
The results of the contact-angle measurement under optimal conditions of copper CT by the inhibitors studied are given in Table 7.

Table 7.
Effect of copper CT on the contact angles of wetting with distilled water. TCT = 100 °C, and τCT = 60 min.
Similar to the case of steel, copper treatment by the CIN resulted in the hydrophobization of its surface. After CT in BTA or ODA vapors, the values of θ acquired were 107 and 104°, respectively. The maximum hydrophobization of copper (θ = 112°) was obtained with the ODA + BTA inhibitor. The same inhibitor gave a superhydrophobic surface (θ = 160°) on copper with preformed polymodal roughness.
The above data showed that the mixed CIN was highly efficient and gave us a reason for testing it under natural conditions. Taking into account the experience obtained in similar tests on steel [12], we placed copper samples in a cardboard box to protect the metal from the deposition of corrosive compounds from the atmosphere.
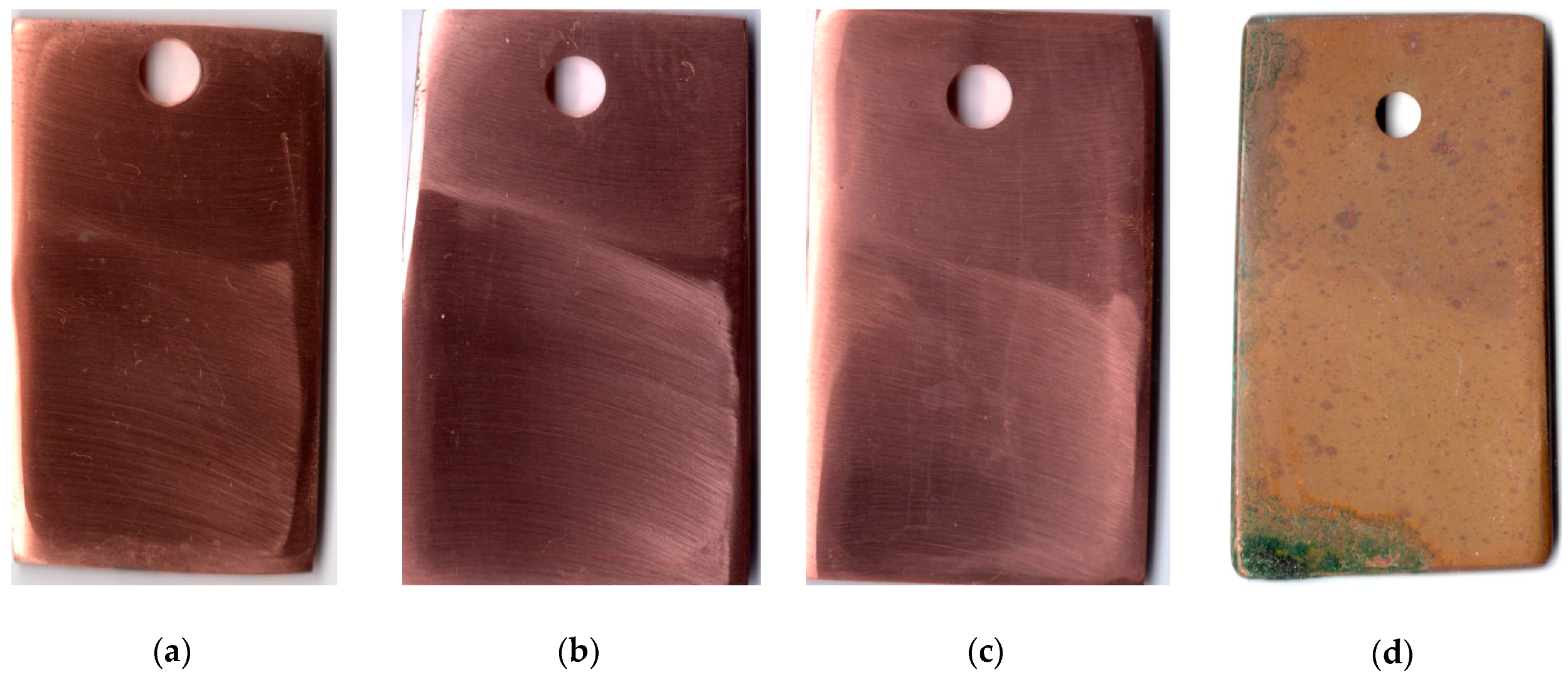
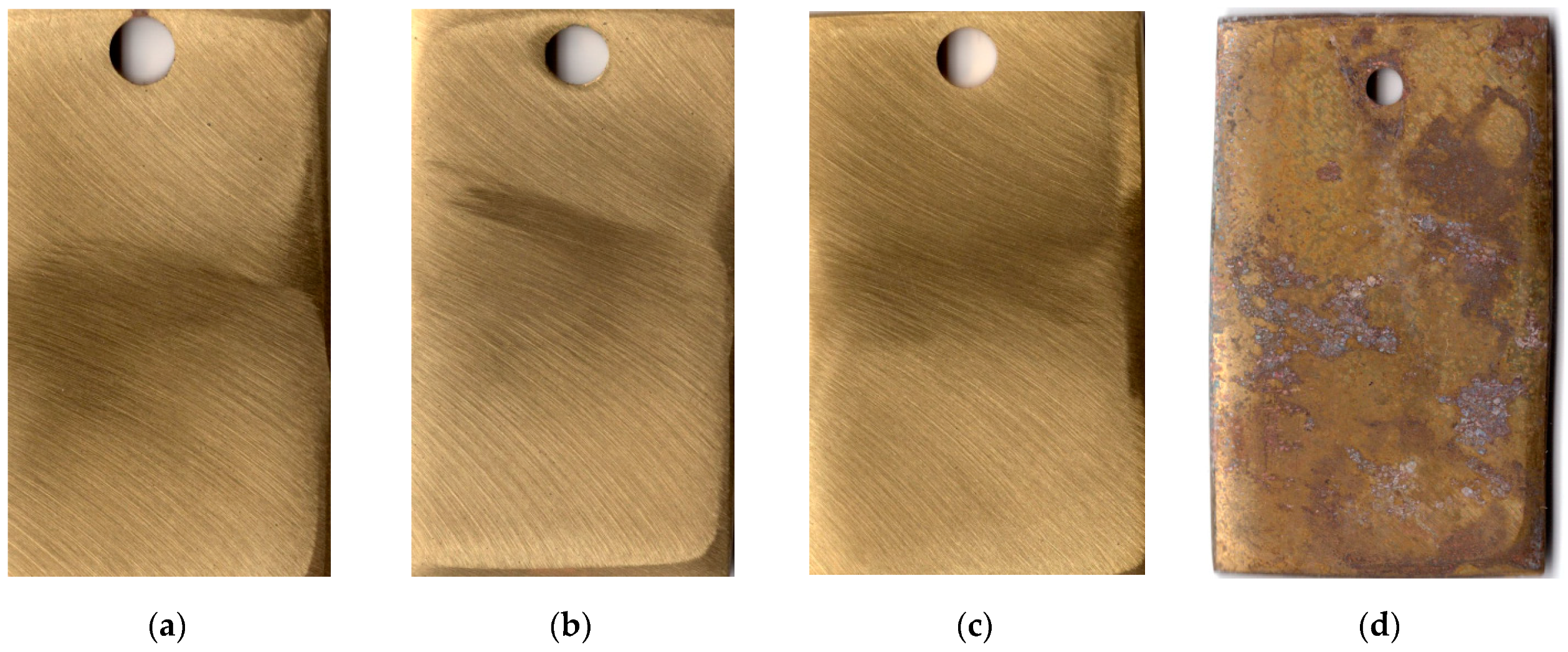
The first indications of corrosion on copper samples that were not subjected to CT appeared after 20 days in the urban atmosphere. The CT of the metal with the ODA + BTA mixture gave total protection of the samples from atmospheric corrosion for a period longer than 25 months, which is quite sufficient for temporary protection. At the time this article was written, the copper samples showed no signs of corrosion, and their exposure under natural conditions continued. Pictures of samples tested for 25 months under the above conditions are presented in Figure 3.
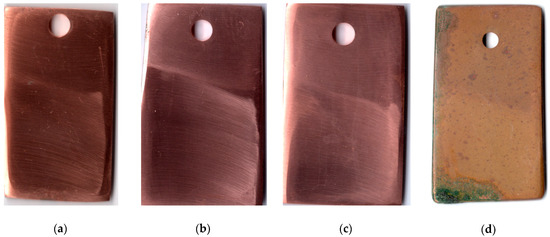
Figure 3.
Copper samples after weathering tests for 25 months: chamber treatment with CIN vapors (a–c); without chamber treatment (d).
3.2. Brass
3.2.1. Accelerated Corrosion Tests
Brass underwent local corrosion under recurrent moisture condensation. Within 2 h of exposure of the alloy under the test conditions, black dots appeared on the samples that did not undergo thermal treatment. The corrosion initiation time did not depend on the alloy treatment temperature.
The protective properties of the adsorption films formed on brass at 80 °C in BTA or ODA vapors did not exceed the background values, but the BTA + ODA mixture provided brass protection for 24 h under these conditions. At tCT = 100 or 120 °C, ODA did not protect brass, while BTA protected it very weakly by slowing down the formation of the first pits 2.5-fold. However, the ODA + BTA mixture demonstrated very efficient protection under these conditions. The films formed in its vapors at 100 °C completely prevented brass corrosion for 1416 h, or for 2232 h after treatment at 120 °C.
CT of brass in the vapors of the inhibitors studied at 140 °C, similar to TT of samples without a CIN, resulted in brass tarnishing. Therefore, these samples were not tested any further.
Thus, TCT = 120 °C is the optimal temperature for the CT of brass in ODA + BTA vapors. Table 8 demonstrates that a significant synergistic effect is observed between the components of the ODA + BTA mixture. The coefficient of mutual effect of the mixture components exceeded 446. The mechanism of this synergy is not entirely clear and requires additional studies that are beyond the scope of this work.

Table 8.
Protective aftereffect of adsorption films formed upon brass treatment in CIN vapors at various temperatures. Recurrent moisture condensation conditions. τCT = 60 min.
Similar to copper, the optimal τCT was determined by testing brass in a salt fog chamber. The first corrosion damage on samples not treated in CIN vapors appeared after one test cycle (Table 9). The corrosion was of a pitting nature.

Table 9.
Protective aftereffect of adsorption films formed on brass in CIN vapors at 120 °C and at various τCT in the salt fog chamber.
In the tests with BTA or ODA at 120 °C, increasing the CT time from 15 to 30 or 60 min did not slow down the metal depassivation. The CT of brass samples with the mixed CIN for the same period of time noticeably slowed down corrosion initiation to 3, 5, or 15 cycles, respectively. However, the character of brass corrosion did not change.
Heat treatment of brass at 120 °C for more than 60 min caused tarnishing of the alloy, regardless of whether the CIN studied was present. Thus, the duration of brass CT with a mixed CIN at the optimum temperature should not exceed 60 min.
3.2.2. Ellipsometry
The results of ellipsometric estimation of the thickness of films formed on brass upon treatment in various modes are given in Table 10.

Table 10.
Effect of brass chamber treatment on the surface film thickness. TCT = 120 °C, and τCT = 60 min.
After heat treatment of brass without a CIN, an oxide film up to 3–3.5 nm thick was found on the surface. It is evident from Table 10 that the thickness of the oxide decreases somewhat after the CT, but the CIN films formed significantly exceed in thickness both the oxide film and the adsorption films of inhibitors on steel [12] or copper.
The thinnest adsorption film (~20 nm) was detected after treatment with BTA. The layers of ODA and the ODA + BTA mixture were almost two times thicker (35–40 nm). They were by an order of magnitude thicker than those formed on copper by the same CIN. Obviously, in this case, the physicochemical nature of the alloy has a decisive effect. However, the thickness of adsorption films on brass does not prevent the use of the mixed CIN for the preservation of precision brass items, where the tolerance of the geometric dimensions is very small, since the films are hundredths of a micron thick.
3.2.3. Voltametric Experiments
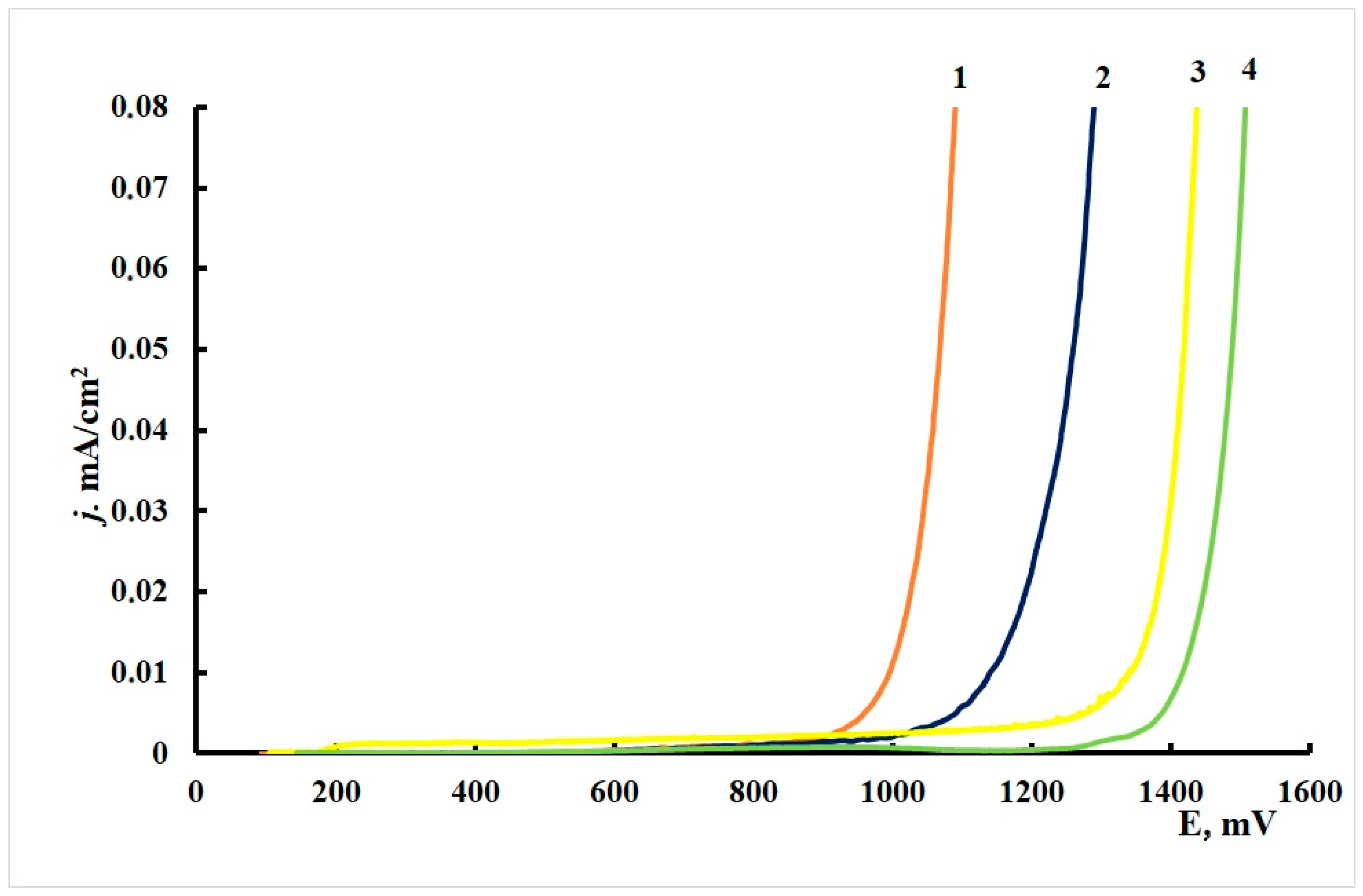
The anodic polarization curves of brass electrodes (Figure 4) had an extended passive region, followed by a region of insignificant current oscillations and gradual current increase in the potential range of 0.9–1.3 V. There was no sharp increase in current density on the polarization curves accompanied by an inflexion on the curve (due to breakdown of the protective film), in contrast to the experiments with copper. Black pits could be observed on the electrodes after the anodic polarization curves were recorded.
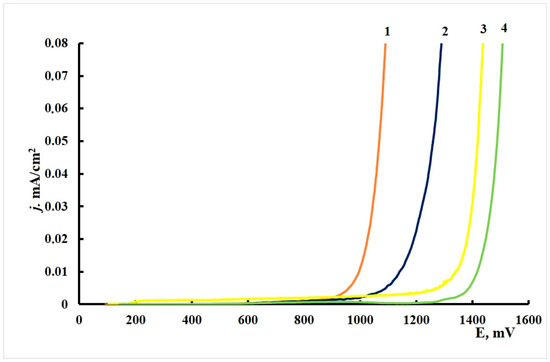
Figure 4.
Polarization curves on brass in borate buffer (pH 7.36) + 0.001 M NaCl. (1) Without CIN treatment, (2) BTA, (3) ODA and (4) ODA + BTA.
The characteristic corrosion potentials, Ecor, and potentials of the beginning of intense dissolution with formation of pits, Epit, are summarized in Table 11.

Table 11.
Effect of brass CT on the parameters of anodic potentiodynamic curves.
One can see from the table that the Ecor values of all brass samples are around 0.1 V, except that after treatment in ODA + BTA vapors, where Ecorr increases by 40–50 mV. This behavior sharply differs from similar tests with copper, where the mixed CIN strongly shifted Ecor in the cathodic direction.
The potentials above, for which a fast increase in current densities was observed listed in the table as Epit, consistently shift anodically after treatment with CIN vapors. Most likely, this potential is associated with the beginning of surface film destruction, as well as intensification of the anodic dissolution of the alloy base. Moreover, the Epit values are similar for the individual CIN but markedly more positive (by 0.4 V) for their mixture.
Thus, all the CIN studied meet the criteria of inhibitory protection if the chamber treatment of brass is performed under the optimal conditions. The mixed inhibitor is much superior to its components in terms of protective effect (higher overvoltage of the anodic reaction).
3.2.4. Electrochemical Impedance Spectroscopy
Figure 5 shows the hodographs of brass specimens subjected to chamber treatment under the optimal conditions. The characteristic shape of the hodographs, i.e., deformed semicircular arcs, allows this system to be simulated by using the same equivalent circuit as discussed above. The error S between the experimental and calculated hodographs obtained in experiments on brass followed by computer simulation with optimization of the circuit elements did not exceed 5%. The calculated values of the circuit elements are presented in Table 12.
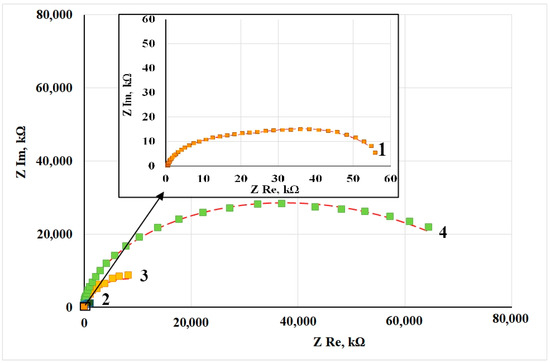
Figure 5.
Nyquist plots of brass samples after TT without a CIN (1) and after CT in the vapors of BTA (2), ODA (3) and ODA + BTA (4).

Table 12.
The values of equivalent circuit elements based on the results of impedance spectroscopy calculated for different variants of brass CT. TCT = 120 °C, and τCT = 60 min.
Since a scheme of the same type was used, let us apply a similar approach and analyze the protective effects of the CINs by the blocking and activation mechanisms summarized in Table 13.

Table 13.
Protective effects of the CINs by the blocking and activation mechanisms after brass chamber treatment in different modes. TCT = 120 °C, and τCT = 60 min.
It is obvious from the data presented above that BTA gives the smallest effect among the inhibitors studied. This is manifested in the degrees of protection both by the blocking and activation mechanism. Nevertheless, the comparison of these parameters to those of copper shows that this inhibitor protects brass much more efficiently and these parameters are quite comparable with those of St3 [12].
Much higher γsl and γct values were achieved for ODA, but the ODA + BTA mixture gave the best results. This CIN has a strong blocking effect (γsl = 1609) and, at the same time, provides a high degree of inhibition of the Faraday corrosion process (γct = 866). This behavior well correlates with the fact that the oxide-adsorption layer formed by ODA + BTA on the surface of brass is considerably thicker than on the other metals studied.
The geometrical capacitance, CPEsl, of brass specimens after treatment with CIN is an order of magnitude smaller than in experiments on steel or copper, probably also due to a thicker coating. The Faraday capacitance CPEdl for CIN on brass is by an order of magnitude lower than on copper and by two orders of magnitude smaller than on steel. This also confirms a high degree of blocking of the active centers of the anodic reaction.
Following the logic used in the interpretation of EIS results on steel and copper, it can be stated that BTA predominantly acts on brass by using the blocking mechanism. The protective efficiency of CIN increases in the series BTA < ODA < ODA + BTA, thus confirming the results of corrosion and potentiodynamic experiments.
3.2.5. Wetting Angle Measurement
The wetting contact angles measured on brass not subjected to CT and that treated for 60 min at 120 °C in the vapors of the CIN studied are shown in Table 14.

Table 14.
Effect of brass CT on the contact angles of wetting with distilled water. TCT = 120 °C, and τCT = 60 min.
Compared to the experiments on copper and steel [12], chamber treatment of brass weakly affected the contact angles. The θ angle slightly increased after CT, but this increase was just a little larger than 10° in the case of the ODA + BTA mixture. Larger contact angles were observed after treatment of brass with a pre-created polymodal roughness with the mixed CIN. However, the measured value, θ = 146°, did not allow us to classify this phenomenon as superhydrophobicity.
Brass samples not subjected to thermal treatment corroded 25 days after the start of exposure under conditions similar to those described above (a cardboard box under a shelter at the Moscow Corrosion Station). Chamber treatment of brass for 60 min at 120 °C in the vapors of the mixed inhibitor provided full protection of the metal for more than 25 months. This time is obviously sufficient for the temporary protection of items made of this material. Pictures of samples tested for 25 months under the indicated conditions are shown in Figure 6.
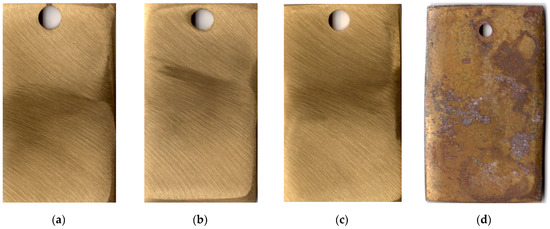
Figure 6.
Brass samples after weathering tests for 25 months: chamber treatment with CIN vapors (a–c); without chamber treatment (d).
At the time this article was written, the samples still showed no signs of corrosion and their exposure under natural conditions continued.
4. Conclusions
- A CIN consisting of an ODA + BTA mixture well protects copper and brass from atmospheric corrosion and can be used to temporarily protect metal items.
- The optimum temperature of CT with the mixed inhibitor is 120 °C for brass and 100 °C for copper. Treatment in its vapors for 1 h at these temperatures creates nanosized adsorption films on the surfaces of these metals. These films stabilize the passive state and provide efficient temporary protection of metal items.
- The protective effect of the ODA + BTA mixture is superior to that of its components. Assessment of their mutual effects indicates that there is an antagonism of the protective effect on copper and a synergistic enhancement in the case of brass.
- The results of impedance measurements make it possible to state that the protection of copper and brass by the CIN studied predominantly occurs by using the blocking mechanism.
- The most significant protective effect of the ODA + BTA formulation was found on brass. This effect is caused by the formation of adsorption films of considerable thickness that have high resistance to ion transfer from the electrolyte to the metal surface and, at the same time, strongly inhibit the Faraday process of metal oxidation.
- Chamber treatment of the metals studied with the ODA + BTA mixture results in surface hydrophobization that is significant on copper but insignificant on brass. Chamber treatment of copper samples with an artificially created polymodal surface makes the latter superhydrophobic.
Author Contributions
Conceptualization, Y.I.K. and N.N.A.; methodology, A.Y.L. and O.A.G.; supervision, Y.I.K.; electrochemical impedance spectroscopy studies, V.E.K., A.Y.L. and S.S.V.; ellipsometry experiments, N.P.A.; voltametric experiments, N.P.A., A.Y.L. and N.N.A.; edge angle measurement, V.E.K.; writing, N.P.A. and S.S.V. All authors have read and agreed to the published version of the manuscript.
Funding
The study was carried out as part of the Program of Fundamental Scientific Research of the Russian Academy of Sciences for 2019–2021, subject “Development of the fundamental scientific basis of the protective action of metal corrosion inhibitors in gas and condensed media, nanocomposites, paint and conversion coatings” (state registration number AAAA-A18-118121090043-0).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rozenfel’d, I.L.; Persiantseva, V.P. Inhibitors of Atmospheric Corrosion; Nauka: Moscow, Russia, 1985; 278p. (In Russian) [Google Scholar]
- Ansari, F.A.; Verma, C.; Siddiqui, Y.S.; Ebenso, E.E.; Quraishi, M.A. Volatile corrosion inhibitors for ferrous and non-ferrous metals and alloys: A review. Int. J. Corros. Scale Inhib. 2018, 7, 126–150. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Mahanwar, P.A. Recent developments in the volatile corrosion inhibitor (VCI) coatings for metal: A review. J. Coat. Technol. Res. 2018, 15, 789–807. [Google Scholar] [CrossRef]
- Andreev, N.N.; Kuznetsov, Y.I. Physicochemical aspects of the action of volatile metal corrosion inhibitors. Russ. Chem. Rev. 2005, 74, 685. [Google Scholar] [CrossRef]
- Valdez, B.; Schorr, M.; Cheng, N.; Beltran, E.; Salinas, R. Technological applications of volatile corrosion inhibitors. Corros. Rev. 2018, 36, 227–238. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Cano, E.; Mora, E.M. Volatile corrosion inhibitors: A review. Anti-Corros. Methods Mater. 2005, 52, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Andreev, N.N.; Goncharova, O.A.; Kuznetsov Yurii, I.; Yu Luchkin, A. A Method for Metal Protection from Atmospheric Corrosion. RF Patent 2649354, 2 April 2018. (In Russian). [Google Scholar]
- Goncharova, O.A.; Luchkin, A.Y.; Archipushkin, I.A.; Andreev, N.N.; Kuznetsov, Y.I.; Vesely, S.S. Vapor-phase protection of steel by inhibitors based on salts of higher carboxylic acids. Int. J. Corros. Scale Inhib. 2019, 8, 586–599. [Google Scholar] [CrossRef]
- Goncharova, O.A.; Luchkin, A.Y.; Andreev, N.N.; Andreeva, N.P.; Vesely, S.S. Triazole derivatives as chamber inhibitors of copper corrosion. Int. J. Corros. Scale Inhib. 2018, 7, 657–672. [Google Scholar] [CrossRef]
- Goncharova, O.A.; Andreev, N.N.; Luchkin, A.Y.; Kuznetsov, Y.I.; Andreeva, N.P.; Vesely, S.S. Protection of copper by treatment with hot vapours of octadecylamine, 1,2,3-benzotriazole and their mixtures. Mater. Corros. 2019, 70, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Luchkin, A.Y.; Goncharova, O.A.; Arkhipushkin, I.A.; Andreev, N.N.; Kuznetsov, Y.I. The effect of oxide and adsorption layers formed in 5-chlorobenzotriazole vapors on the corrosion resistance of copper. J. Taiwan Inst. Chem. Eng. 2020, 117, 231–241. [Google Scholar] [CrossRef]
- Goncharova, O.A.; Luchkin, A.Y.; Andreeva, N.P.; Kasatkin, V.E.; Vesely, S.S.; Andreev, N.N.; Kuznetsov, Y.I. Mutual Effects of Components of Protective Films Applied on Steel in Octadecylamine and 1,2,3-Benzotriazole Vapors. Materials 2021, 14, 7181. [Google Scholar] [CrossRef]
- GOST 859-2014; Copper. Grades. Rosstandart: Moscow, Russia, 2014.
- GOST 15527-2004; Pressure Treated Copper Zinc Alloys (Brasses). Grades. Rosstandart: Moscow, Russia, 2004.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).