Surface Cleaning Effect of Bare Aluminum Micro-Sized Powder by Low Oxygen Induction Thermal Plasma
Abstract
:1. Introduction
2. Materials and Methods
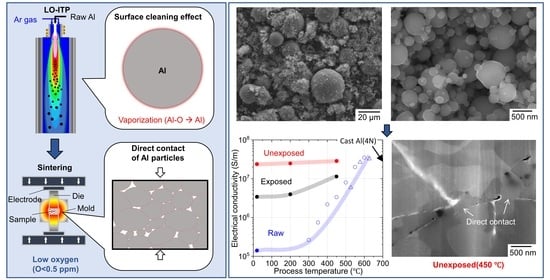
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Espera, A.H.; Dizon, J.R.C.; Chen, Q.; Advincula, R.C. 3D-printing and advanced manufacturing for electronics. Prog. Addit. Manuf. 2019, 4, 245–267. [Google Scholar] [CrossRef]
- Areir, M.; Xu, Y.; Harrison, D.; Fyson, J. 3D printing of highly flexible supercapacitor designed for wearable energy storage. Mater. Sci. Eng. B 2017, 226, 29–38. [Google Scholar] [CrossRef]
- Faller, L.-M.; Krivec, M.; Abram, A.; Zangl, H. AM metal substrates for inkjet-printing of smart devices. Mater. Charact. 2018, 143, 211–220. [Google Scholar] [CrossRef]
- DeBruin, D. Formulating a Particle-Free and Low Temperature Nickel Reactive Ink for Inkjet Printing Conductive Features; Arizona State University: Tempe, AZ, USA, 2019. [Google Scholar]
- Zhao, Y.; Gao, W.; Xi, J.; Li, H.; Ren, F. Development of copper powder paste for direct printing and soft mold casting. Addit. Manuf. 2020, 31, 100992. [Google Scholar] [CrossRef]
- Ji, H.; Zhou, J.; Liang, M.; Lu, H.; Li, M. Ultra-low temperature sintering of Cu@Ag core-shell nanoparticle paste by ultrasonic in air for high-temperature power device packaging. Ultrason. Sonochem. 2018, 41, 375–381. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Ji, H.; Wang, C. Rapid pressureless low-temperature sintering of Ag nanoparticles for high-power density electronic packaging. Scr. Mater. 2013, 69, 789–792. [Google Scholar] [CrossRef]
- Chakradhary, V.K.; Ansari, A.; Akhtar, M.J. Design, synthesis, and testing of high coercivity cobalt doped nickel ferrite nanoparticles for magnetic applications. J. Magn. Magn. Mater. 2019, 469, 674–680. [Google Scholar] [CrossRef]
- Panwar, N.; Chauhan, A. Fabrication methods of particulate reinforced Aluminium metal matrix composite—A review. Mater. Today Proc. 2018, 5, 5933–5939. [Google Scholar] [CrossRef]
- Singh, N.; Singh, R.; Ahuja, I.P.S.; Farina, I.; Fraternali, F. Metal matrix composite from recycled materials by using additive manufacturing assisted investment casting. Compos. Struct. 2019, 207, 129–135. [Google Scholar] [CrossRef]
- Tian, W.; Chen, F.; Cheng, F.; Li, Z.; Pang, G. Corrosion Properties of Pure Aluminum Prepared by Spark Plasma Sintering (SPS) Using Different Grain Size of Aluminium Powders as Raw Material. Int. J. Electrochem. Sci. 2020, 15, 9120–9134. [Google Scholar] [CrossRef]
- Demirskyi, D.; Agrawal, D.; Ragulya, A. Neck growth kinetics during microwave sintering of copper. Scr. Mater. 2010, 62, 552–555. [Google Scholar] [CrossRef]
- Brandes, E.A.; Brook, G. Smithells Metals Reference Book; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Munir, Z.A. Analytical treatment of the role of surface oxide layers in the sintering of metals. J. Mater. Sci. 1979, 14, 2733–2740. [Google Scholar] [CrossRef]
- Xie, G.; Ohashi, O.; Yoshioka, T.; Song, M.; Mitsuishi, K.; Yasuda, H.; Furuya, K.; Noda, T. Effect of Interface Behavior between Particles on Properties of Pure Al Powder Compacts by Spark Plasma Sintering. Mater. Trans. 2001, 42, 1846–1849. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Kondoh, K. Sintering Behaviors of Carbon Nanotubes—Aluminum Composite Powders. Metals 2016, 6, 213. [Google Scholar] [CrossRef] [Green Version]
- Nagae, T.; Yokota, M.; Nose, M.; Tomida, S.; Kamiya, T.; Saji, S. Effects of Pulse Current on an Aluminum Powder Oxide Layer During Pulse Current Pressure Sintering. Mater. Trans. 2002, 43, 1390–1397. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.; Park, D.H.; Park, Y.; Silvain, J.-F.; Kawasaki, A. Spark plasma sintering behavior of pure aluminum depending on various sintering temperatures. Met. Mater. Int. 2010, 16, 71–75. [Google Scholar] [CrossRef]
- Hasani, S.; Panjepour, M.; Shamanian, M. The Oxidation Mechanism of Pure Aluminum Powder Particles. Oxid. Met. 2012, 78, 179–195. [Google Scholar] [CrossRef]
- Liu, Q.-M.; Zhou, D.-B.; Yamamoto, Y.; Ichino, R.; Okido, M. Preparation of Cu nanoparticles with NaBH4 by aqueous reduction method. Trans. Nonferrous Met. Soc. China 2012, 22, 117–123. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Kim, D.; Hirayama, Y.; Liu, Z.; Kwon, H.; Kobashi, M.; Takagi, K. Highly Conductive Al/Al Interfaces in Ultrafine Grained Al Compact Prepared by Low Oxygen Powder Metallurgy Technique. Nanomaterials 2021, 11, 1182. [Google Scholar] [CrossRef]
- Hirayama, Y.; Suzuki, K.; Yamaguchi, W.; Takagi, K. Cold welding behavior of fine bare aluminum powders prepared by new low oxygen induction thermal plasma system. J. Alloy. Compd. 2018, 768, 608–612. [Google Scholar] [CrossRef]
- Park, K.; Hirayama, Y.; Shigeta, M.; Liu, Z.; Kobashi, M.; Takagi, K. Anisotropic Sm-Co nanopowder prepared by induction thermal plasma. J. Alloy. Compd. 2021, 882, 160633. [Google Scholar] [CrossRef]
- Hirayama, Y.; Takahashi, Y.; Hirosawa, S.; Hono, K. NdFe12Nx hard-magnetic compound with high magnetization and anisotropy field. Scr. Mater. 2015, 95, 70–72. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, Y.; Takahashi, Y.; Hirosawa, S.; Hono, K. Intrinsic hard magnetic properties of Sm (Fe1 − xCox)12 compound with the ThMn12 structure. Scr. Mater. 2017, 138, 62–65. [Google Scholar] [CrossRef]
- Lamoreaux, R.; Hildenbrand, D.; Brewer, L. High-Temperature Vaporization Behavior of Oxides II. Oxides of Be, Mg, Ca, Sr, Ba, B, Al, Ga, In, Tl, Si, Ge, Sn, Pb, Zn, Cd, and Hg. J. Phys. Chem. Ref. Data 1987, 16, 419–443. [Google Scholar] [CrossRef]
- Simmons, J.G. Generalized Formula for the Electric Tunnel Effect between Similar Electrodes Separated by a Thin Insulating Film. J. Appl. Phys. 1963, 34, 1793–1803. [Google Scholar] [CrossRef] [Green Version]
- Shu, Q.Q.; Ma, W.G. Barrier parameter variation in Al-Al,03-metal tunnel jainctions. Appl. Phys. Lett. 1992, 61, 2542–2544. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, Y.-C.; Lu, C.-C.; Yeh, C.-H.; Chiu, P.-W.; Iijima, S.; Suenaga, K. In situ observation of step-edge in-plane growth of graphene in a STEM. Nat. Commun. 2014, 5, 4055. [Google Scholar] [CrossRef] [Green Version]




| Sample | Process Temperature (°C) | Oxygen Content (wt%) | Absolute Density (g/cm3) | Correct Relative Density (%) | Electrical Conductivity (S/m) |
|---|---|---|---|---|---|
| Unexposed | 20 | 1.4 | 2.46 | 90.5 ± 4.4 | 2.4∙107 |
| 200 | 1.1 | 2.53 | 95.5 ± 1.5 | 2.5∙107 | |
| 450 | 0.7 | 2.50 | 96.0 ± 1.1 | 2.9∙107 | |
| Exposed | 20 | 2.5 | 2.39 | 89.0 ± 5.5 | 0.3∙107 |
| 200 | 2.2 | 2.52 | 89.8 ± 4.8 | 0.4∙107 | |
| 450 | 1.3 | 2.64 | 94.0 ± 1.5 | 1.1∙107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Hirayama, Y.; Takagi, K.; Kwon, H. Surface Cleaning Effect of Bare Aluminum Micro-Sized Powder by Low Oxygen Induction Thermal Plasma. Materials 2022, 15, 1553. https://doi.org/10.3390/ma15041553
Kim D, Hirayama Y, Takagi K, Kwon H. Surface Cleaning Effect of Bare Aluminum Micro-Sized Powder by Low Oxygen Induction Thermal Plasma. Materials. 2022; 15(4):1553. https://doi.org/10.3390/ma15041553
Chicago/Turabian StyleKim, Dasom, Yusuke Hirayama, Kenta Takagi, and Hansang Kwon. 2022. "Surface Cleaning Effect of Bare Aluminum Micro-Sized Powder by Low Oxygen Induction Thermal Plasma" Materials 15, no. 4: 1553. https://doi.org/10.3390/ma15041553
APA StyleKim, D., Hirayama, Y., Takagi, K., & Kwon, H. (2022). Surface Cleaning Effect of Bare Aluminum Micro-Sized Powder by Low Oxygen Induction Thermal Plasma. Materials, 15(4), 1553. https://doi.org/10.3390/ma15041553