Corrosion Behavior of Titanium Dental Implants with Implantoplasty
Abstract
:1. Introduction
2. Materials and Methods
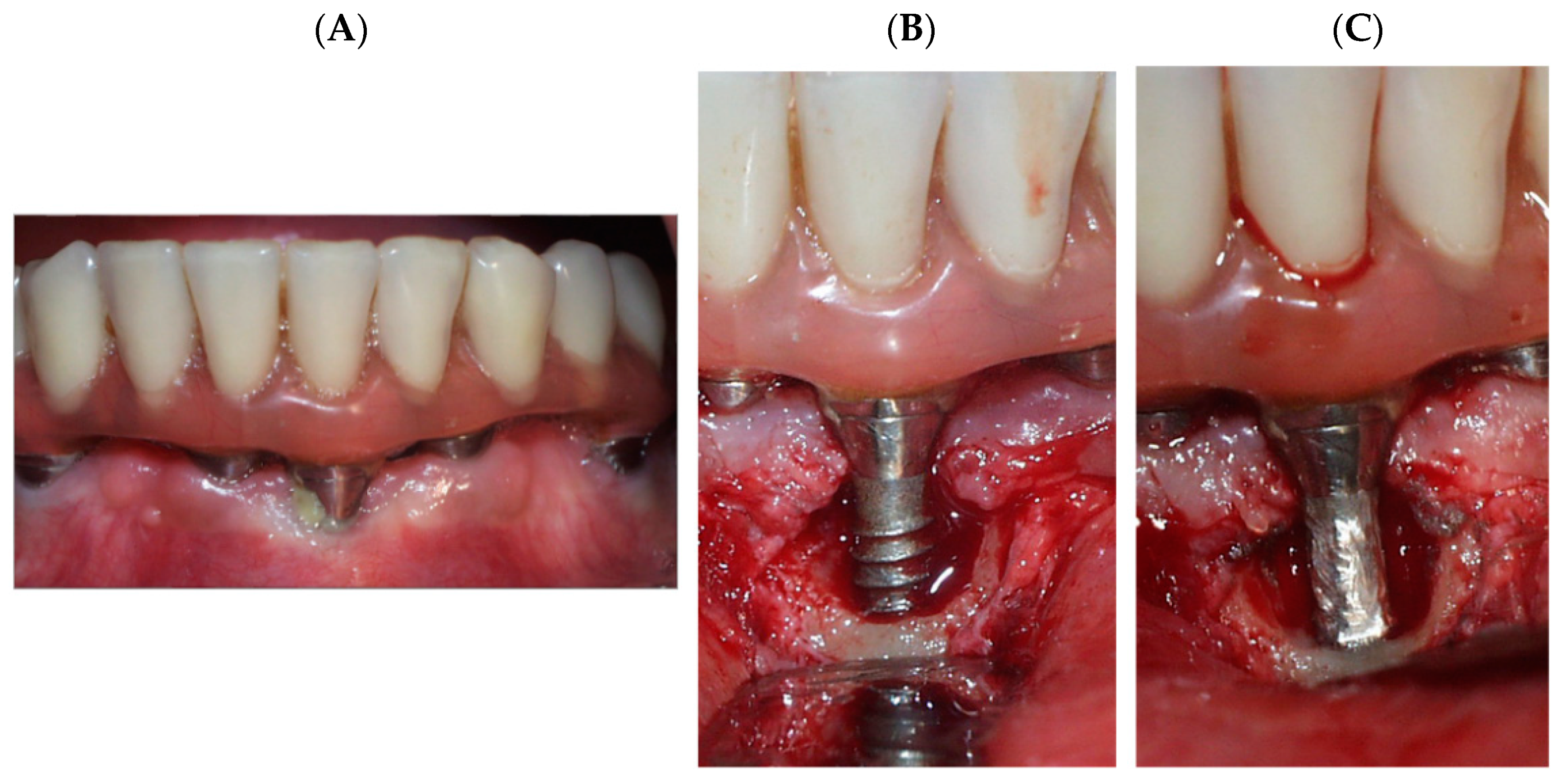
- Cp-Ti dental implant as received. (grade 4). (As-received)
- Cp-Ti dental implant treated by implantoplasty. (Implantoplasty)
- Cp-Ti debris obtained by implantoplasty. (Debris)
- -
- icorr (μA/cm2)/corrosion current density.
- -
- Ecorr (mV)/Corrosion potential: value at which the current density changes from cathodic to anodic.
- -
- Erep (mV)/Repassivation potential: potential at which the passive layer regenerates.
- -
- Ep (mV)/Pitting potential: value at which pitting corrosion may occur.
- -
- ip (μA/cm2)/passivation current density.
- -
- ip (μA/cm2)/repassivation current density.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco-Ortega, E.; Alfonso-Rodríguez, C.; Monsalve-Guil, L.; España-López, A.; Jiménez-Guerra, A.; Garzón, I.; Alaminos, M.; Gil, F. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater. Sci. Eng. C 2016, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Rodriguez, D.; Gil, F.J. Variation of roughness and adhesion strength of deposited apatite layers on titanium dental implants. Mater. Sci. Eng. C 2011, 31, 320–324. [Google Scholar] [CrossRef]
- Von Wilmowsky, C.; Moest, T.; Nkenke, E.; Stelzle, F.; Schlegel, K.A. Implants in bone: Part I. A current overview about tissue response, surface modifications and future perspectives. Oral Maxillofac. Surg. 2013, 18, 243–257. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; Lázaro, P.; Rios, J.V.; Lluch, S.; Marqués, M.; Guillem-Martí, J.; Gil, F.J. Influence of acid-etching after grit-blasted on osseointegration of titanium dental implants: In vitro and in vivo studies. J. Mater. Sci. Mater. Electron. 2013, 24, 2047–2055. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 286–291. [Google Scholar] [CrossRef]
- Lindhe, J.; Meyle, J.; Group D of the European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 282–285. [Google Scholar] [CrossRef] [Green Version]
- Lang, N.P.; Berglundh, T.; Working Group 4 of the Seventh European Workshop on Periodontology. Periimplant diseases: Where are we now?—Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 178–181. [Google Scholar] [CrossRef] [Green Version]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S158–S171. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 292–304. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Lindhe, J.; Marinello, C.; Ericsson, I.; Liljenberg, B. Soft tissue reaction to de novo plaque formation on implants and teeth. An experimental study in the dog. Clin. Oral Implant. Res. 1992, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Brägger, U.; Walther, D.; Beamer, B.; Kornman, K.S. Ligature-induced peri-implant infection in cynomolgus monkeys. I. Clinical and radiographic findings. Clin. Oral Implant. Res. 1993, 4, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; van Oosten, M.A.; Schurch, E.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol. 2000 2010, 53, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Soto-Alvaredo, J.; Blanco, E.; Bettmer, J.; Hevia, D.; Sainz, R.M.; López Cháves, C.; Sánchez, C.; Llopis, J.; Sanz-Medel, J.; Montes-Bayón, M. Evaluation of the biological effect of Ti generated debris from metal implants: Ions and nanoparticles. Metallomics 2014, 6, 1702–1708. [Google Scholar] [CrossRef]
- Barrak, F.N.; Li, S.; Muntane, A.M.; Jones, J.R. Particle release from implantoplasty of dental implants and impact on cells. Int. J. Implant. Dent. 2020, 6, 50. [Google Scholar] [CrossRef]
- Costa-Berenguer, X.; García-García, M.; Sánchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellón, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implant. Res. 2018, 29, 46–54. [Google Scholar] [CrossRef]
- ASTM-E3-11; Standard Guide for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM G5-14e1; Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2014.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneve, Switzerland, 2009.
- ASTM G-102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2010.
- Gil, F.J.; Rodríguez, D.; Planell, J.A.; Cortada, M.; Giner, L.; Costa, S. Galvanic corrosion behaviour of Titanium implants coupled to dental alloys. J. Mat. Sci. Mat. Med. 2000, 11, 287–293. [Google Scholar]
- Gil, F.J.; Sánchez, L.A.; Espias, A.; Planell, J.A. In vitro corrosion behaviour and metallic ion release of different prosthodontic alloys. Int. Dent. J. 1999, 49, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Al-Hity, R.R.; Kappert, H.F.; Viennot, S.; Dalard, F.; Grosgogeat, B. Corrosion resistance measurements of dental alloys, are they correlated? Dent. Mater. 2007, 23, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Gil, F.J.; Fonseca, C.; Barbosa, M.; Planell, J.A. Corrosion behaviour of commercially pure tianium shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials 2003, 24, 263–273. [Google Scholar] [CrossRef]
- Rodrigues, D.; Valderrama, P.; Wilson, T.; Palmer, K.; Thomas, A.; Sridhar, S.; Sadhwani, C. Titanium Corrosion Mechanisms in the Oral Environment: A Retrieval Study. Materials 2013, 6, 5258–5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godoy-Gallardo, M.; Manzanares-Céspedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodríguez, D. Evaluation of bone loss in antibacterial coated dental implants: An experimental study in dogs. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Hoyos, M.; Velasco, F.; Ginebra, M.P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating bone via multifunctional coatings: The blending of cell integration and bacterial inhibition properties on the Surface of biomaterials. ACS Appl. Mater. Interf. 2019, 11, 36449–36457. [Google Scholar]
- Garcia-Godoy, M.; Rodríguez-Hernández, A.; Delgado, L.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Silver deposition on titanium surface by electrochemical anodizing process reduces bacterial adhesion of Streptococcus sanguinis and Lactobacillus salivarius. Clin.Oral Impl. Res. 2014, 26, 1170–1179. [Google Scholar] [CrossRef] [Green Version]
- Punset, M.; Villarrasa, J.; Nart, J.; Manero, J.M.; Bosch, B.; Padrós, R.; Perez, R.A.; Gil, F.J. Citric Acid Passivation of Titanium Dental Implants for Minimizing Bacterial Colonization Impact. Coatings 2021, 11, 214. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-functional silane immobilized onto titanium surfaces induces osteoblast cell differentiation and reduces bacterial adhesion and biofilm formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Buxadera-Palomero, J.; Godoy-Gallardo, M.; Molmeneu, M.; Punset, M.; Gil, F.J. Antibacterial properties of triethoxysilylpropyl succinic anhidride silane (TESPSA) on Titanium dental implants. Polymers 2020, 12, 773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco-Ortega, E.; Flichy-Fernández, A.; Punset, M.; Jiménez-Guerra, A.; Manero, J.M.; Gil, F.J. Fracture and Fatigue of Titanium Narrow Dental Implants: New Trends in Order to Improve the Mechanical Response. Materials 2019, 12, 3728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, R.A.; Gargallo, J.; Altuna, P.; Herrero-Climent, M.; Gil, F.J. Fatigue of Narrow Dental Implants: Influence of the Hardening Method. Materials 2020, 13, 1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannon, B.P.; Mild, E.E. Titanium alloys for Biomaterial Application: An overview. In Titanium Alloys in Surgical Implants. ASTM-STP796; Luckey, H.A., Kubli, F., Eds.; ASTM: Philadelphia, PA, USA, 1981; pp. 7–14. [Google Scholar]
- Lemons, J.E. Application of materials in medicine and dentistry. Dental implants. In Biomaterials Science: An Introduction to Materials in Medicine; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 308–318. [Google Scholar]
- Boutinguiza, M.; Fernández-Arias, M.; del Val, J.; Buxadera-Palomero, J.; Rodríguez, D.; Lusquiños, F.; Gil, F.J.; Pou, J. Synthesis and deposition of silver nanoparticles on cp Ti by laser ablation in open air for antibacterial effect in dental implants. Mater. Lett. 2018, 231, 126–129. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Boutinguiza, M.; del Val, J.; Medina, E.; Rodriguez, D.; Riveiro, A.; Comesaña, R.; Gil, F.J.; Pou, J. RE-irradiation of silver nanoparticles obtained by laser ablation in water and assessment of their antibacterial effect. Appl. Surf. Sci. 2019, 473, 548–554. [Google Scholar] [CrossRef]
- Zabielska, J.; Kunicka-Styczynska, A.; Otlewska, A. Adhesive and hydrophobic properties of Pseudomonas aeruginosa and Pseudomonas cedrina associated with cosmetics. Ecol. Quest. 2017, 28, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Toledano-Serrabona, J.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellon, E.; Gay-Escoda, C.; Sánchez-Garcés, M.A. Physicochemical and Biological Characterization of Ti6Al4V particles obtained by Implantoplasty: An In vivo study. Part I. Materials 2021, 14, 6507. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.A.; Gay-Escoda, C.; Valmaseda-Castellon, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical properties and corrosión behavior of Ti6Al4V particles obtained by Implatoplasty. An in vivo study. Part II. Materials 2021, 14, 6519. [Google Scholar] [CrossRef]
- Burgueño-Barris, G.; Camps-Font, O.; Figueiredo, R.; Valmaseda-Castellón, E. The Influence of Implantoplasty on Surface Roughness, Biofilm Formation, and Biocompatibility of Titanium Implants: A Systematic Review. J. Oral Maxil. Impl. 2021, 36, 111–119. [Google Scholar] [CrossRef]
- González Regueiro, I.; Martínez Rodriguez, N.; Barona Dorado, C.; Sanz-Sánchez, I.; Montero, E.; Ata-Ali, J.; Duarte, F.; Martínez-González, J.M. Surgical approach combining implantoplasty and reconstructive therapy with locally delivered antibiotic in the treatment of peri-implantitis: A prospective clinical case series. Clin. Implant. Dent. Relat. Res. 2021, 23, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Sukiman, N.L.; Bushroa, A.R.; Nasiri-Tabrizi, B.; Dabbagh, A.; Abu Kasim, N.H.; Basirun, W.J. In vitro bioactivity and corrosion resistance enhancement of Ti-6Al-4V by highly ordered TiO2 nanotube arrays. J. Aust. Cer. Soc. 2019, 55, 187–200. [Google Scholar] [CrossRef]
- Verdeguer, P.; Gil, J.; Punset, M.; Manero, J.M.; Nart, J.; Vilarrasa, J.; Ruperez, E. Citric Acid in the Passivation of Titanium Dental Implants: Corrosion Resistance and Bactericide Behavior. Materials 2022, 15, 545. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.; Gil, J.; Punset, M.; Manero, J.M.; Tondela, J.P.; Verdeguer, P.; Aparicio, C.; Rúperez, E. Relevant Aspects of Piranha Passivation in Ti6Al4V Alloy Dental Meshes. Coatings 2022, 12, 154. [Google Scholar] [CrossRef]








| Chemical Product | Composition (mM) |
|---|---|
| K2HPO4 | 0.44 |
| KCl | 5.4 |
| CaCl2 | 1.3 |
| Na2HPO4 | 0.25 |
| NaCl | 137 |
| NaHCO3 | 4.2 |
| MgSO4 | 1.0 |
| C6H12O6 | 5.5 |
| Material | σ (MPa) |
|---|---|
| As-received | −77.2 ± 5.2 |
| Implantoplasty | −120.0 ± 10.3 |
| Debris | −202.1 ± 12.2 |
| Samples | EOCP (mV) | icorr (μA/cm2) | Rp (MΩ/cm2) | ECORR (V) | Vc (mm/year) |
|---|---|---|---|---|---|
| As-received | −195 ± 9 | 0.049 ± 0.007 | −340 ± 32 | ||
| Implantoplasty | −273 ± 10 | 0.056 ± 0.005 | −368 ± 47 | ||
| Debris | −334 ± 17 | 0.063 ± 0.009 | −411 ± 21 |
| Mesh | 1 Day | 3 Days | 7 Days | 14 Days | 21 Days |
|---|---|---|---|---|---|
| As-receieved | 1.3 ± 0.2 | 2.7 ± 0.5 | 2,9 ± 0.5 | 4.5 ± 0.4 | 7.0 ± 0.6 |
| Implantoplasty | 1.7 ± 0.4 | 3.3 ± 0.4 | 4.1 ± 0.2 | 5.7 ± 0.3 | 9.1 ± 0.5 |
| Debris | 2.9 ± 0.8 | 4.8 ± 0.8 | 5.3 ± 0.9 | 10.4 ± 5.9 | 15.3 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, P.; Peña, M.; Herrero-Climent, M.; Rios-Santos, J.V.; Rios-Carrasco, B.; Brizuela, A.; Gil, J. Corrosion Behavior of Titanium Dental Implants with Implantoplasty. Materials 2022, 15, 1563. https://doi.org/10.3390/ma15041563
Lozano P, Peña M, Herrero-Climent M, Rios-Santos JV, Rios-Carrasco B, Brizuela A, Gil J. Corrosion Behavior of Titanium Dental Implants with Implantoplasty. Materials. 2022; 15(4):1563. https://doi.org/10.3390/ma15041563
Chicago/Turabian StyleLozano, Pablo, Marta Peña, Mariano Herrero-Climent, Jose Vicente Rios-Santos, Blanca Rios-Carrasco, Aritza Brizuela, and Javier Gil. 2022. "Corrosion Behavior of Titanium Dental Implants with Implantoplasty" Materials 15, no. 4: 1563. https://doi.org/10.3390/ma15041563
APA StyleLozano, P., Peña, M., Herrero-Climent, M., Rios-Santos, J. V., Rios-Carrasco, B., Brizuela, A., & Gil, J. (2022). Corrosion Behavior of Titanium Dental Implants with Implantoplasty. Materials, 15(4), 1563. https://doi.org/10.3390/ma15041563










