Efficient Removal of Hg(II) from Water under Mildly Acidic Conditions with Hierarchical SiO2 Monoliths Functionalized with –SH Groups
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Hierarchical Silica Monoliths
2.2. Preparation of Thiol-Functionalized Adsorbents
2.3. Mercury Batch Adsorption Experiments
2.4. Characterization Methods
3. Result and Discussion
3.1. Sorbent Characterization
3.1.1. Textural Properties
3.1.2. Framework Vibration
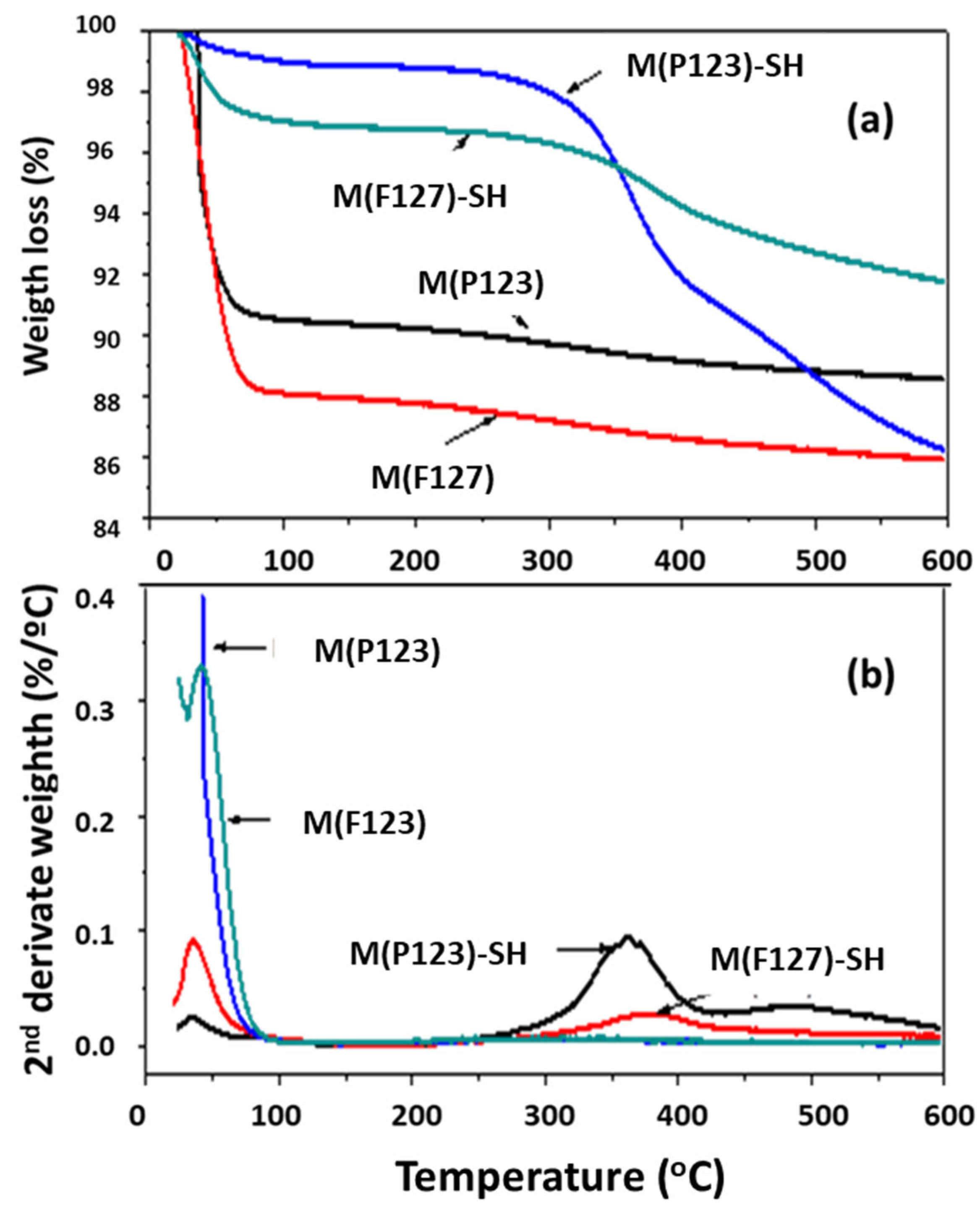
3.1.3. Thermal Stability of –SH Groups
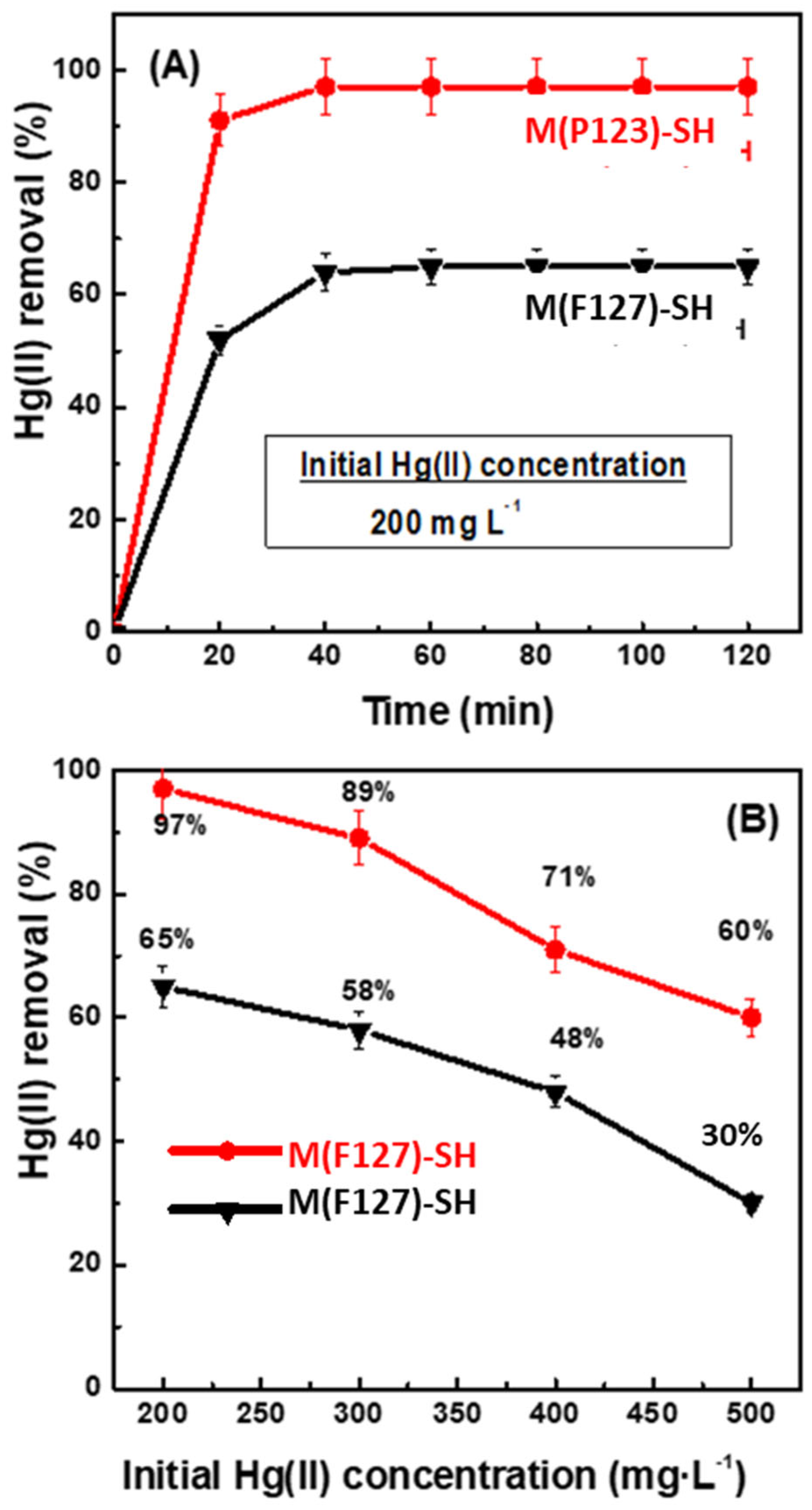
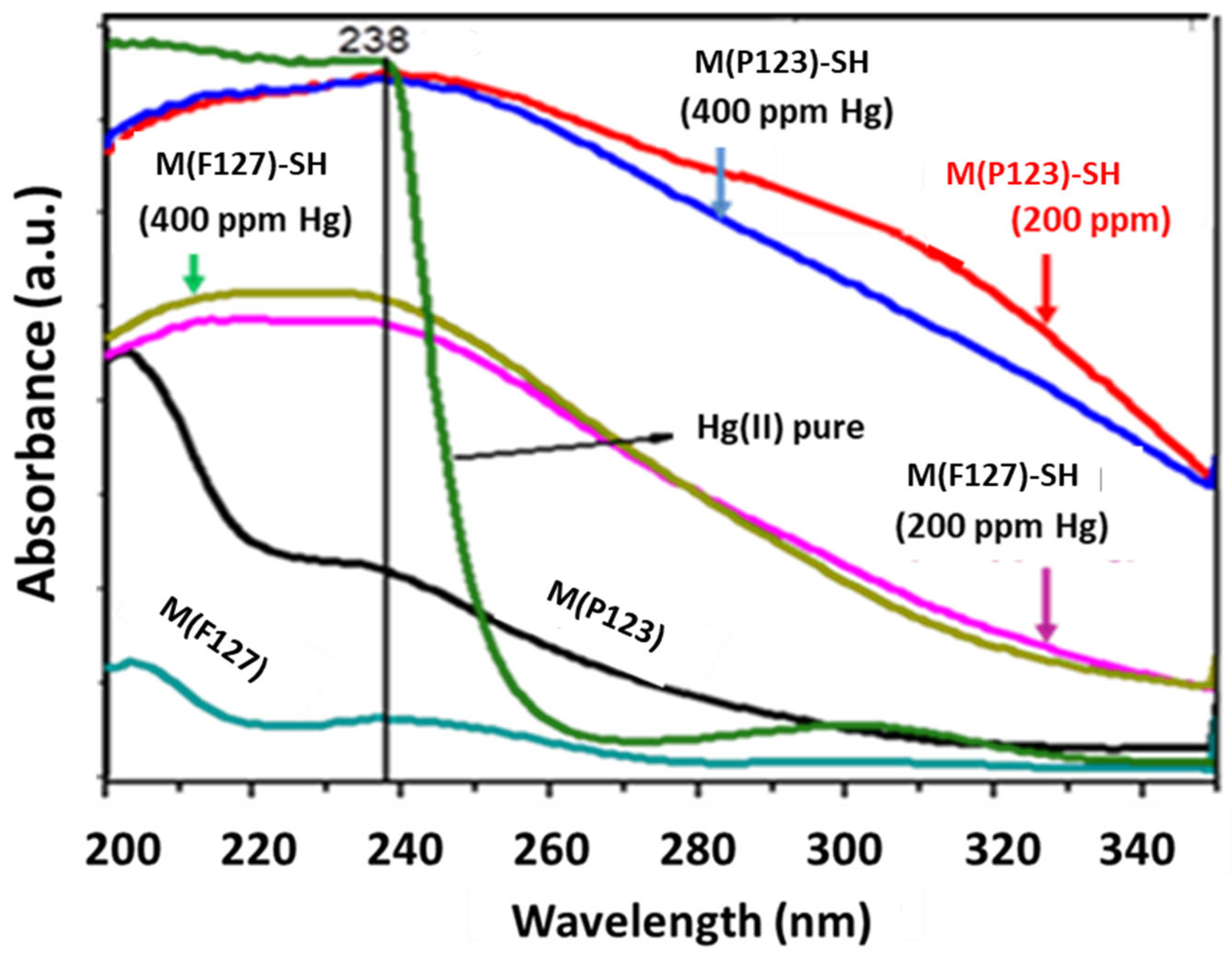
3.2. Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Y.; Yu, H.; Hua, M.; Lv, L.; Zhang, W. Highly efficient and selective Hg(II) removal from water by thiol-functionalized MOF–808: Kinetic and mechanism study. Chem. Eng. J. 2021, 430, 132960. [Google Scholar] [CrossRef]
- Šćiban, M.; Klašnja, M.; Škrbić, B. Modified softwood sawdust as adsorbent of heavy metal ions from water. J. Hazard. Mater. 2006, 136, 266. [Google Scholar] [CrossRef]
- Bernard, E.; Jimoh, A. Adsorption of Pb, Fe, Cu, and Zn from industrial electroplating wastewater by orange peel activated carbon. Int. J. Eng. Appl. Sci. 2013, 4, 95–103. [Google Scholar]
- Xia, N.N.; Zhang, B.; Hu, Z.H.; Kong, F.; Xu, G.; He, F. A biomass-assembled macro/mesoporous nano-scavenger for Hg ion trapping. New J. Chem. 2021, 45, 17002–17008. [Google Scholar] [CrossRef]
- Ho, K.Y.; Yeung, K.Y.; McKay, G. Selective Adsorbents from Ordered Mesoporous Silica. Langmuir 2003, 19, 3019–3024. [Google Scholar] [CrossRef]
- Xue, X.; Li, F. Removal of Cu(II) from aqueous solution by adsorption onto functionalized SBA–16 mesoporous silica. Micropor. Mesopor. Mater. 2008, 116, 1016. [Google Scholar] [CrossRef]
- Zeng, W.; Bai, H. Adsorption of acetone vapors by SBA–16 and MCM–48 synthetized from rice husk ash. Int. J. Environ. Chem. Ecol. Geol Geophys. Eng. 2015, 9, 308. [Google Scholar] [CrossRef]
- Shah, A.T.; Din, M.I.; Kanwal, F.N.; Mirza, M.L. Direct synthesis of mesoporous sieves of Ni–SBA–16 by internal pH adjustment method and its performance for adsorption of toxic Brillant Green dye. Arabian. J. Chem. 2015, 8, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Lesaint, C.; Frébault, F.; Delacôte, C.; Lebeau, B.; Marichal, C.; Walcarius, A.; Patarin, J. Synthesis and characterization of mesoporous silicas functionalized by thiol groups, and application as sorbents for mercury (II). Stud. Surf. Sci. Catal. 2005, 156, 925–932. [Google Scholar] [CrossRef]
- Han, S.C.; Sujandi, S.; Park, S.E. Functionalization of SBA–16 mesoporous materials with cobalt(III) cage amine complex. Bull. Korean Chem. Soc. 2005, 26, 1381–1384. [Google Scholar] [CrossRef] [Green Version]
- Marjani, A.; Mohammadi, R.K. Synthesis of novel adsorbent based on tetrasulfide-functionalized fibrous silica KCC–1 for removal of Hg(II) cations. Sci. Rep. 2021, 11, 10735. [Google Scholar] [CrossRef]
- Saman, N.; Johari, K.; Mat, H. Adsorption characteristics of sulfur-functionalized silica microspheres with respect to the removal of Hg(II) from aqueous solutions. Ind. Eng. Chem. Res. 2014, 53, 1225–1233. [Google Scholar] [CrossRef]
- Mureseanu, M.; Reiss, A.; Cioatera, N.; Trandafr, L.; Hulea, V. Mesoporous silica functionalized with 1-furoyl thiourea urea for Hg(II) adsorption from aqueous media. J. Hazard. Mater. 2010, 182, 197–203. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; del Hierro, I.; Fajardo, M.; Sierra, I. Preparation of 2-mercaptobenzothiazole-derivatized mesoporous silica and removal of Hg(II) from aqueous solution. J. Environ. Monit. 2006, 8, 214–222. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; del Hierro, I.; Fajardo, M.; Sierra, I. 2-Mercaptothiazoline modifed mesoporous silica for mercury removal from aqueous media. J. Hazard. Mater. 2006, 13, 245–256. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; Del Hierro, I.; Fajardo, M.; Sierra, I. Mesoporous silica functionalized with 2-mercaptopyridine: Synthesis, characterization and employment for Hg(II) adsorption. Micropor. Mesopor. Mater. 2006, 89, 58–68. [Google Scholar] [CrossRef]
- Modak, A.; Bhanja, P.; Selvaraj, M.; Bhaumik, A. Functionalized porous organic materials as efficient media for the adsorptive removal of Hg(II) ions. Environ. Sci. Nano 2020, 7, 2887–2923. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Micropor. Mesopor. Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Smått, J.H.; Schunk, S.; Linde’n, M. Versatile Double-Templating synthesis route to silica monoliths exhibiting a multimodal hierarchial porosity. Chem. Mater. 2003, 15, 2354–2361. [Google Scholar] [CrossRef]
- Higgins, S.; Kennard, R.; Hill, N.; DiCarlo, J.; DeSisto, W.J. Preparation and characterization of non-ionic block co-polymer templated mesoporous silica membranes. J. Membr. Sci. 2006, 279, 669–674. [Google Scholar] [CrossRef]
- Hristov, P.; Yoleva, A.; Djambazov, S.; Chukovska, I.; Dimitrov, D. Preparation and characterization of porous ceramic membranes for microfiltration from natural zeolite. J. Univ. Chem. Techn. Metall. 2012, 47, 476–480. [Google Scholar]
- Kumar, P.; Guliants, V.V. Periodic mesoporous organic-inorganic hybrid materials: Applications in membrane separations and adsorption. Micropor. Mesopor. Mater. 2010, 132, 1–2. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Tam, S.Y.; Lu, Y.; Brinker, C.J. Dual-layer asymmetric microporous silica membranes. J. Membr. Sci. 2000, 169, 255–268. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Morales, V.; Nava, R.; Acosta-Silva, Y.J.; Macías-Sánchez, S.A.; Pérez-Bueno, J.J.; Pawelec, B. Adsorption of lead (II) on SBA–15 mesoporous molecular sieve functionalized with –NH2 groups. Micropor. Mesopor. Mater. 2012, 160, 133–142. [Google Scholar] [CrossRef]
- Heidari, A.; Younesi, H.; Mehraban, Z. Removal of Ni(II), Cd(II), and Pb(II) from a ternary aqueous solution by amino-functionalized mesoporous and nano mesoporous silica. Chem. Eng. J. 2009, 153, 70–79. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Peter, C.A.; Alberius, K.L.; Frindell, R.C.; Hayward, E.J.; Kramer, G.D.; Stucky, B.F.; Chmelka, B.F. General predictive syntheses of cubic, hexagonal and lamellar silica and titania mesostructured thin films. Chem. Mater. 2002, 14, 3284–3294. [Google Scholar] [CrossRef]
- Arencibia, A.; Aguado, J.; Arsuaga, J.M. Regeneration of thiol-functionalized mesostructured silica adsorbents of mercury. Appl. Surf. Sci. 2010, 256, 5453–5457. [Google Scholar] [CrossRef]
- Arsuaga, J.M.; Aguado, J.; Arencibia, A.; López-Gutiérrez, M.S. Aqueous mercury adsorption in a fixed bed column of thiol functionalized mesoporous silica. Adsorption 2014, 20, 311–319. [Google Scholar] [CrossRef]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A. Heavy metals removal from water by adsorption on propylthiol-functionalised mesoporous silica obtained by co-condensation. Int. J. Environ. Technol. Manag. 2010, 12, 381–392. [Google Scholar] [CrossRef]
- Lourenço, M.A.O.; Figueira, P.; Pereira, E.; Gomes, J.R.B.; Lopes, C.B.; Ferreira, P. Simple, mono and bifunctional periodic mesoporous organosilicas for removal of priority hazardous substances from water: The case of mercury(II). Chem. Eng. J. 2017, 322, 263–274. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, N.; Liu, S.; Zheng, C.; Wang, X.; Huang, T.; Guo, Y.; Bai, R. Thiol functionalization of short channel SBA–15 through a safe, mild and facile method and application for the removal of mercury (II). J. Environ. Chem. Eng. 2018, 6, 5420–5433. [Google Scholar] [CrossRef]
- Ling, R.; Zou, H. Removal of aqueous Hg(II) by thiol-functionalized nonporous silica microspheres prepared by one-step sol–gel method. RSC Adv. 2020, 10, 18534–18542. [Google Scholar] [CrossRef]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A. Infuence of synthesis conditions on mercury adsorption capacity of propylthiol functionalized SBA–15 obtained by co-condensation. Mesopor. Mesopor. Mater. 2008, 109, 513–524. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Islam, S.M.; Liu, Y.; Ma, S.; Kanatzidis, M.G. Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS42− ion. J. Am. Chem. Soc. 2016, 138, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A. Adsorption of aqueous mercury (II) on propylthiol-functionalized mesoporous silica obtained by cocondensation. Ind. Engin. Chem. Res. 2009, 44, 3665–3671. [Google Scholar] [CrossRef]
- Lee, B.; Kim, Y. Synthesis of functionalized porous silicas via templating method as heavy metal ion adsorbents: The introduction of surface hydrophilicity onto the surface of adsorbents. Micropor. Mesopor. Mater. 2001, 50, 77–90. [Google Scholar] [CrossRef]
- Sajjadi, S.A.; Mohammadzadeh, A.; Tran, H.N.; Anastopoulos, I.; Dotto, G.L.; Lopičić, Z.R.; Sivamani, S.; Rahmani-Sani, A.; Ivanets, A.; Hosseini-Bandegharaei, A. Efficient mercury removal from wastewater by agent. J. Environ. Manag. 2018, 223, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- McCool, B.A.; Hill, N.; Di Carlo, J.; De Sisto, W.J. Synthesis and characterization of mesoporous silica membranes pistachio wood wastes-derived activated carbon prepared by chemical activation using a novel activating via dip-coating and hydrothermal deposition techniques. J. Membr. Sci. 2003, 218, 55–67. [Google Scholar] [CrossRef]
- Smarsly, B.; Xomeritakis, G.; Yu, K.; Liu, N.; Fan, H.; Assink, R.A.; Drewien, C.A.; Ruland, W.; Brinker, C.J. Microstructural characterization of polystyrene-block-poly(ethylene oxide)-Templated silica films with cubic-oredere spherical mesopores. Langmuir 2003, 19, 7295–7301. [Google Scholar] [CrossRef]
- Boissiere, C.; Martines, M.U.; Larbot, A.; Prouzet, E. On the specific filtration mechanism of mesoporous silica membrane, prepared with non-connecting parallel pores. J. Membr. Sci. 2005, 251, 17–28. [Google Scholar] [CrossRef]
- Nakanishi, K.J. Pore Structure control of silica gels based on phase separation. Porous Mater. 1997, 4, 67–112. [Google Scholar] [CrossRef]
- Minakuchi, H.; Nakanishi, K.; Soga, N.; Ishizuka, N.; Tanaka, N. Octadecylsilylated porous silica rods as separation media for reversed-phase liquid chromatography. Anal. Chem. 1996, 68, 3498–3501. [Google Scholar] [CrossRef]
- Nakamura, N.; Takahashi, R.; Sato, S.; Sodesawa, T.; Yoshida, S. Ni/SiO2 catalyst with hierarchical pore structure prepared by phase separation in sol-gel process. Phys. Chem. Chem. Phys. 2000, 2, 4983–4990. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Shirsat, M.D.; Ramanaviciene, A.; Ramanavicius, A. Composites Based on Conducting Polymers and Carbon Nanomaterials for Heavy Metal Ion Sensing (Review). Crit. Rev. Anal. Chem. 2018, 48, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.A.; Celiesiute, R.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA_PANI/SWCNTs Nanocomposite Modified Electrode for Electrochemical Determination of Copper (II), Lead (II) and Mercury (II) Ions. Electr. Acta 2018, 259, 930–938. [Google Scholar] [CrossRef]
- Wang, J.; Dong, B.; Chen, H.; Wang, X.; Heng, J. Removal of aqueous Hg(II) by polyaniline: Sorption characteristics and mechanisms. Environ. Sci. Technol. 2009, 43, 5223–5228. [Google Scholar] [CrossRef]
- Hernandez-Morales, V. Synthesis and Characterization of the Gold Catalysts Supported on SBA–15 Modified with SH Groups. Master’s Dissertation, Instituto Tecnológico de Querétaro, Querétaro, México, 2008. [Google Scholar]
- Boss, C.B.; Fredeen, K.J. Concept, Instrumentation and Techniques in Inductively Coupled Plasma Optical Emission Spectrometry, 2nd ed.; Perkin-Elmer: Norwalk, CT, USA, 1997. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Nooney, I.R.; Kalyanaraman, M.; Kennedy, G.; Edward, J. Heavy metal remediation using fuctionalized mesoporous silicas with controlled macrostructure. Langmuir 2001, 17, 528–533. [Google Scholar] [CrossRef]
- Acosta-Silva, Y.J.; Nava, R.; Hernández-Morales, V.; Macías-Sánchez, S.A.; Pawelec, B. TiO2/DMS–1 disordered mesoporous silica materials: Structural characteristics and methylene blue photodegradation activity. Micropor. Mesopor. Mater. 2013, 170, 181–188. [Google Scholar] [CrossRef]
- Boutros, M.; Onfroy, T.; Da Costa, P. Mesostructured or alumina-mesostructured silica SBA–16 as potential support for NOx reduction and ethanol oxidation. Catal Lett. 2010, 139, 50–55. [Google Scholar] [CrossRef]
- Stevens, W.J.J.; Lebeau, K.; Mertens, M.; Van Tendeloo, G.; Cool, P.; Vansant, E.F. Investigation of the morphology of the mesoporous SBA–16 and SBA–15 material. J. Phys. Chem. B 2006, 110, 9183–9187. [Google Scholar] [CrossRef] [PubMed]
- Mauraya, M.R.; Chandrakar, A.K.; Chand, S. Oxovanadium(IV) and copper(II) complexes of 1,2-diaminocyclohexane based ligand encapsulated in zeolite Y for the catalytic oxidation of styrene, cyclohexene and cyclohexane. J. Mol. Catal. A Chem. 2007, 270, 225–235. [Google Scholar] [CrossRef]
- Colthup, B.N.; Daly, H.L.; Wiberly, E.S. Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Jesenák, K.L.; Kuchta, L.; Hudec, P.; Fagnor, V.S. Calcination of SiO2-Aerogel in Oxidizing Atmosphere. J. Therm. Anal. Cal. 1999, 55, 773. [Google Scholar] [CrossRef]
- Kouznetsova, T.; Sauka, J.; Ivanets, A. Template synthesis and gas adsorption properties of ordered mesoporous aluminosilicates. Appl. Nanosci. (Switzerland) 2021, 11, 1903–1915. [Google Scholar] [CrossRef]
- Łuczkowski, M.; Stachura, M.; Schirf, V.; Demeler, B.; Hemmingsen, L. Design of Thiolate Rich Metal Binding Sites within a Peptidic Framework. Inorg. Chem. 2008, 47, 10875–10888. [Google Scholar] [CrossRef] [Green Version]
- Abdulghani, A.J.; Jasim, H.H.; Shebeeb Hassan, A. Determination of Tetracycline in Pharmaceutical Preparation by Molecular and Atomic Absorption Spectrophotometry and High Performance Liquid Chromatography via Complex Formation with Au(III) and Hg(II) Ions in Solutions. Int. J. Anal. Chem. 2013. Article ID 305124, 11 pages. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Salyn, Y.V.; Zylberajch-Antoine, C.; Barraud, A.; Roulet, H.; Dufour, G. XPS characterization of inserted mercury sulfide single layers in a Langmuir-Blodgett matrix. Appl. Surf. Sci. 1999, 52, 323–327. [Google Scholar] [CrossRef]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-ray photoelectron spectroscopy sulfur 2p study of organic thiol and disulfide binding interactions with gold surfaces. Langmuir 1996, 12, 5083–5086. [Google Scholar] [CrossRef]
- Humbert, P. An XPS and UPS photoemission study of HgO. Solid State Commun. 1986, 60, 21–24. [Google Scholar] [CrossRef]
- Mercier, L.; Pinnavaia, T.J. Access in mesoporous materials: Advantages of a uniform pore structure in the design of a heavy metal ion adsorbent for environmental remediation. Adv. Mater. 1997, 9, 500–503. [Google Scholar] [CrossRef]
- Antochshuk, V.; Olkhovyk, O.; Jaroniec, M.; Park, I.S.; Ryo, R. Benzoylthiourea-modified mesoporous silica for mercury (II) removal. Langmuir 2003, 19, 3031–3034. [Google Scholar] [CrossRef]
- Green-Ruiz, C. Effect of salinity and temperature on the adsorption of Hg(II) from aqueos solutions by Ca-montmorillonite. Environ. Tech. 2009, 30, 63–68. [Google Scholar] [CrossRef]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Jinjing, L.; Huazhen, S.; Hanna, M.; Zhongye, W.N. Removal of Cu2+ from Aqueous Solution using Fly Ash. J. Miner. Mater. Character Eng. 2011, 10, 561–571. [Google Scholar] [CrossRef]
- Ren, X.; Shi, Y.; Zheng, H.; Zhang, H.; Zuo, Q. A novel covalent organic polymer with hierarchical pore structure for rapid and selective trace Hg(II) removal from drinking water. Sep. Purif. Technol. 2022, 285, 120306. [Google Scholar] [CrossRef]
- He, H.; Meng, X.; Yue, Q.; Yin, W.; Gao, Y.; Fang, P.; Shen, L. Thiol-ene click chemistry synthesis of a novel magnetic mesporous silica/chitosan composite for selective Hg(II) capture and high catalytic activity of spent Hg(II) adsorbent. Chem. Eng. J. 2021, 405, 126743. [Google Scholar] [CrossRef]
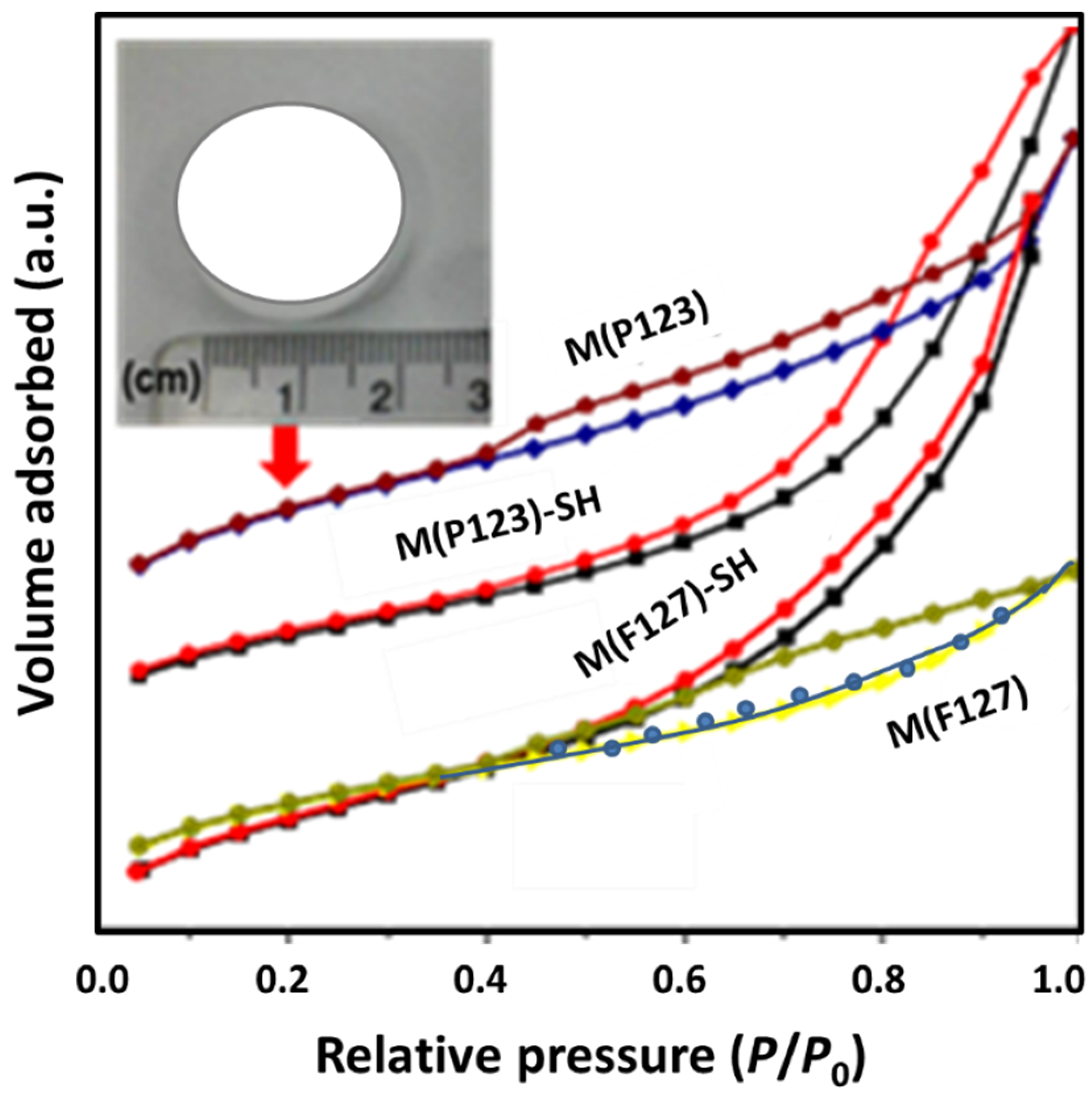
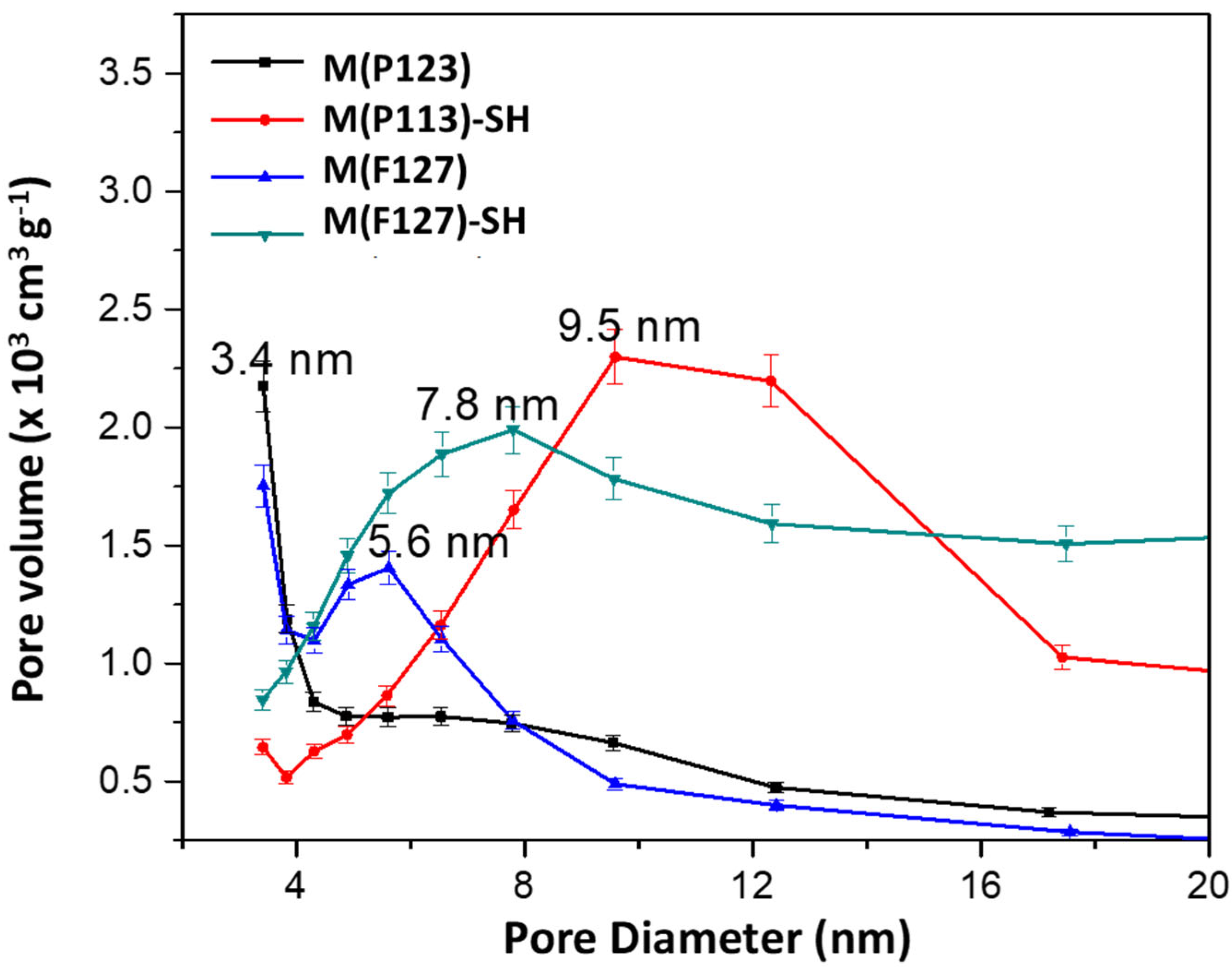

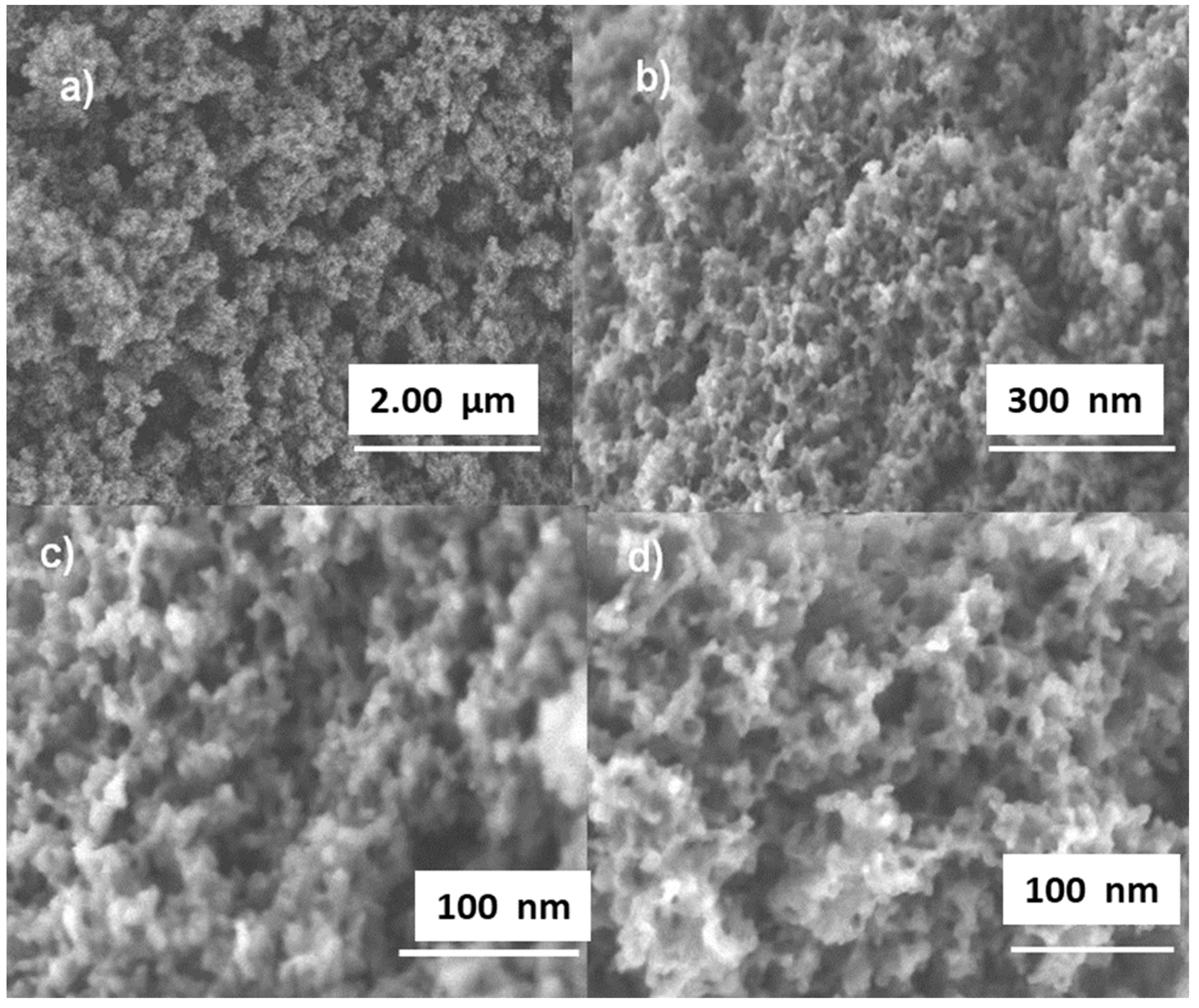
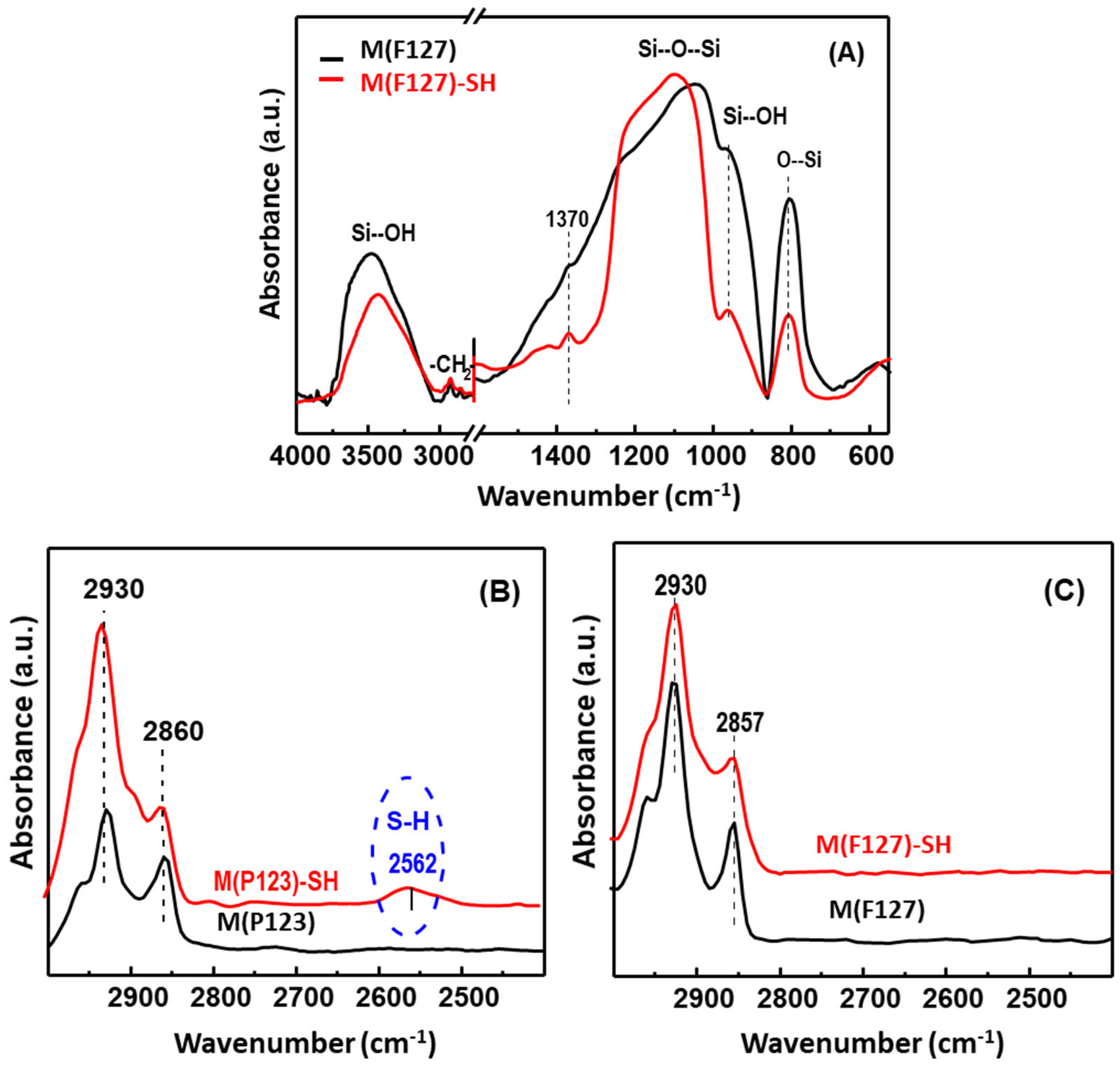
| Sorbent | SBET (m2/g) | d (nm) | V (m3/g) |
|---|---|---|---|
| M(P123) | 530 | 3.4 | 0.42 |
| M(P123)–SH | 281 | 9.6 | 0.69 |
| M(F127) | 527 | 3.4 | 0.36 |
| M(F127)–SH | 515 | 7.9 | 2.26 |
| Sorbent | Initial Hg(II) Concentration (mg L−1) | Adsorption Conditions pH/T/Time | Sorption Capacity (mg/g) | Reference |
|---|---|---|---|---|
| M(P123)–SH (a) | 200 | pH 3.5; 28 °C; 1 h | 194.0 | This work |
| M(F127)–SH (a) | 200 | pH 3.5; 28 °C; 1 h | 70.0 | This work |
| TS–KCC–1 (b) | 200 | pH 5; 25 °C; 40 min | 132.6 | [12] |
| MPTMS–SMs (c) | 160 | pH 7.5; 30 °C; 1500 min | 62.3 | [13] |
| FTU–SBA–15 (d) | 200 | pH 6; 25 °C; | 122.4 | [14] |
| MBT–SBA–15 (e) | 100 | pH 6; 25 °C; 4 h | 48.1 | [15] |
| MBT–MCM–41 (f) | 100 | pH 6; 25 °C; 4 h | 42.1 | [16] |
| SiO2–SH (g) | 150 | pH 5.5; 20 °C, 24 h | 274.1 | [34] |
| MTZ–MCM–41 (h) | 647 | pH 6; 25 °C, 4 h | 140.4 | [18] |
| MP–MCM–41 (i) | 100 | pH 6; 25 °C; 4 h | 38.1 | [18] |
| MP–SBA–15 (j) | 100 | pH 6: 25 °C; 4 h | 20.0 | [18] |
| MoS4–LDH (j) | 1162 | pH 4.11; 20 °C; 6 h | 500 | [35] |
| XPS Core Level | Before Hg(II) Adsorption | After Hg(II) Adsorption 200 mg/L | After Hg(II) Adsorption 400 mg/L |
|---|---|---|---|
| Si 2p | 103.3 | 103.3 | 103.3 |
| O 1s | 532.9 | 532.8 | 532.9 |
| Hg 4f7/2 | - | 101.0 | 101.0 |
| Hg 4f5/2 | - | 104.4 | 104.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, I.S.; Morales, V.H.; Muñoz, E.M.R.; Mendoza, R.N.; Soto, L.L.; Ledesma, C.L.P.; Casados, D.S.; Pawelec, B. Efficient Removal of Hg(II) from Water under Mildly Acidic Conditions with Hierarchical SiO2 Monoliths Functionalized with –SH Groups. Materials 2022, 15, 1580. https://doi.org/10.3390/ma15041580
Gutiérrez IS, Morales VH, Muñoz EMR, Mendoza RN, Soto LL, Ledesma CLP, Casados DS, Pawelec B. Efficient Removal of Hg(II) from Water under Mildly Acidic Conditions with Hierarchical SiO2 Monoliths Functionalized with –SH Groups. Materials. 2022; 15(4):1580. https://doi.org/10.3390/ma15041580
Chicago/Turabian StyleGutiérrez, Ireri Segura, Verónica Hernández Morales, Eric Mauricio Rivera Muñoz, Rufino Nava Mendoza, Ludwig Lagarde Soto, Carmen Leticia Peza Ledesma, Doris Solís Casados, and Barbara Pawelec. 2022. "Efficient Removal of Hg(II) from Water under Mildly Acidic Conditions with Hierarchical SiO2 Monoliths Functionalized with –SH Groups" Materials 15, no. 4: 1580. https://doi.org/10.3390/ma15041580







