Nitrogen-Doped Nanoporous Anodic Stainless Steel Foils towards Flexible Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Methods
2.2. Characterization Techniques
2.3. Electrochemical Measurements
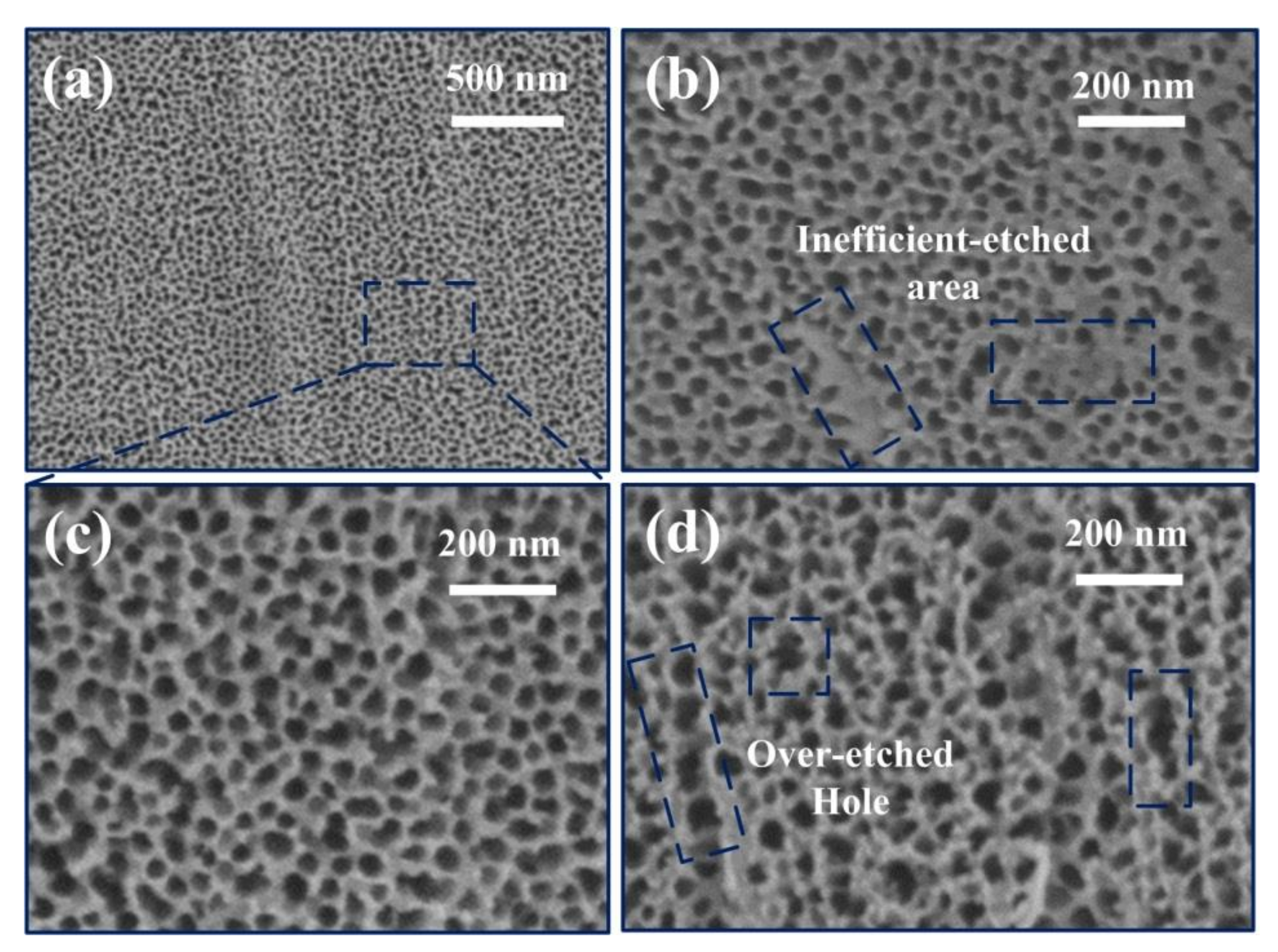
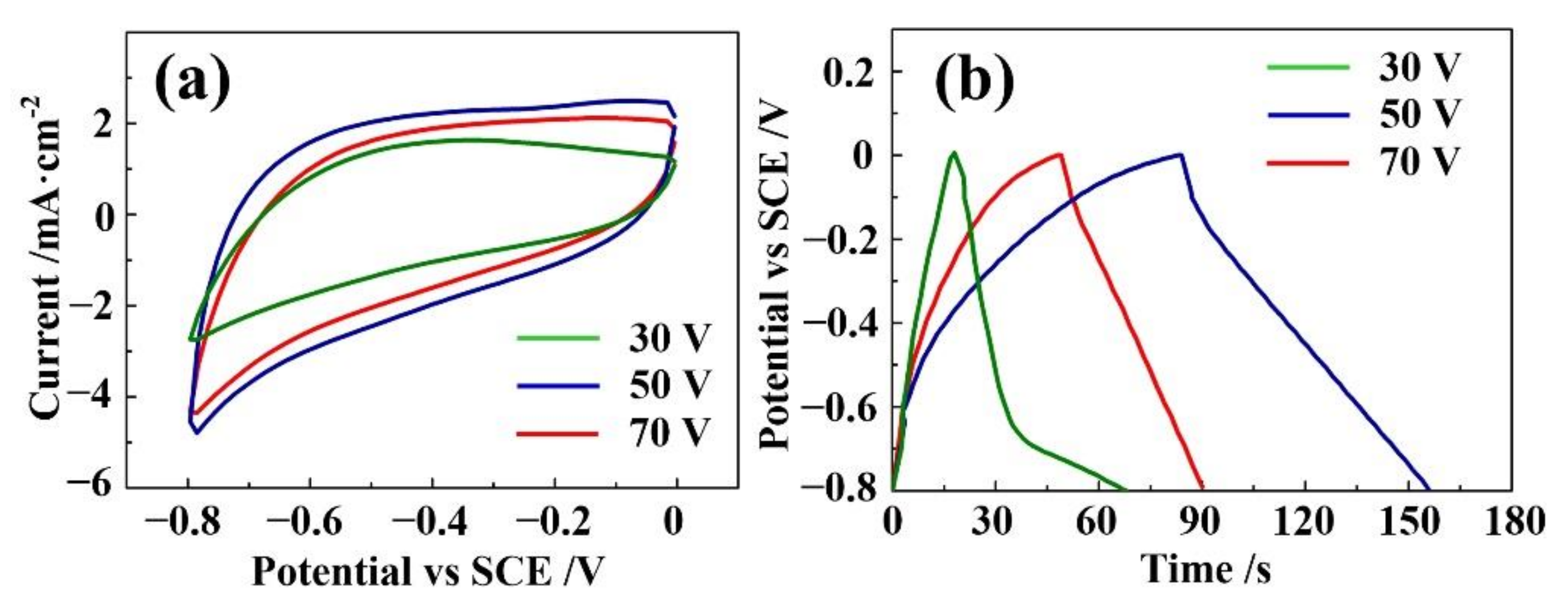
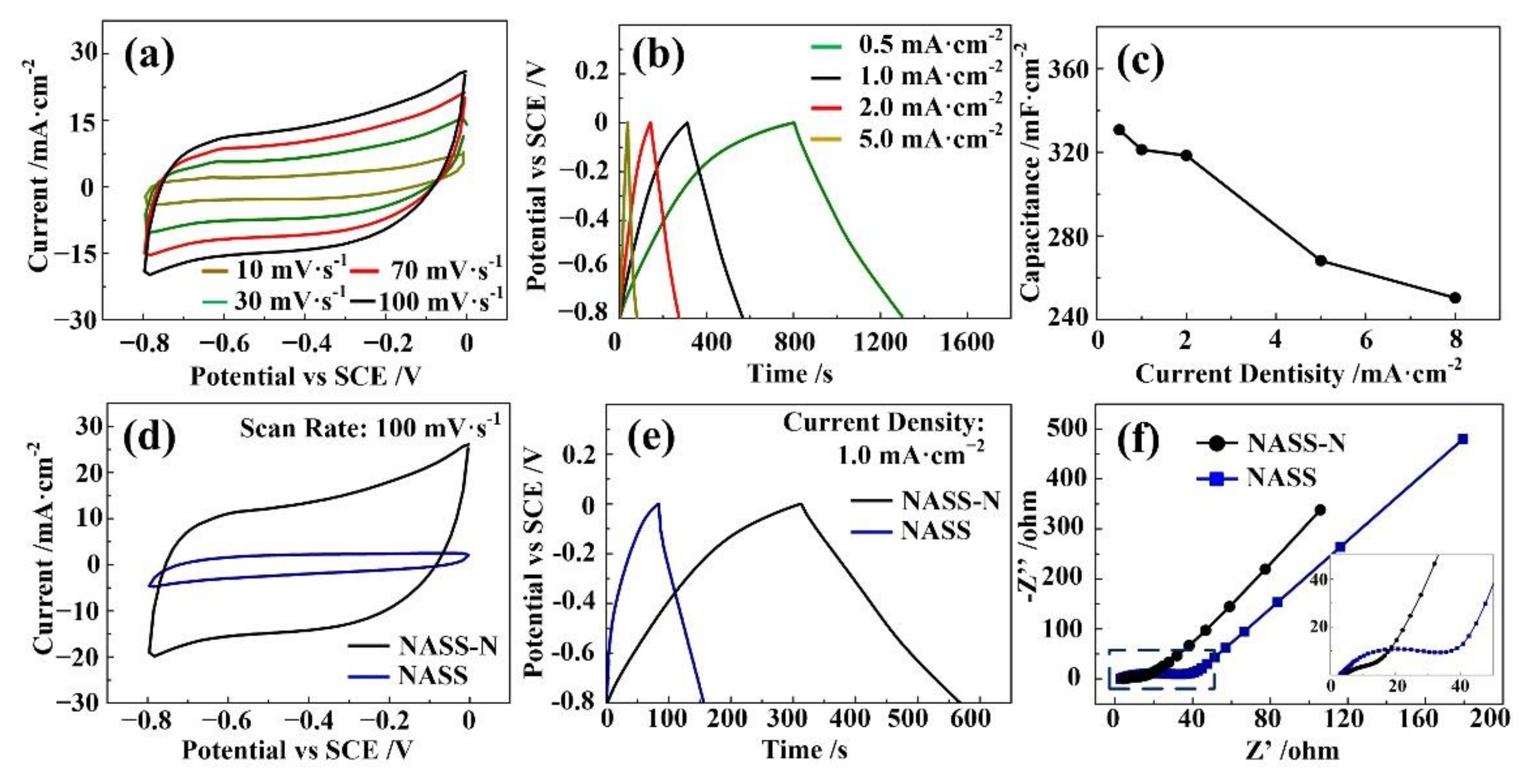
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, G.; Na, W.; Kim, J.; Lee, S.; Jang, J. Improved electrochemical performances of MOF-derived Ni–Co layered double hydroxide complexes using distinctive hollow-in-hollow structures. J. Mater. Chem. A 2019, 7, 17637–17647. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xu, Y.; Xue, H.; Liu, C.; Pang, H. Ultrathin two-dimensional cobalt-organic frameworks nanosheets for electrochemical energy storage. Chem. Eng. J. 2019, 373, 1319–1328. [Google Scholar] [CrossRef]
- Haider, W.A.; Tahir, M.; He, L.; Yang, W.; Minhas-Khan, A.; Owusu, K.A.; Chen, Y.; Hong, X.; Mai, L. Integration of VS2 nanosheets into carbon for high energy density micro-supercapacitor. J. Alloys Compd. 2020, 823, 151769. [Google Scholar] [CrossRef]
- Huang, Y.; Buffa, A.; Deng, H.; Sarkar, S.; Ouyang, Y.; Jiao, X.; Hao, Q.; Mandler, D. Ultrafine Ni(OH)2 nanoplatelets grown on 3D graphene hydrogel fabricated by electrochemical exfoliation for high-performance battery-type asymmetric supercapacitor applications. J. Power Sources 2019, 439, 227046. [Google Scholar] [CrossRef]
- Maile, N.; Shinde, S.K.; Patil, S.S.; Kim, D.Y.; Fulari, A.V.; Lee, D.S.; Fulari, V.J. Capacitive property studies of electrochemically synthesized Co3O4 and Mn3O4 on inexpensive stainless steel current collector for supercapacitor application. Ceram. Int. 2020, 46, 14640–14649. [Google Scholar] [CrossRef]
- Nuramdhani, I.; Gokceoren, A.T.; Odhiambo, S.A.; Mey, G.D.; Hertleer, C.; Langenhove, L.V. Electrochemical impedance analysis of a PEDOT:PSS-based textile energy storage device. Materials 2018, 11, 48. [Google Scholar] [CrossRef] [Green Version]
- Amade, R.; Avetisyan, A.M.; Gonzalez, J.M.; Pino, A.P.; Gyorgy, E.; Pascual, E.; Andujar, J.L.; Serra, E.B. Super-capacitive performance of manganese dioxide/graphene nano-walls electrodes deposited on stainless steel current collectors. Materials 2019, 12, 483. [Google Scholar] [CrossRef] [Green Version]
- Waghmode, R.; Jadhav, H.; Kanade, K.; Torane, A. Morphology-controlled synthesis of NiCo2O4 nanoflowers on stainless steel substrates as high-performance supercapacitors. Mater. Sci. Energy Technol. 2019, 2, 556–564. [Google Scholar] [CrossRef]
- Mo, Y.; Meng, W.; Xia, Y.; Du, X.; Lin, Z.; Li, W. Facile flame deposit of CNFs/Fe2O3 coating on 304 stainless steel mesh and their high capacitive performance. Electrochim. Acta 2020, 335, 135527. [Google Scholar] [CrossRef]
- Zhu, X.; Hou, D.; Tao, H.; Li, M. Simply synthesized N-doped carbon supporting Fe3O4 nanocomposite for high performance supercapacitor. J. Alloys Compd. 2020, 821, 153580. [Google Scholar] [CrossRef]
- Sagu, J.S.; Wijayantha, K.G.U.; Bohm, M.; Bohm, S.; Rout, T.K. Anodized Steel Electrodes for Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 6277–6285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmukh, P.R.; Sohn, Y.; Shin, W.G. Flexible Solid-State Symmetric Supercapacitor Based on (Fe,Cr)2O3 Oxide Layer Developed on the Stainless Steel Mesh. ACS Sustain. Chem. Eng. 2018, 6, 300–310. [Google Scholar] [CrossRef]
- Long, B.; Yang, H.; Wang, F.; Mao, Y.; Balogun, M.-S.; Song, S.; Tong, Y. Chemically-modified stainless steel mesh derived substrate-free iron-based composite as anode materials for affordable flexible energy storage devices. Electrochim. Acta 2018, 284, 271–278. [Google Scholar] [CrossRef]
- Kageyama, H.; Hayashi, K.; Maeda, K.; Attfield, J.P.; Hiroi, Z.; Rondinelli, J.M.; Poeppelmeier, K.R. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 2018, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Parveen, N.; Al-Othoum, M.; Ansari, M. Effect of Washing on the Electrochemical Performance of a Three-Dimensional Current Collector for Energy Storage Applications. Nanomaterials 2021, 11, 1596. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Ansari, S.A.; Ansari, M.Z.; Ansari, M.O. Manganese oxide as an effective electrode material for energy storage: A review. Environ. Chem. Lett. 2021, 20, 283–309. [Google Scholar] [CrossRef]
- Scaife, D. Oxide semiconductors in photoelectrochemical conversion of solar energy. Sol. Energy 1980, 25, 41–54. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Qiu, W.; Wang, W.; Fang, P.; Lu, X.; Tong, Y. Recent advances in metal nitrides as high-performance electrode materials for energy storage devices. J. Mater. Chem. A 2015, 3, 1364–1387. [Google Scholar] [CrossRef]
- Wang, K.; Liu, G.; Hoivik, N.; Johannessen, E.; Jakobsen, H. Electrochemical engineering of hollow nanoarchitectures: Pulse/step anodization (Si, Al, Ti) and their applications. Chem. Soc. Rev. 2014, 43, 1476–1500. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Wang, K.; Chen, X. Fabrication and formation mechanisms of ultra-thick porous anodic oxides film with controllable morphology on type-304 stainless steel. Appl. Surf. Sci. 2020, 505, 144497. [Google Scholar] [CrossRef]
- Martin, F.; Del Frari, D.; Cousty, J.; Bataillon, C. Self-organisation of nanoscaled pores in anodic oxide overlayer on stainless steels. Electrochim. Acta 2009, 54, 3086–3091. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Suzumura, T.; Terada, Y.; Fujimoto, S. Formation of self-organized pores on type 316 stainless steel in organic solvents. Electrochim. Acta 2012, 82, 333–338. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Schmuki, P. High aspect ratio, self-ordered iron oxide nanopores formed by anodization of Fe in ethylene glycol/NH4F electrolytes. Phys. Status Solidi (RRL)—Rapid Res. Lett. 2009, 3, 64–66. [Google Scholar] [CrossRef]
- Farrag, H.H.; Sayed, S.Y.; Allam, N.K.; Mohammad, A.M. Emerging nanoporous anodized stainless steel for hydrogen production from solar water splitting. J. Clean. Prod. 2020, 274, 122826. [Google Scholar] [CrossRef]
- Sarma, B.; Smith, Y.R.; Jurovitzki, A.L.; Ray, R.S.; Mohanty, S.K.; Misra, M. Supercapacitance behavior of porous oxide layer grown on 302 type stainless steel substrate. J. Power Sources 2013, 236, 103–111. [Google Scholar] [CrossRef]
- Xia, S.; Du, W.; Zheng, L.; Chen, P.; Hou, Z. A thermally stable and easily recycled core–shell Fe2O3@CuMgAl catalyst for hydrogenolysis of glycerol. Catal. Sci. Technol. 2014, 4, 912–916. [Google Scholar] [CrossRef]
- Takahashi, N.; Toda, Y.; Nakamura, T. Preparation of FeN thin films by chemical vapor deposition using a chloride source. Mater. Lett. 2000, 42, 380–382. [Google Scholar] [CrossRef]
- Marco, J.F.; Herranz, T.; Gracia, M.; Gancedo, J.R.; Moutinho, F.; Prieto, P.; Sanz, J.M. Corrosion behavior under accelerated SO2 corrosion tests of thin iron nitride films prepared by DIBS. Surf. Interface Anal. 2010, 42, 616–620. [Google Scholar] [CrossRef]
- Yao, M.; Sun, B.; Wang, N.; Hu, W.; Komarneni, S. Self-generated N-doped anodized stainless steel mesh for an efficient and stable overall water splitting electrocatalyst. Appl. Surf. Sci. 2019, 480, 655–664. [Google Scholar] [CrossRef]
- Zeng, Y.; Han, Y.; Zhao, Y.; Zeng, Y.; Yu, M.; Liu, Y.; Tang, H.; Tong, Y.; Lu, X. Advanced Ti-Doped Fe2O3@PEDOT Core/Shell Anode for High-Energy Asymmetric Supercapacitors. Adv. Energy Mater. 2015, 5, 1402176. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultraca-pacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Zhan, Y.; Hu, Y.; Chen, Y.; Yang, Q.; Shi, Z.; Xiong, C. In-situ synthesis of flexible nanocellulose/carbon nano-tube/polypyrrole hydrogels for high-performance solid-state supercapacitors. Cellulose 2021, 28, 7097–7108. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, D.; Liu, Y.; Shen, L.; Zhu, T.; Xu, X.; Zheng, J.; Gong, X. Solid-State Double-Network Hydrogel Redox Electrolytes for High-Performance Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 34168–34177. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Zhu, H.; Wei, B. Synthesis and electrochemical characterizations of amorphous manganese oxide and single walled carbon nanotube composites as supercapacitor electrode materials. Electrochem. Commun. 2006, 8, 827–832. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, C.; Teng, F.; Zhao, Y.; Chen, G.; Shi, R.; Waterhouse, G.I.N.; Huang, W.; Zhang, T. Defect-engineered ultrathin δ-MnO2 nanosheet arrays as bifunctional electrodes for efficient overall water splitting. Adv. Energy Mater. 2017, 7, 1700005. [Google Scholar] [CrossRef]
- Raut, S.S.; Bommineedi, L.K.; Pande, S.; Sankapal, B.R. Prototype symmetric configured MWCNTs/Fe2O3 based solid-state supercapacitor. Synth. Met. 2021, 271, 116629. [Google Scholar] [CrossRef]
- Nithya, V.; Arul, N.S. Review on α-Fe2O3 based negative electrode for high performance supercapacitors. J. Power Sources 2016, 327, 297–318. [Google Scholar] [CrossRef]
- Li, Z.; Fang, Y.; Zhang, J.; Lou, X.W. (David) Necklace-Like Structures Composed of Fe3 N@C Yolk-Shell Particles as an Advanced Anode for Sodium-Ion Batteries. Adv. Mater. 2018, 30, e1800525. [Google Scholar] [CrossRef]
- Tian, H.; Cheng, R.; Lin, M.; Li, P.; Lv, Y.; Ran, S. Oxygen-vacancy-rich ultrathin BiOBr nonosheets for high-performance supercapacitor electrodes. Inorg. Chem. Commun. 2020, 118, 108018. [Google Scholar] [CrossRef]
- Liu, D.; Wang, C.; Yu, Y.; Zhao, B.-H.; Wang, W.; Du, Y.; Zhang, B. Understanding the Nature of Ammonia Treatment to Synthesize Oxygen Vacancy-Enriched Transition Metal Oxides. Chem 2019, 5, 376–389. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Xu, J.; Li, G.; Wang, K. Nitrogen-Doped Nanoporous Anodic Stainless Steel Foils towards Flexible Supercapacitors. Materials 2022, 15, 1615. https://doi.org/10.3390/ma15041615
Zhang W, Xu J, Li G, Wang K. Nitrogen-Doped Nanoporous Anodic Stainless Steel Foils towards Flexible Supercapacitors. Materials. 2022; 15(4):1615. https://doi.org/10.3390/ma15041615
Chicago/Turabian StyleZhang, Wenlei, Jianle Xu, Gang Li, and Kaiying Wang. 2022. "Nitrogen-Doped Nanoporous Anodic Stainless Steel Foils towards Flexible Supercapacitors" Materials 15, no. 4: 1615. https://doi.org/10.3390/ma15041615
APA StyleZhang, W., Xu, J., Li, G., & Wang, K. (2022). Nitrogen-Doped Nanoporous Anodic Stainless Steel Foils towards Flexible Supercapacitors. Materials, 15(4), 1615. https://doi.org/10.3390/ma15041615




