Effect of Nano-Y2O3 on the Microstructure and Properties of WC-Reinforced Ni-Based Composite Surfacing Layer
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Y2O3 Content on the Formability of Surfacing Layer
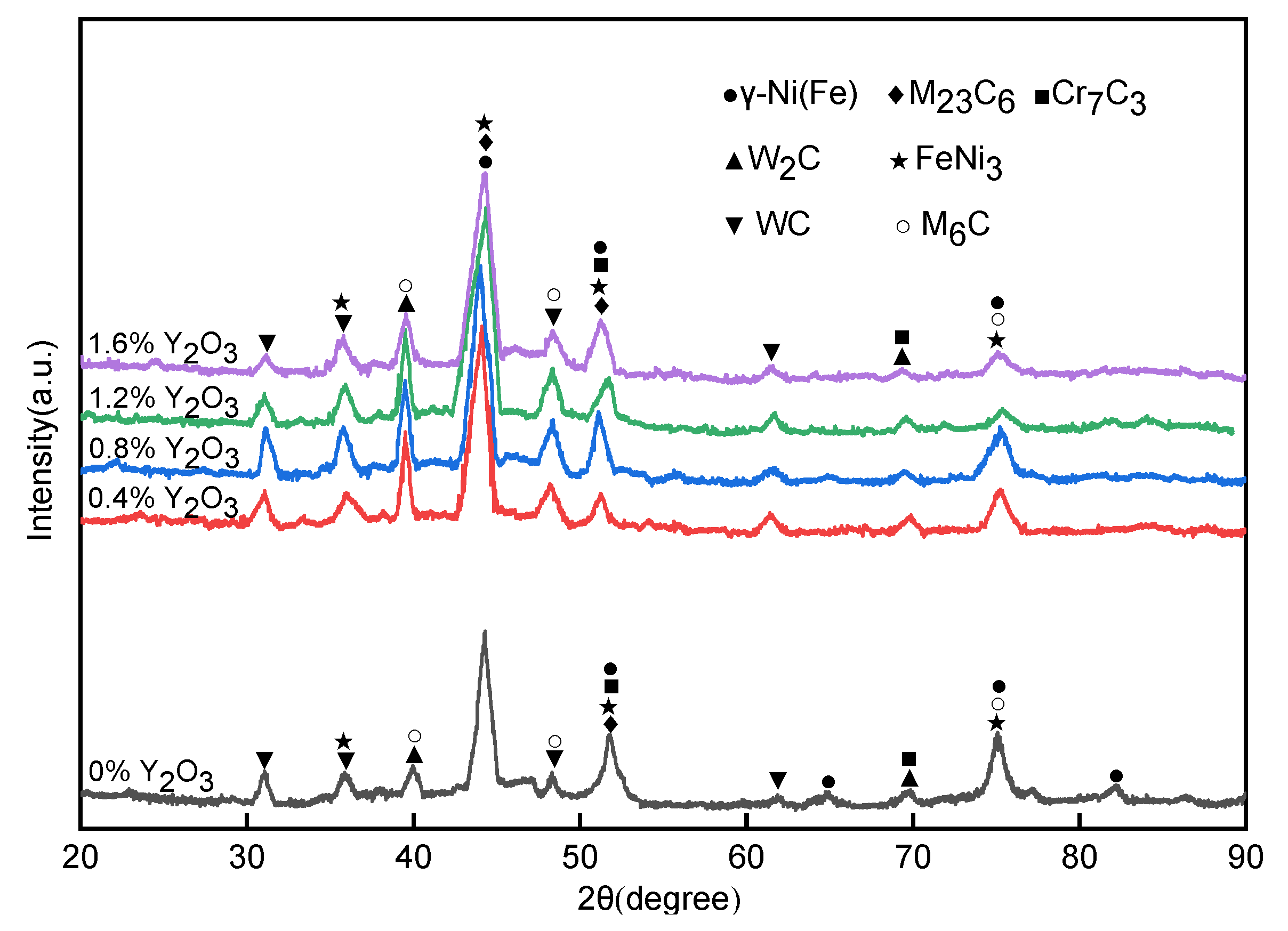
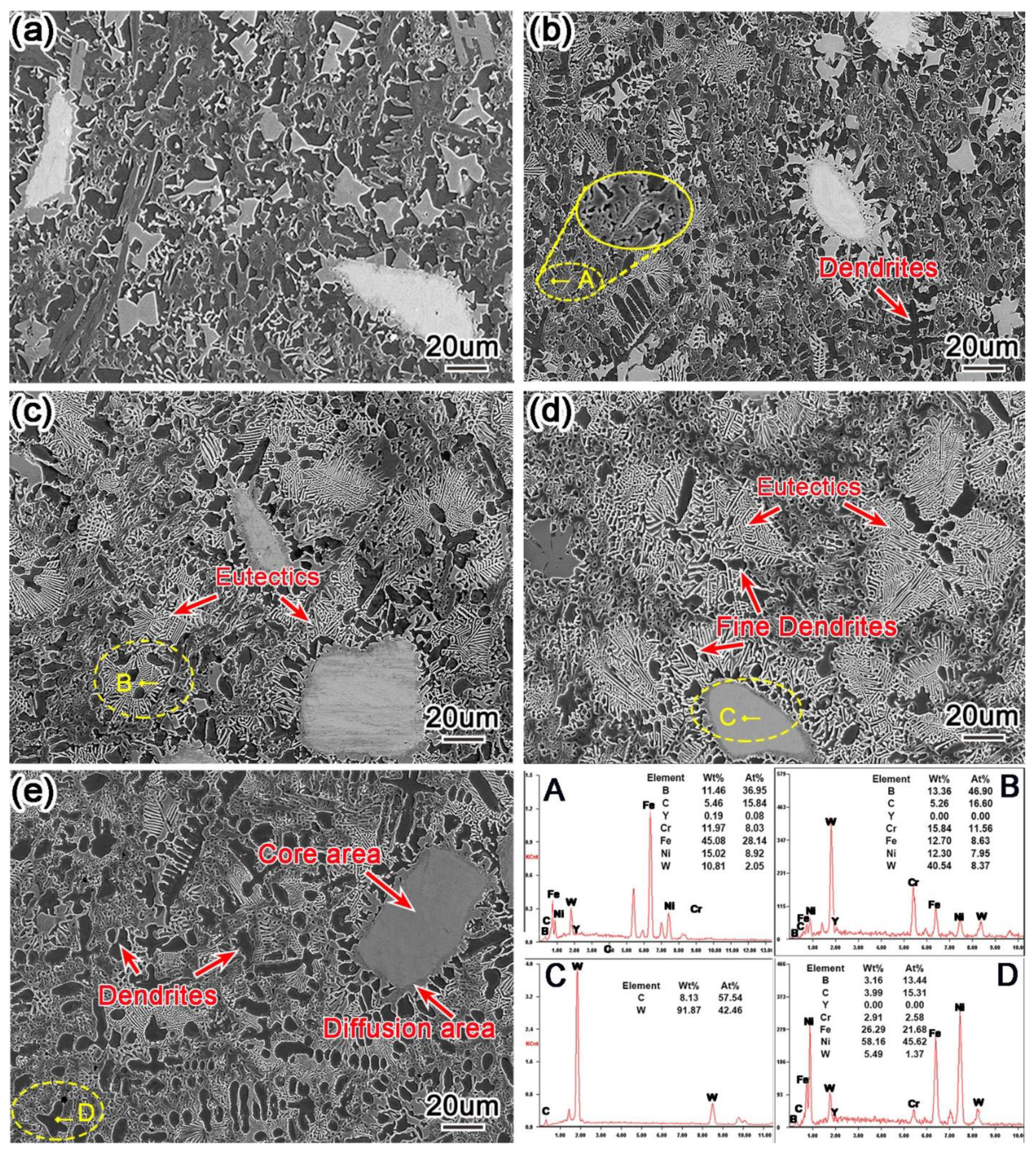
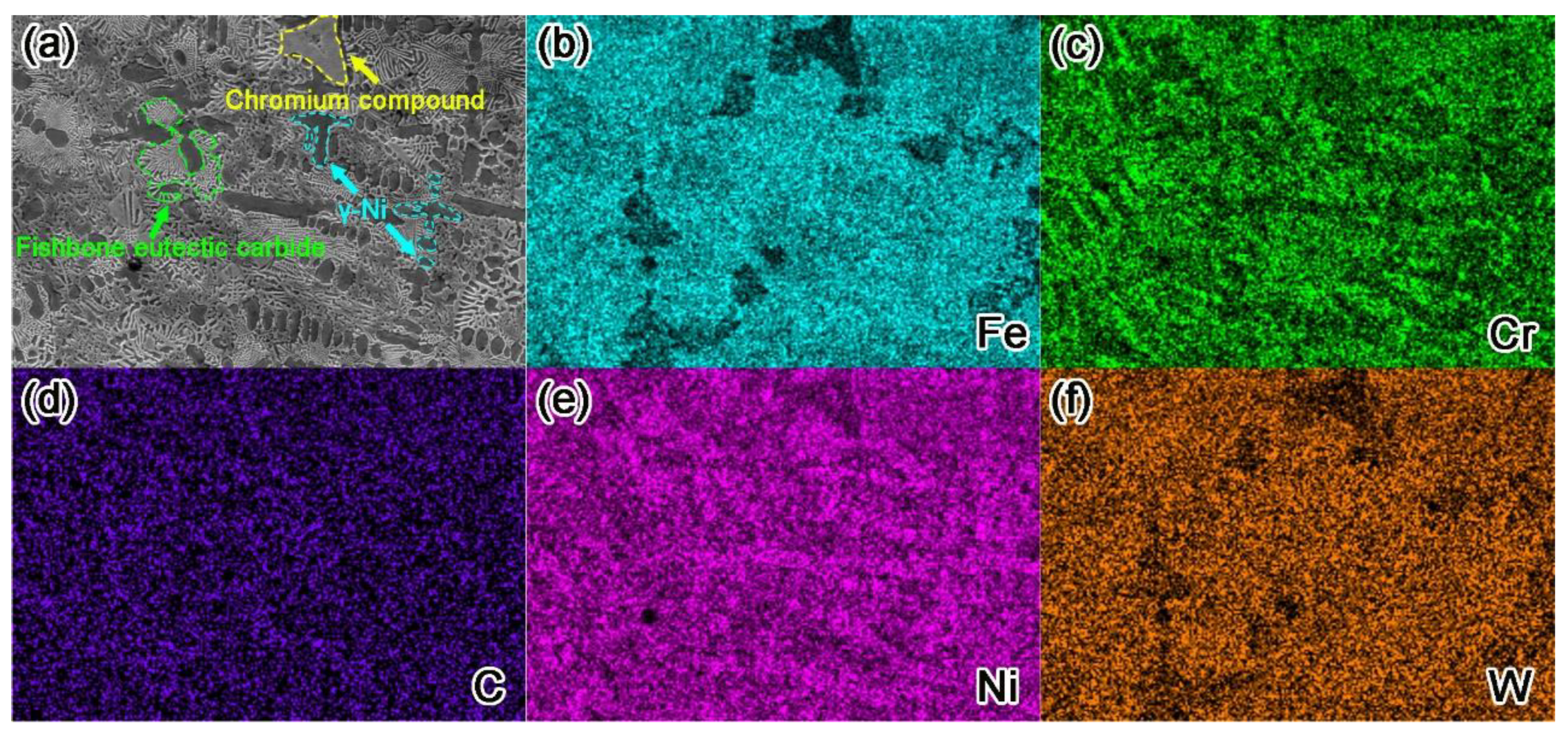
3.2. Analysis of Phase and Microstructure
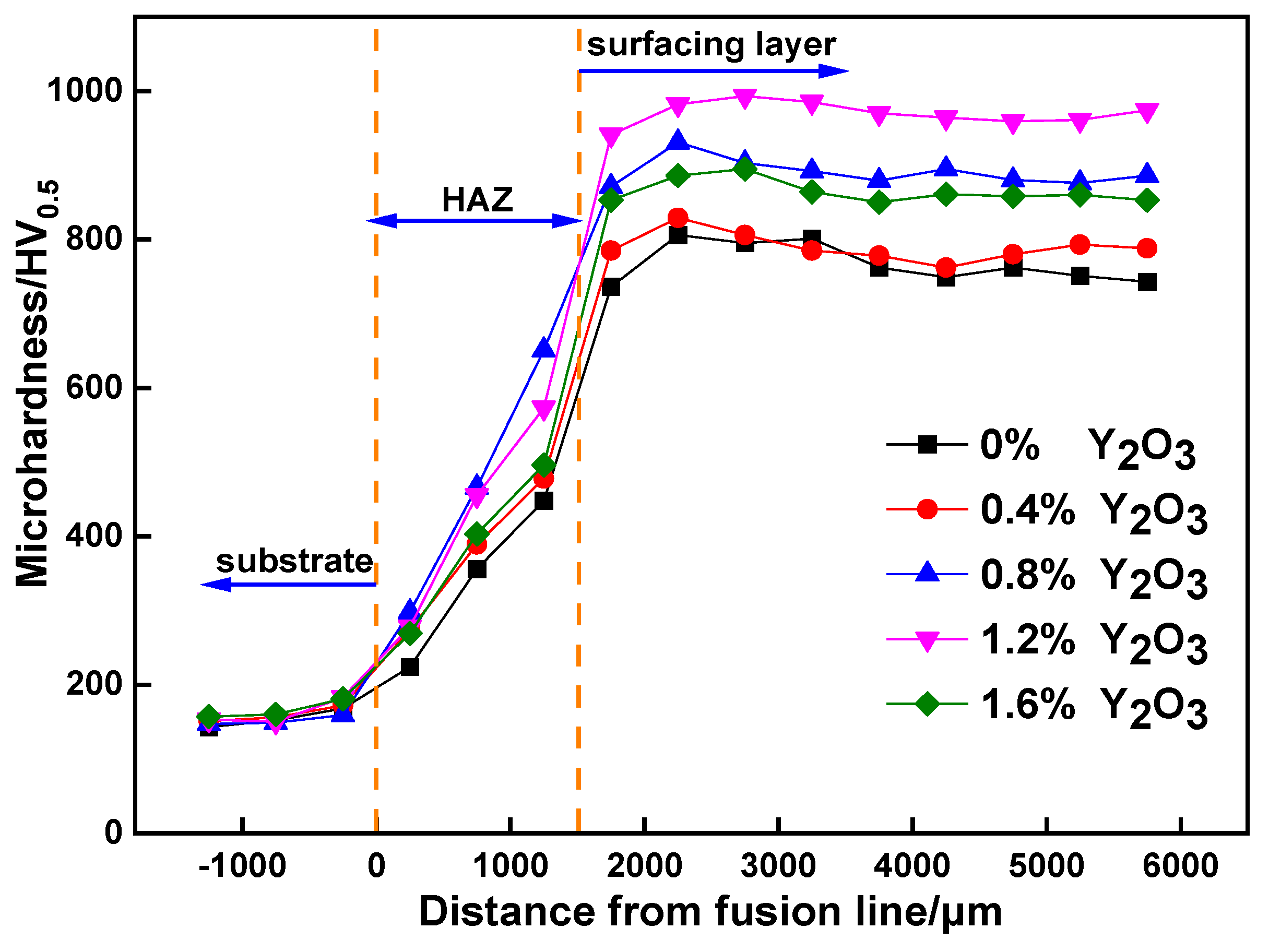
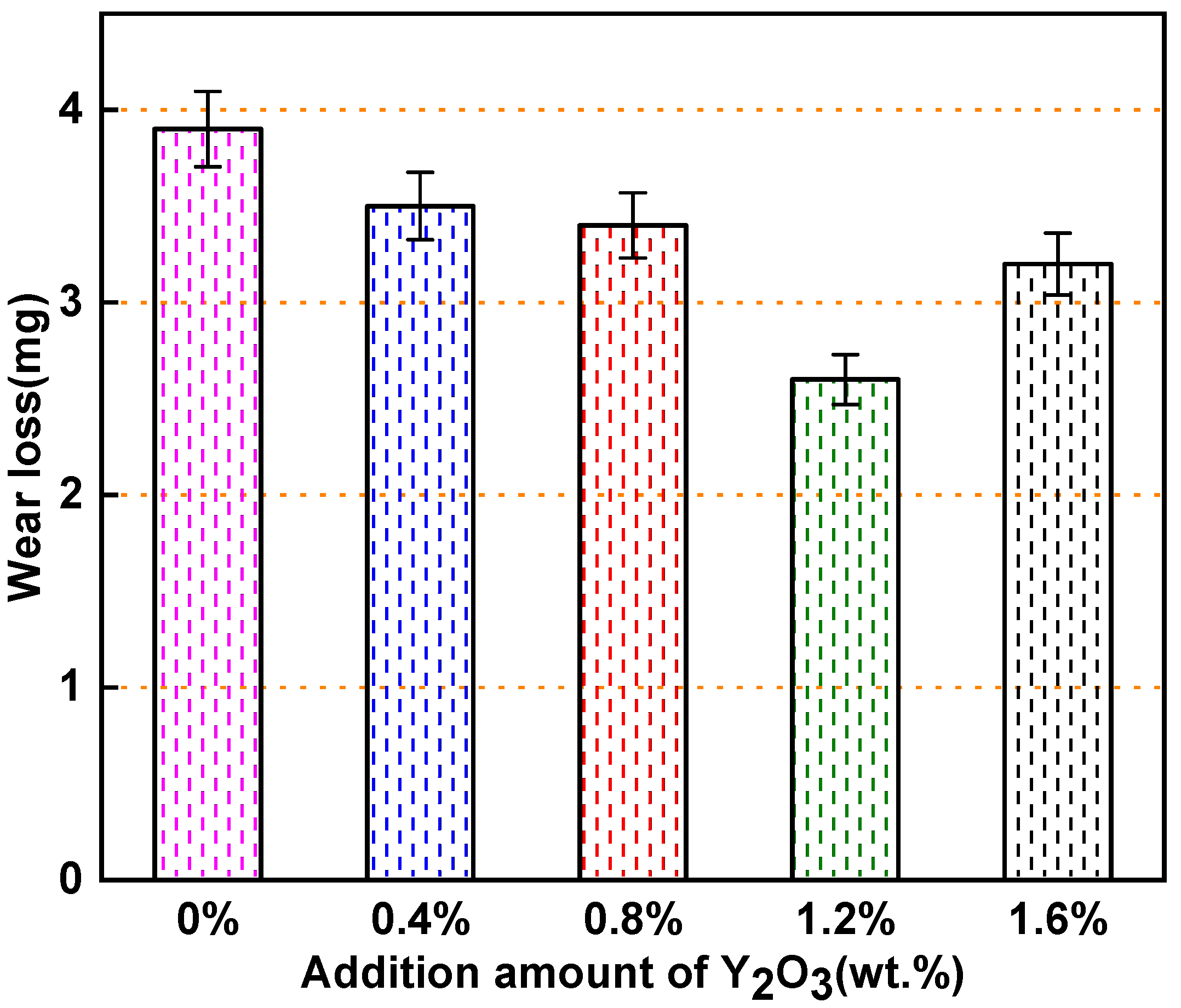
3.3. Microhardness and Wear Resistance Analysis of the Surfacing Layer
4. Conclusions
- By adding different contents of Y2O3 into the 30 wt.% WC-Ni based alloy surfacing layer, it can be observed that the addition of Y2O3 with a content of 1.2% can effectively reduce the wetting angle and improve weld formability. However, an excessive amount of Y2O3 can cause welding defects.
- The formation of phase was found to be independent of the amount of RE addition, and its main phase is γ-Ni (Fe), FeNi3 solid solution. Carbides were formed with various morphology, namely, WC, W2C, M23C6, M6C, and Cr7C3.
- An appropriate amount of Y2O3 can significantly refine microstructure and increase the content of eutectic carbides. However, excessive Y2O3 will cause coarse microstructure and reduce the content of carbides.
- The addition of nano-Y2O3 significantly improved the average hardness of the surfacing layers. With 1.2 wt.% nano-Y2O3, the surfacing layer reaches the maximum average of Vickers hardness, 970HV0.5, which is about six-times harder than the base metal.
- According to the pin-on-disk wear experiment results, an appropriate amount of nano-Y2O3 at room temperature can reduce the coefficient of friction (COF) and wear of the surfacing layer. The minimum wear of the surfacing layer containing 1.2 wt.% nano-Y2O3 was 2.6 mg, whereas the minimum COF was approximately 0.3092. The wear mechanism is mainly abrasive wear, accompanied by slight adhesive wear.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassini, E.; Sivo, A.; Ugues, D. Assessment of the Hardening Behavior and T ensile Properties of a Cold-Rolled Bainitic–Ferritic Steel. Materials 2021, 14, 6662. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Lu, J. Nanostructured surface layer on metallic materials induced by surface mechanical attrition treatment. Mater. Sci. Eng. A 2004, 375, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Weng, F.; Yu, H.; Chen, C.; Liu, J.; Zhao, L. Microstructures and properties of TiN reinforced Co-based composite coatings modified with Y2O3 by laser cladding on Ti–6Al–4V alloy. J. Alloys Compd. 2015, 650, 178–184. [Google Scholar] [CrossRef]
- Yao, F.; Fang, L.; Li, G.S. Study on structure and process performance of laser cladding nickel-based coating. J. Mater. Res. Technol. 2021, 13, 138–143. [Google Scholar] [CrossRef]
- Chen, B.; Shen, J.; Ye, X.; Jia, L.; Li, S.; Umeda, J.; Takahashi, M.; Kondoh, K. Length Effect of Carbon Nanotubes on the Strengthening Mechanisms in Metal Matrix Composites. Acta Mater. 2017, 140, 317–325. [Google Scholar] [CrossRef]
- Ozerov, M.; Stepanov, N.; Kolesnikov, A.; Sokolovsky, V.; Zherebtsov, S. Brittle-to-ductile transition in a Ti–TiB metal-matrix composite. Mater. Lett. 2017, 187, 28–31. [Google Scholar] [CrossRef]
- Min, Y.K.; Park, J.S.; Min, K.P.; Kim, K.T.; Hong, S.H. Effect of aspect ratios of in situ formed TiB whiskers on the mechanical properties of TiBw/Ti–6Al–4V composites. Scripta 2012, 66, 487–490. [Google Scholar]
- Ozerov, M.S.; Klimova, M.V.; Stepanov, N.D.; Zherebtsov, S.V. Microstructure evolution of a TI/TIB metal-matrix composite during high-temperature deformation. Mater. Phys. Mech. 2018, 38, 54–63. [Google Scholar]
- Zherebtsov, S.; Ozerov, M.; Povolyaeva, E.; Sokolovsky, V.; Stepanov, N.; Moskovskikh, D.; Salishchev, G. Effect of Hot Rolling on the Microstructure and Mechanical Properties of a Ti-15Mo/TiB Metal-Matrix Composite. Metals-Open Access Metall. J. 2019, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Morsi, K. Review: Titanium–titanium boride composites. J. Mater. Sci. 2019, 54, 6753–6771. [Google Scholar] [CrossRef]
- Ozerov, M.; Povolyaeva, E.; Stepanov, N.; Ventzke, V.; Zherebtsov, S. Laser Beam Welding of a Ti-15Mo/TiB Metal–Matrix Composite. Metals 2021, 11, 506. [Google Scholar] [CrossRef]
- Marchese, G.; Bassini, E.; Parizia, S.; Manfredi, D.; Ugues, D.; Lombardi, M.; Fino, P.; Biamino, S. Role of the chemical homogenization on the microstructural and mechanical evolution of prolonged heat-treated laser powder bed fused Inconel 625-ScienceDirect. Mater. Sci. Eng. A 2020, 796, 140007. [Google Scholar] [CrossRef]
- He, X.; Song, R.G.; Kong, D.J. Microstructures and properties of Ni/TiC/La2O3 reinforced Al based composite coatings by laser cladding. Opt. Laser Technol. 2019, 117, 18–27. [Google Scholar] [CrossRef]
- Wang, X.; Keya, T.; Chou, K. Build Height Effect on the Inconel 718 Parts Fabricated by Selective Laser Melting. Procedia Manuf. 2016, 5, 1006–1017. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.M.; Zhou, J.; Zhang, M.M.; Peng, S.X.; Zhu, S.; Zhang, J.S. A comparison study on wear characteristics of Ni-based, Co-based and Fe-based alloys for heated hot stamping tools manufactured by surfacing technology. Int. J. Adv. Manuf. Technol. 2020, 106, 3659–3668. [Google Scholar] [CrossRef]
- Verma, V.; Kumar, B. Tribological characteristics of conventionally sintered TiCN-WC-Ni/Co cermets against cemented carbide. Ceram. Int. 2017, 43, 368–375. [Google Scholar] [CrossRef]
- Ward, L.P.; Pilkington, A. The Dry Sliding Wear Behavior of HVOF-Sprayed WC: Metal Composite Coatings. J. Mater. Eng. Perform. 2014, 23, 3266–3278. [Google Scholar] [CrossRef]
- Xiang, W.; Zhu, L.; Zhou, Z.; Liu, G.; Wu, X. Tribological Properties of WC-Reinforced Ni-Based Coatings Under Different Lubricating Conditions. J. Therm. Spray Technol. 2015, 24, 1323–1332. [Google Scholar]
- Jiménez, H.; Olaya, J.J.; Alfonso, J.E. Tribological Behavior of Ni-Based WC-Co Coatings Deposited via Spray and Fuse Technique Varying the Oxygen Flow. Adv. Tribol. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Shu, D.; Li, Z.; Zhang, K.; Yao, C.; Li, D.; Dai, Z. In situ synthesized high volume fraction WC reinforced Ni-based coating by laser cladding. Mater. Lett. 2017, 195, 178–181. [Google Scholar] [CrossRef]
- Lv, C.; Ren, X.; Wang, C.; Peng, Z. Improved mechanical performance and wear resistance of Ti-coated cBN–WC–Ni composites. J. Mater. Res. 2019, 34, 1–9. [Google Scholar] [CrossRef]
- Acker, K.V.; Vanhoyweghen, D.; Persoons, R.; Vangrunderbeek, J. Influence of tungsten carbide particle size and distribution on the wear resistance of laser clad WC/Ni coatings. Wear 2005, 258, 194–202. [Google Scholar] [CrossRef]
- Kwon, H.; Suh, C.; Kim, W. Preparation of a highly toughened (Ti, W) C-20Ni cermet through in situ formation of solid solution and WC whiskers. Ceram. Int. 2015, 41, 4223–4226. [Google Scholar] [CrossRef]
- Fang, Y.C.; Cui, X.F.; Cai, Z.B.; Wang, C.; Jin, G. Influence of La2O3 addition on nano indentation hardness and residual stress of Stellite 6 coating prepared by plasma cladding. J. Rare Earth 2018, 36, 873–878. [Google Scholar] [CrossRef]
- Farahmand, P.; Liu, S.; Zhang, Z.; Kovacevic, R. Laser cladding assisted by induction heating of Ni–WC composite enhanced by nano-WC and La2O3. Ceram. Int. 2014, 40, 15421–15438. [Google Scholar] [CrossRef]
- Cui, Y. Study on the Effect of Rare Earth Elements on the Microstructure and Performance of Cladding Layer. Appl. Mech. Mater. 2014, 608, 1035–1038. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wang, T.H. Effects of Rare Earth Y2O3 on the Microstructure and Wear Behaviors of Ti-6Al-4V Cladding with SiC. Mater. Sci. Forum 2018, 934, 89–94. [Google Scholar] [CrossRef]
- Zhao, N.; Tao, L.; Guo, H.; Zhang, M.Q. Microstructure and Wear Resistance of Laser Cladded Ni-based Coatings with Nanometer La2O3 Addition. Rare Metal Mater. Eng. 2017, 46, 2092–2096. [Google Scholar]
- Wang, K.L.; Zhang, Q.B.; Sun, M.L.; Wei, X.G. Microstructural characteristics of laser clad coatings with rare earth metal elements. J. Mater. Process. Technol. 2003, 139, 448–452. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhang, Q.B.; Sun, M.L. Microstructure and corrosion resistance of laser clad coatings with rare earth elements. Corros. Sci. 2001, 43, 255–267. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Li, G.J. Effect of Y2O3 on the Sliding Wear Resistance of TiB/TiC-Reinforced Composite Coatings Fabricated by Laser Cladding. Wear 2014, 310, 72–82. [Google Scholar] [CrossRef]
- Yun, X.; Zhou, Y.F.; Zhao, B.; Xing, X.L.; Yang, J.; Yang, Y.L.; Yang, Q.X. Influence of Nano-Y2O3 on Wear Resistance of Hypereutectic Fe–Cr–C Hardfacing Coating. Tribol. Lett. 2015, 58, 23. [Google Scholar] [CrossRef]
- Wang, J.F.; Xue, W.H.; Gao, S.Y.; Li, S.; Duan, D.L. Effect of groove surface texture on the fretting wear of Ti–6Al–4V alloy. Wear 2021, 486, 204079. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, K.S.; Inoue, M.; Jiang, J.X. Effects of Ag and Cu addition on microstructure properties and oxidation resistance of Sn-Zn eutectic alloy. J. Alloys Compd. 2008, 454, 310–320. [Google Scholar] [CrossRef]
- Chen, C.M.; Chen, C.H. Interfacial Reactions between Eutectic SnZn Solder and Bulk or Thin-Film Cu Substrates. J. Electron. Mater. 2007, 36, 1363–1371. [Google Scholar] [CrossRef]
- Sharif, A.; Chan, Y.C. Effect of substrate metallization on interfacial reactions and reliability of Sn-Zn-Bi solder joints. Microelectron. Eng. 2007, 84, 328–335. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, Y.; Zeng, Z.D.; Shi, C.W. Effects of CeO2 on microstructure and corrosion resistance of TiC-VC reinforced Fe-based laser cladding layers. J. Rare Earth 2014, 32, 1095–1100. [Google Scholar] [CrossRef]
- Xia, M.J.; Gu, D.D.; Ma, C.L.; Zhang, H.M.; Dai, D.H.; Chen, H.Y.; Li, C.P.; Zhou, Z.J.; Chen, G.F.; Kelbassa, I. Fragmentation and refinement behavior and underlying thermodynamic mechanism of WC reinforcement during selective laser melting of Ni-based composites. J. Alloys Compd. 2018, 777, 693–702. [Google Scholar] [CrossRef]
- Wang, C.L.; Gao, Y.; Wang, R.; Wei, D.Q.; Cai, M.; Fu, Y.K. Microstructure of laser-clad Ni60 cladding layers added with different amounts of rare-earth oxides on 6063 Al alloys. J. Alloy Compd. 2018, 740, 1099–1107. [Google Scholar] [CrossRef]
- Shi, X.L.; Jiang, Y.H.; Zhou, R. Effects of rare earth, titanium, and magnesium additions on microstructures and properties of high-boron medium-carbon alloy. Journal of Iron and Steel. Res. Int. 2016, 23, 1226–1237. [Google Scholar] [CrossRef]
- Hou, Q.Y.; Huang, Z.Y.; Gao, J.S. Effects of Y2O3 on the microstructure and wear resistance of cobalt-based alloy coatings deposited by plasma transferred arc process. J. Rare Earth 2007, 26, 103–109. [Google Scholar] [CrossRef]
- Ulutan, M.; Kiliçay, K.; Çelik, O.N.; Er, Ü. Microstructure and wear behaviour of plasma transferred arc (PTA)-deposited eCrC composite coatings on AISI5115 steel. J. Mater. Process. Technol. 2016, 236, 26–34. [Google Scholar] [CrossRef]


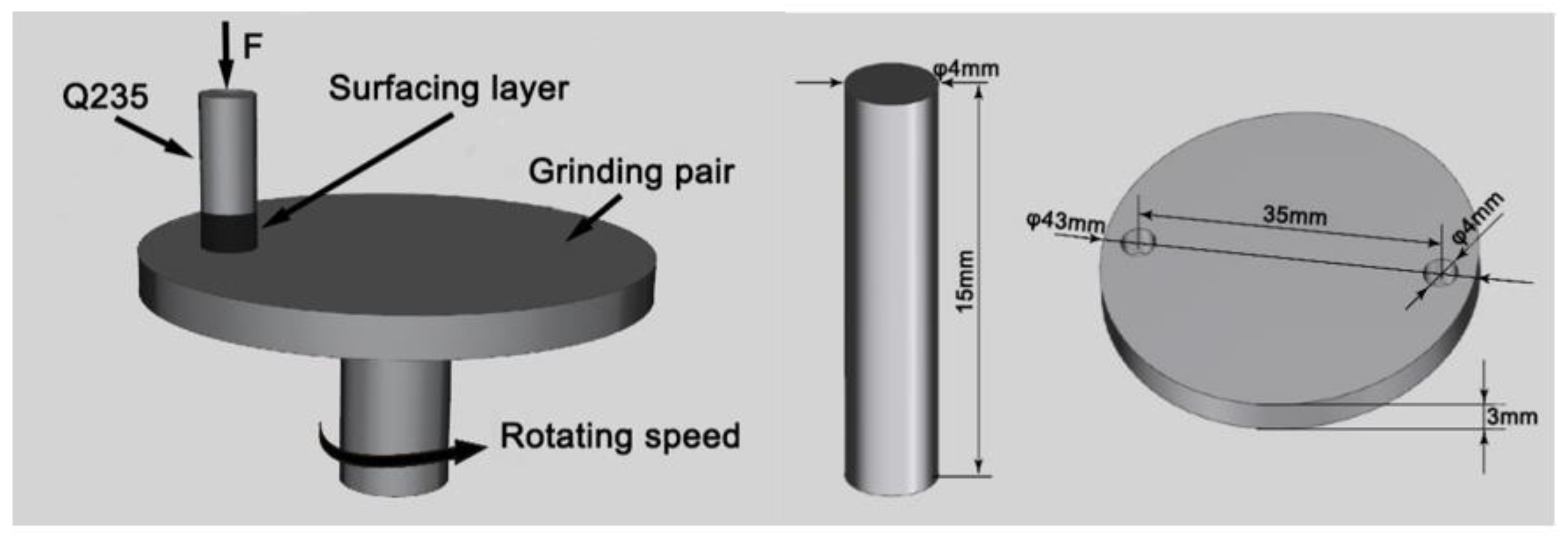
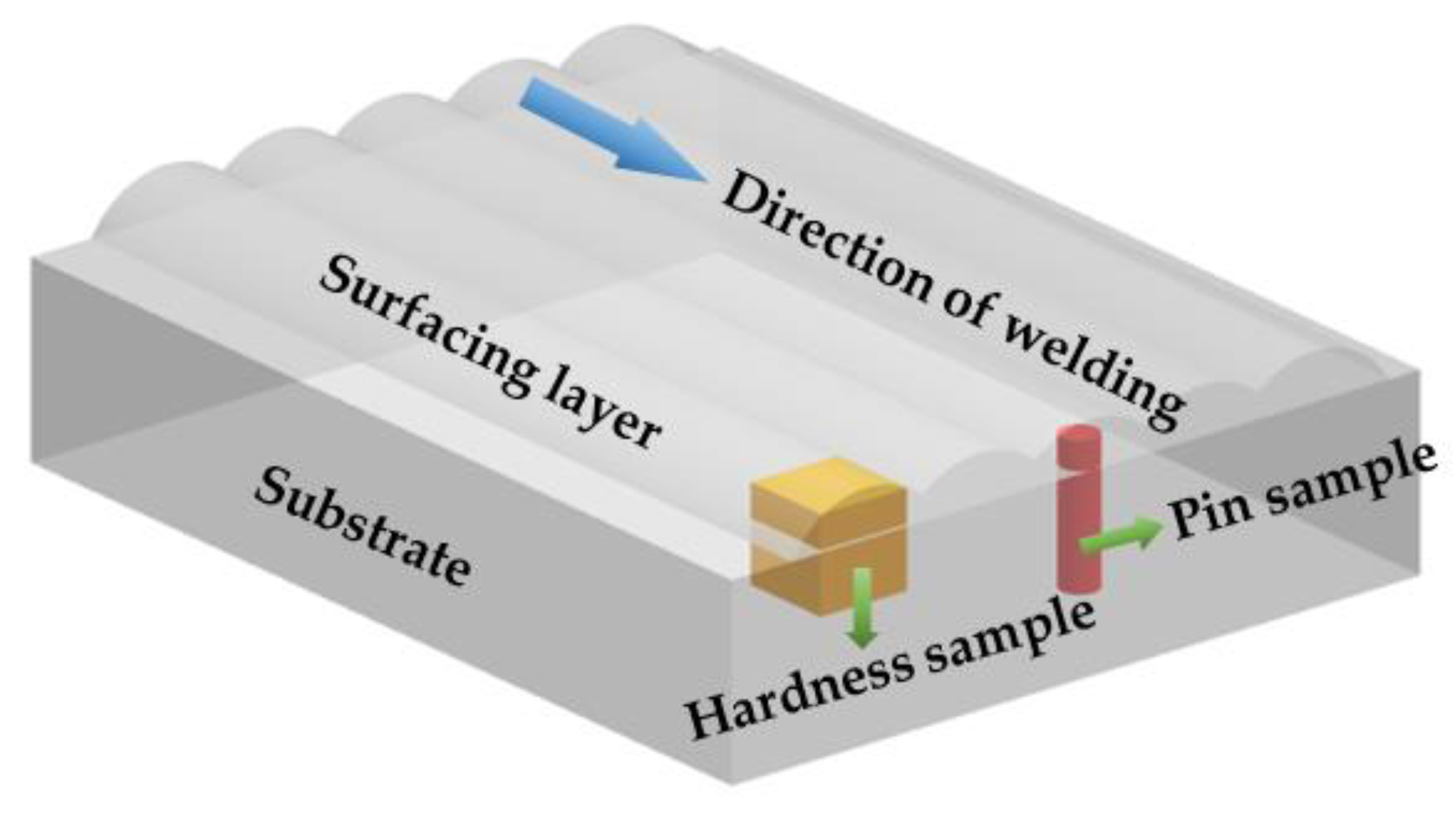





| Element | C | Mn | Si | S | P | Fe |
|---|---|---|---|---|---|---|
| Content | 0.12~0.22 | 0.30~0.70 | ≤0.37 | ≤0.050 | ≤0.045 | bal. |
| Element | C | B | Si | Cr | Fe | Ni |
|---|---|---|---|---|---|---|
| Content | 0.7~1.0 | 3.0~4.5 | 3.5~4.5 | 15~17 | ≤8 | Bal. |
| Element | C | Si | Mn | Cr | Mo | S | P | Ni | Cu | Ni + Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.95~1.05 | 0.15~0.35 | 0.25~0.45 | 1.40~1.65 | ≤0.10 | ≤0.025 | ≤0.025 | ≤0.30 | ≤0.25 | ≤0.50 | bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, X.; Liu, Z.; Zou, Z.; Wang, Z. Effect of Nano-Y2O3 on the Microstructure and Properties of WC-Reinforced Ni-Based Composite Surfacing Layer. Materials 2022, 15, 1665. https://doi.org/10.3390/ma15051665
Ai X, Liu Z, Zou Z, Wang Z. Effect of Nano-Y2O3 on the Microstructure and Properties of WC-Reinforced Ni-Based Composite Surfacing Layer. Materials. 2022; 15(5):1665. https://doi.org/10.3390/ma15051665
Chicago/Turabian StyleAi, Xingyu, Zhengjun Liu, Zongxuan Zou, and Zhenyu Wang. 2022. "Effect of Nano-Y2O3 on the Microstructure and Properties of WC-Reinforced Ni-Based Composite Surfacing Layer" Materials 15, no. 5: 1665. https://doi.org/10.3390/ma15051665






