Geopolymers and Functionalization Strategies for the Development of Sustainable Materials in Construction Industry and Cultural Heritage Applications: A Review
Abstract
:1. Introduction
- Chemistry of geopolymers, focusing on the factors affecting the reaction mechanism of the polymerization process;
- The development of new hybrid materials deriving from functionalization with organic and inorganic nanomaterials or polymers;
- Their possible applications in the cultural heritage sector through a critical analysis of the various data present in the literature.
2. Geopolymers Chemistry and Reaction Mechanism
Assessment of Factors Affecting the Geopolymerization Process
3. Synthesis of Functional Geopolymeric Hybrid Materials
- Wetting and drying cycles refer to baking.
- Heat treatments.
- Hydrothermal process in which the fibers are heated in a liquid or vapor medium.
3.1. Functionalization by Sol–Gel Technique
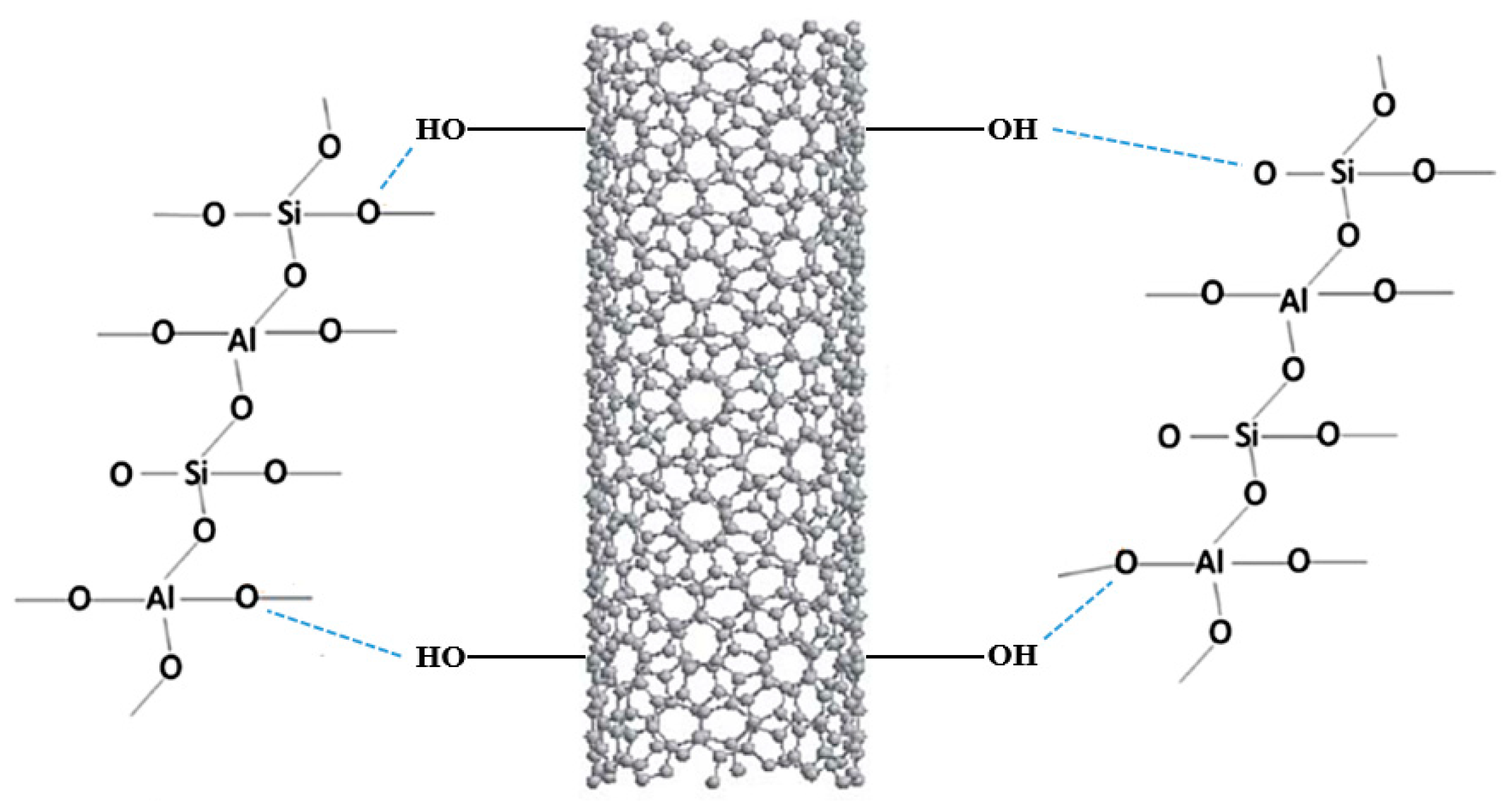
3.2. Functionalization with Nanoparticles (NPs)
4. Applications in Construction Industry and Cultural Heritage Fields
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozga, I.; Ghedini, N.; Giosuè, C.; Sabbioni, C.; Tittarelli, F.; Bonazza, A. Assessment of air pollutant sources in the deposit on monuments by multivariate analysis. Sci. Total Environ. 2014, 490, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Hanzlíček, T.; Steinerová, M.; Straka, P.; Perná, I.; Siegl, P.; Švarcová, T. Reinforcement of the terracotta sculpture by geopolymer composite. Mater. Des. 2009, 30, 3229–3234. [Google Scholar] [CrossRef]
- Occhipinti, R.; Stroscio, A.; Finocchiaro, C.; Fugazzotto, M.; Leonelli, C.; José Lo Faro, M.; Megna, B.; Barone, G.; Mazzoleni, P. Alkali activated materials using pumice from the Aeolian Islands (Sicily, Italy) and their potentiality for cultural heritage applications: Preliminary study. Constr. Build. Mater. 2020, 259, 120391. [Google Scholar] [CrossRef]
- Valluzzi, M.R.; Modena, C.; de Felice, G. Current practice and open issues in strengthening historical buildings with composites. Mater. Struct. 2014, 47, 1971–1985. [Google Scholar] [CrossRef]
- Ielo, I.; Galletta, M.; Rando, G.; Sfameni, S.; Cardiano, P.; Sabatino, G.; Drommi, D.; Rosace, G.; Plutino, M. Design, synthesis and characterization of hybrid coatings suitable for geopolymeric-based supports for the restoration of cultural heritage. IOP Conf. Ser. Mater. Sci. Eng. 2020, 777, 012003. [Google Scholar] [CrossRef]
- Geraldes, C.F.M.; Lima, A.M.; Delgado-Rodrigues, J.; Mimoso, J.M.; Pereira, S.R.M. Geopolymers as potential repair material in tiles conservation. Appl. Phys. A 2016, 122, 197. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar]
- Nodehi, M.; Taghvaee, V.M. Alkali-Activated Materials and Geopolymer: A Review of Common Precursors and Activators Addressing Circular Economy. Circ. Econ. Sustain. 2021, 1–32. [Google Scholar] [CrossRef]
- Obonyo, E.A.; Kamseu, E.; Lemougna, P.N.; Tchamba, A.B.; Melo, U.C.; Leonelli, C. A Sustainable Approach for the Geopolymerization of Natural Iron-Rich Aluminosilicate Materials. Sustainability 2014, 6, 5535–5553. [Google Scholar] [CrossRef] [Green Version]
- Kaze, R.C.; à Moungam, L.M.B.; Djouka, M.L.F.; Nana, A.; Kamseu, E.; Melo, U.F.C.; Leonelli, C. The corrosion of kaolinite by iron minerals and the effects on geopolymerization. Appl. Clay Sci. 2017, 138, 48–62. [Google Scholar] [CrossRef]
- Jihui, Z. Eco-friendly geopolymer materials: A review of performance improvement, potential application and sustainability assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry and Applications; Institut Geopolymère: Saint-Quentin, France, 2020. [Google Scholar]
- Provis, J.L.; Duxson, P.; van Deventer, J.S.J. The role of particle technology in developing sustainable construction materials. Adv. Powder Technol. 2010, 21, 2–7. [Google Scholar] [CrossRef]
- Bakhtyar, B.; Kacemi, T.; Nawaz, M.A. A Review on Carbon Emissions in Malaysian Cement Industry. Int. J. Energy Econ. Policy 2017, 7, 282–286. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Glukhovsky, V.D. Soil Silicates (Gruntosilikaty); Budivelnik Publisher: Kiev, Ukraine, 1959. [Google Scholar]
- Davidovits, J. Geopolymers—Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Pavel, K. Alkaline Activators. In Alkali Activated Cenents & Concrete; CRC Press: London, UK, 2006. [Google Scholar]
- Shi, C.; Krivenko, P.V.; Roy, D.M. Alkali-Activated Cements and Concretes; Taylor and Francis: Abingdon, UK, 2006. [Google Scholar]
- Silverstrim, T.; Rostami, H.; Larralde, J.; Samadi, A. Fly Ash Cementitious Material and Method of Making A Product. U.S. Patent 5,601,643, 11 February 1997. [Google Scholar]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes—A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L. Designing Precursors for Geopolymer Cements. J. Am. Ceram. Soc. 2008, 91, 3864–3869. [Google Scholar] [CrossRef]
- Kaze, C.R.; Nana, A.; Lecomte-Nana, G.L.; Deutou, J.G.N.; Kamseu, E.; Melo, U.C.; Andreola, F.; Leonelli, C. Thermal behaviour and microstructural evolution of metakaolin and meta-halloysite-based geopolymer binders: A comparative study. J. Therm. Anal. Calorim. 2021, 147, 2055–2071. [Google Scholar] [CrossRef]
- Ekinci, E.; Kantarci, F.; Karakoc, M.B.; Turkmen, I. Effect of silica modulus on compressive strength of volcanic tuff based geopolymer concrete. In Proceedings of the 185th IIER International Conference, Montreal, QC, Canada, 29–30 August 2018. [Google Scholar]
- Ricciotti, L.; Molino, A.J.; Roviello, V.; Chianese, E.; Cennamo, P.; Roviello, G. Geopolymer Composites for Potential Applications in Cultural Heritage. Environments 2017, 4, 91. [Google Scholar] [CrossRef] [Green Version]
- Laskar, A.I.; Talukdar, S. Rheological behavior of high performance concrete with mineral admixtures and their blending. Constr. Build. Mater. 2008, 22, 2345–2354. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Messina, F.; Ferone, C.; Asprone, D.; Cioffi, R. Lightweight geopolymer-based hybrid materials. Compos. Part B Eng. 2017, 128, 225–237. [Google Scholar] [CrossRef]
- Colangelo, F.; Roviello, G.; Ricciotti, L.; Ferone, C.; Cioffi, R. Preparation and Characterization of New Geopolymer-Epoxy Resin Hybrid Mortars. Materials 2013, 6, 2989–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trovato, V.; Rosace, G.; Colleoni, C.; Sfameni, S.; Migani, V.; Plutino, M. Sol-gel based coatings for the protection of cultural heritage textiles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 777, 012007. [Google Scholar] [CrossRef]
- Nikolova, A.; Rostovskya, I.; Nugterenb, H. Geopolymer materials based on natural zeolite. Case Stud. Constr. Mater. 2017, 6, 198–205. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgoa, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Rowles, M.R.; Hanna, J.V.; Pike, K.J.; Smith, M.E. 29Si, 27Al, 1H and 23Na MAS NMR Study of the Bonding Character in Aluminosilicate Inorganic Polymers. Appl. Magn. Reson. 2007, 32, 663–689. [Google Scholar] [CrossRef]
- Škvára, F. Alkali activated materials or geopolymers? Ceramics−Silikáty 2007, 51, 173–177. [Google Scholar]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio: Part I: FTIR study. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Alkaline activation of aluminosilicates as an alternative to Portland cement: A Review. Rom. J. Mater. 2017, 47, 3–15. [Google Scholar]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Glukhovsky, V.D.; Rostovkaya, G.S.; Rumyna, G.V. High strength slag-alkali cement. In Proceedings of the 7th International Congress on the Chemistry of Cements, Paris, France, 30 June–4 July 1980. [Google Scholar]
- Pacheco Torgal, F.; Labrincha, J.A.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Garcí, T.A.; de Lourdes Chávez-Garcí, M. Compressive Strength of Metakaolin-Based Geopolymers: Influence of KOH Concentration, Temperature, Time and Relative Humidity. Mater. Sci. Appl. 2016, 7, 772–791. [Google Scholar]
- Shi, C.; Day, R.L. Pozzolanic reactions in the presence of chemical activators—Part II: Reaction mechanisms. Cem. Concr. Res. 2000, 30, 607–613. [Google Scholar] [CrossRef]
- Błaszczyński, T.Z.; Król, M.R. Alkaline Activator Impact on the Geopolymer Binders. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245, 022036. [Google Scholar] [CrossRef] [Green Version]
- Palomo, A.; Alonso, S.; Fernández-Jiménez, A.; Sobrados, I.; Sanz, J. Alkali activated of fly ashes, A NMR study of the reaction products. J. Am. Ceram. Soc. 2004, 87, 1141–1145. [Google Scholar] [CrossRef]
- Brouwers, H.J.H.; Van Eijk, R.J. Reactivity of fly ash: Extension and application of a shrinking core model. Concr. Sci. Eng. 2002, 14, 106–113. [Google Scholar]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J. Effect of alkali-metal activator on the properties of fly ash-based geopolymers. Ind. Eng. Chem. Res. 1989, 38, 3932–3941. [Google Scholar] [CrossRef]
- Runzhang, Y.; Shi-Zi, O.; Qiong-Ying, G. Study on structure an latent hydraulic activity of slag and its activation mechanism. J. Wuhan Univ. Technol. 1987, 3, 297–303. [Google Scholar]
- Jiao, Z.; Wang, Y.; Zheng, W.; Huang, W. Effect of Dosage of Alkaline Activator on the Properties of Alkali-Activated Slag Pastes. Adv. Mater. Sci. Eng. 2018. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Sohn, D.; Jennings, H.M.; Mason, T.O. Hydration of alkaliactivated ground granulated blast furnace slag. J. Mater. Sci. 2000, 35, 249–257. [Google Scholar] [CrossRef]
- Shi, C.; Li, Y.; Tang, X. A preliminary investigation on the activation mechanism of granulated phosphorus slag. J. Southeast Univ. Nanjing PR China 1989, 19, 141–145. [Google Scholar]
- Joshi, S.V.; Kadu, M.S. Role of Alkaline Activator in Development of Eco-friendly Fly Ash Based Geo Polymer Concrete. Int. J. Environ. Sci. Dev. 2012, 3, 417–421. [Google Scholar] [CrossRef]
- Neville, A.M. Properties of Concrete; Prentice-Hall: Harlow, UK, 2011. [Google Scholar]
- Kresse, P. Efflorescence and its prevention. Betonw. Fert.-Tech. 1991, 57, 84–88. [Google Scholar]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Molino, A.J.; Menna, C.; Ferone, C.; Cioffi, R.; Tarallo, O. Hybrid Geopolymers from Fly Ash and Polysiloxanes. Molecules 2019, 24, 3510. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-Based Mesoporous Organic–Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Z.; Zhu, H.; Wang, X.; Gao, J. Effects of silane on reaction process and microstructure of metakaolin-based geopolymer composites. J. Build. Eng. 2020, 32, 101695. [Google Scholar] [CrossRef]
- Ramos, F.J.H.T.V.; da Silva, M.H.P.; Monteiro, S.N.; Grafov, A.; Grafova, I. Recycled polypropylene matrix nanocomposites reinforced with silane functionalized geopolymer concrete waste. J. Mater. Res. Technol. 2020, 9, 7540–7550. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Y.; Xu, F.; Song, X.; Ye, J.; Duan, P.; Zhang, Z. Sulfate resistance of hybrid fiber reinforced metakaolin geopolymer composites. Compos. Part B Eng. 2020, 183, 107689. [Google Scholar] [CrossRef]
- Fiset, J.; Cellier, M.; Vuillaume, P.Y. Macroporous geopolymers designed for facile polymers post-infusion. Cem. Concr. Compos. 2020, 110, 103591. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, M.; Ge, X.; Niu, Z.; Guo, Y. Study on the microstructure of metakaolin-based geopolymer enhanced by polyacrylate. J. Ceram. Soc. Jpn. 2019, 127, 165–172. [Google Scholar] [CrossRef] [Green Version]
- dos Reis, G.S.; Lima, E.C.; Sampaio, C.H.; Rodembusch, F.S.; Petter, C.O.; Cazacliu, B.G.; Dotto, G.L.; Hidalgo, G.E.N. Novel kaolin/polysiloxane based organic-inorganic hybrid materials: Sol-gel synthesis, characterization and photocatalytic properties. J. Solid State Chem. 2018, 260, 106–116. [Google Scholar] [CrossRef]
- Bergamonti, L.; Taurino, R.; Cattani, L.; Ferretti, D.; Bondioli, F. Lightweight hybrid organic-inorganic geopolymers obtained using polyurethane waste. Constr. Build. Mater. 2018, 185, 285–292. [Google Scholar] [CrossRef]
- Amritphale, S.S.; Mishra, D.; Mudgal, M.; Chouhan, R.K.; Chandra, N. A novel green approach for making hybrid inorganic- organic geopolymeric cementitious material utilizing fly ash and rice husk. J. Environ. Chem. Eng. 2016, 4, 3856–3865. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Lamanna, G.; Bollino, F. Geopolymer/PEG Hybrid Materials Synthesis and Investigation of the Polymer Influence on Microstructure and Mechanical Behavior. Mater. Res. 2015, 18, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Ouellet-Plamondon, C.; Aranda, P.; Favier, A.; Habert, G.; van Damme, H.; Ruiz-Hitzky, E. The Maya blue nanostructured material concept applied to colouring geopolymers. RSC Adv. 2015, 5, 98834–98841. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.W.; Easteal, A.J.; Bhattacharyya, D. Geopolymer Reinforced Polyethylene Nanocomposites. Compos. Technol. For. 2020 2004, 796–802. [Google Scholar] [CrossRef]
- Ferone, C.; Roviello, G.; Colangelo, F.; Cioffi, R.; Tarallo, O. Novel hybrid organic-geopolymer materials. Appl. Clay Sci. 2013, 73, 42–50. [Google Scholar] [CrossRef]
- Lamanna, G.; Soprano, A.; Bollino, F.; Catauro, M. Mechanical Characterization of Hybrid (Organic-Inorganic) Geopolymers. Key Eng. Mater. 2013, 569–570, 119–125. [Google Scholar] [CrossRef]
- Hussain, M.; Varely, R.; Cheng, Y.B.; Mathys, Z.; Simon, G.P. Synthesis and thermal behavior of inorganic–organic hybrid geopolymer composites. J. Appl. Polym. Sci. 2005, 96, 112–121. [Google Scholar] [CrossRef]
- Shaikh, F.U.A. Review of mechanical properties of short fibre reinforced geopolymer composites. Constr. Build. Mater. 2013, 43, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Švegl, F.; Šuput-Strupi, J.; Škrlep, L.; Kalcher, K. The influence of aminosilanes on macroscopic properties of cement paste. Cem. Concr. Res. 2008, 38, 945–954. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Asprone, D.; di Maggio, R.; Cappelletto, E.; Prota, A.; et al. Preparation, structure and properties of hybrid materials based on geopolymers and polysiloxanes. Mater. Des. 2015, 87, 82–94. [Google Scholar] [CrossRef]
- Kaewkuk, S.; Sutapun, W.; Jarukumjorn, K. Effects of interfacial modification and fiber content on physical properties of sisal fiber/polypropylene composites. Compos. Part B Eng. 2013, 45, 544–549. [Google Scholar] [CrossRef]
- Nicole, L.; Laberty-Robert, C.; Rozes, L.; Sanchez, C. Hybrid materials science: A promised land for the integrative design of multifunctional materials. Nanoscale 2014, 6, 6267–6292. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.M.; Adefrs Taye, E.; Roether, J.A.; Tilahun Redda, D.; Boccaccini, A.R. A Review on Natural Fiber-Reinforced Geopolymer and Cement-Based Composites. Materials 2020, 13, 4603. [Google Scholar] [CrossRef]
- Magdaleno-López, C.; Pérez-Bueno, J.d.J.; Flores-Segura, J.C.; Reyes-Araiza, J.L.; Mendoza-López, M.L.; Arés, O.; Manzano-Ramírez, A. A geopolymeric composite of non-calcined rice husks made of metakaolin/sol–gel silica. J. Compos. Mater. 2018, 53, 603–611. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Dell’Era, A.; Ciprioti, S.V. Pure Al2O3·2SiO2 synthesized via a sol-gel technique as a raw material to replace metakaolin: Chemical and structural characterization and thermal behavior. Ceram. Int. 2016, 42, 16303–16309. [Google Scholar] [CrossRef]
- Chen, X.; Mondal, P. Effects of NaOH amount on condensation mechanism to form aluminosilicate, case study of geopolymer gel synthesized via sol–gel method. J. Sol. Gel. Sci. Technol. 2020, 96, 589–603. [Google Scholar] [CrossRef]
- Huang, B.; Li, C.; Zhang, Y.; Ding, W.; Yang, M.; Yang, Y.; Zhai, H.; Xu, X.; Wang, D.; Debnath, S.; et al. Advances in fabrication of ceramic corundum abrasives based on sol–gel process. Chin. J. Aeronaut. 2021, 34, 1–17. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Cattaneo, A.S.; Mustarelli, P. Al2O3·2SiO2 powders synthesized via sol–gel as pure raw material in geopolymer preparation. J. Am. Ceram. Soc. 2017, 100, 1919–1927. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ansary, J.; Ghoreishi, R. Sol-Gel Process Applications: A mini-Review. Proc. Nat. Res. Soc. 2018, 2, 02008. [Google Scholar] [CrossRef]
- Plutino, M.R.; Guido, E.; Colleoni, C.; Rosace, G. Effect of GPTMS functionalization on the improvement of the pH-sensitive methyl red photostability. Sens. Actuators B Chem. 2017, 238, 281–291. [Google Scholar] [CrossRef]
- Rosace, G.; Guido, E.; Colleoni, C.; Brucale, M.; Piperopoulos, E.; Milone, C.; Plutino, M.R. Halochromic resorufin-GPTMS hybrid sol-gel: Chemical-physical properties and use as pH sensor fabric coating. Sens. Actuators B Chem. 2017, 241, 85–95. [Google Scholar] [CrossRef]
- Guido, E.; Colleoni, C.; De Clerck, K.; Plutino, M.R.; Rosace, G. Influence of catalyst in the synthesis of a cellulose-based sensor: Kinetic study of 3-glycidoxypropyltrimethoxysilane epoxy ring opening by Lewis acid. Sens. Actuators B Chem. 2014, 203, 213–222. [Google Scholar] [CrossRef]
- Trovato, V.; Colleoni, C.; Castellano, A.; Plutino, M.R. The key role of 3-glycidoxypropyltrimethoxysilane sol–gel precursor in the development of wearable sensors for health monitoring. J. Sol. Gel. Sci. Technol. 2018, 87, 27–40. [Google Scholar] [CrossRef]
- Rosace, G.; Trovato, V.; Colleoni, C.; Caldara, M.; Re, V.; Brucale, M.; Piperopoulos, E.; Mastronardo, E.; Milone, C.; De Luca, G.; et al. Structural and morphological characterizations of MWCNTs hybrid coating onto cotton fabric as potential humidity and temperature wearable sensor. Sens. Actuators B Chem. 2017, 252, 428–439. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Plutino, M.R. Nanostructured Surface Finishing and Coatings: Functional Properties and Applications. Materials 2021, 14, 2733. [Google Scholar] [CrossRef]
- Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C.; et al. Sol–Gel Treatment of Textiles for the Entrapping of an Antioxidant/Anti-Inflammatory Molecule: Functional Coating Morphological Characterization and Drug Release Evaluation. Appl. Sci. 2020, 10, 2287. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Zhu, X.; Du, J.; Kong, D.; Wang, N.; Wang, Z.; Wang, Q.; Liu, W.; Li, Q.; Zhou, Z. Facile and green preparation of novel adsorption materials by combining sol-gel with ion imprinting technology for selective removal of Cu(II) ions from aqueous solution. Appl. Surf. Sci. 2018, 435, 574–584. [Google Scholar] [CrossRef]
- Tan, W.K.; Muto, H.; Kawamura, G.; Lockman, Z.; Matsuda, A. Nanomaterial Fabrication through the Modification of Sol–Gel Derived Coatings. Nanomaterials 2021, 11, 181. [Google Scholar] [CrossRef]
- Kessler, V.G. The Synthesis and Solution Stability of Alkoxide Precursors. In Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 31–80. [Google Scholar]
- Zhou, F.; Cheng, G.; Jiang, B. Effect of silane treatment on microstructure of sisal fibers. Appl. Surf. Sci. 2014, 292, 806–812. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Facile preparation of slag or fly ash geopolymer composite coatings with flame resistance. Constr. Build. Mater. 2019, 203, 655–661. [Google Scholar] [CrossRef]
- Alvi, M.A.A.; Khalifeh, M.; Agonafir, M.B. Effect of nanoparticles on properties of geopolymers designed for well cementing applications. J. Pet. Sci. Eng. 2020, 191, 107128. [Google Scholar] [CrossRef]
- Rashad, A.M. Effect of nanoparticles on the properties of geopolymer materials. Mag. Concr. Res. 2019, 71, 1283–1301. [Google Scholar] [CrossRef]
- Rahmawati, C.; Aprilia, S.; Saidi, T.; Aulia, T.B. Current development of geopolymer cement with nanosilica and cellulose nanocrystals. J. Phys. Conf. Ser. 2021, 1783, 012056. [Google Scholar] [CrossRef]
- Guzmán-Carrillo, H.R.; Manzano-Ramírez, A.; Garcia Lodeiro, I.; Fernández-Jiménez, A. ZnO Nanoparticles for Photocatalytic Application in Alkali-Activated Materials. Molecules 2020, 25, 5519. [Google Scholar] [CrossRef] [PubMed]
- Maiti, M.; Sarkar, M.; Maiti, S.; Malik, M.A.; Xu, S. Modification of geopolymer with size controlled TiO2 nanoparticle for enhanced durability and catalytic dye degradation under UV light. J. Clean. Prod. 2020, 255, 120183. [Google Scholar] [CrossRef]
- Tay, C.H.; Norkhairunnisa, M. Mechanical Strength of Graphene Reinforced Geopolymer Nanocomposites: A Review. Front. Mater. 2021, 8, 276. [Google Scholar] [CrossRef]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent progress in environmentally friendly geopolymers: A review. Sci. Total Environ. 2021, 762, 143166. [Google Scholar] [CrossRef]
- Kumar, M.M.; Yuvaraj, S. Behaviour of Different Nanomaterials in Geopolymer Concrete. Int. J. Innov. Technol. Explor. Eng. 2020, 9, 605–611. [Google Scholar]
- Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes: Interfacial Properties and Applications in Cultural Heritage. Langmuir 2020, 36, 3677–3689. [Google Scholar] [CrossRef]
- Janczarek, M.; Klapiszewski, Ł.; Jędrzejczak, P.; Klapiszewska, I.; Ślosarczyk, A.; Jesionowski, T. Progress of functionalized TiO2-based nanomaterials in the construction industry: A comprehensive review. Chem. Eng. J. 2021, 430, 132062. [Google Scholar] [CrossRef]
- Kantarcı, F.; Maraş, M.M. Formulation of a novel nano TiO2-modified geopolymer grout for application in damaged beam-column joints. Constr. Build. Mater. 2022, 317, 125929. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Zidi, Z.; Ltifi, M.; Ayadi, Z.B.; Mir, L.E.; Nóvoa, X.R. Effect of nano-ZnO on mechanical and thermal properties of geopolymer. J. Asian Ceram. Soc. 2020, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Armayani, M.; Pratama, M.A. Subaer The Properties of Nano Silver (Ag)-Geopolymer as Antibacterial Composite for Functional Surface Materials. MATEC Web Conf. 2017, 97, 01010. [Google Scholar] [CrossRef] [Green Version]
- Kürklü, G.; Görhan, G. Investigation of usability of quarry dust waste in fly ash-based geopolymer adhesive mortar production. Constr. Build. Mater. 2019, 217, 498–506. [Google Scholar] [CrossRef]
- Baltazar, L.G.; Henriques, F.M.A.; Temporão, D.; Cidade, M.T. Experimental Assessment of Geopolymer Grouts for Stone Masonry Strengthening. Key Eng. Mater. 2019, 817, 507–513. [Google Scholar] [CrossRef]
- Moutinho, S.; Costa, C.; Andrejkovičová, S.; Mariz, L.; Sequeira, C.; Terroso, D.; Rocha, F.; Velosa, A. Assessment of properties of metakaolin-based geopolymers applied in the conservation of tile facades. Constr. Build. Mater. 2020, 259, 119759. [Google Scholar] [CrossRef]
- Clausi, M.; Tarantino, S.C.; Magnani, L.L.; Riccardi, M.P.; Tedeschi, C.; Zema, M. Metakaolin as a precursor of materials for applications in Cultural Heritage: Geopolymer-based mortars with ornamental stone aggregates. Appl. Clay Sci. 2016, 132–133, 589–599. [Google Scholar] [CrossRef]
- Pagnotta, S.; Tenorio, A.; Tiné, M.; Lezzerini, M. Geopolymers as a potential material for preservation and restoration of Urban Build Heritage: An overview. In Proceedings of the IOP Conference Series: Earth and Environmental Science, 6th World Multidisciplinary Earth Sciences Symposium, Prague, Czech Republic, 7–11 September 2020; Volume 609, p. 012057. [Google Scholar]

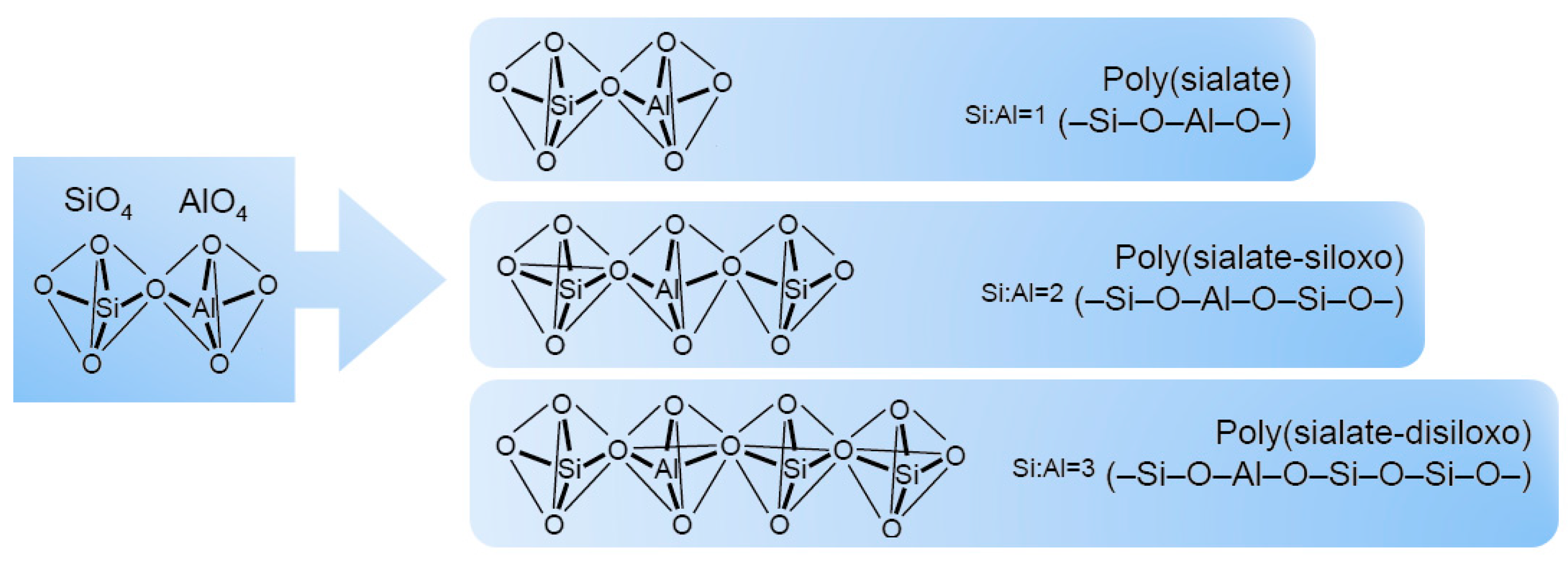

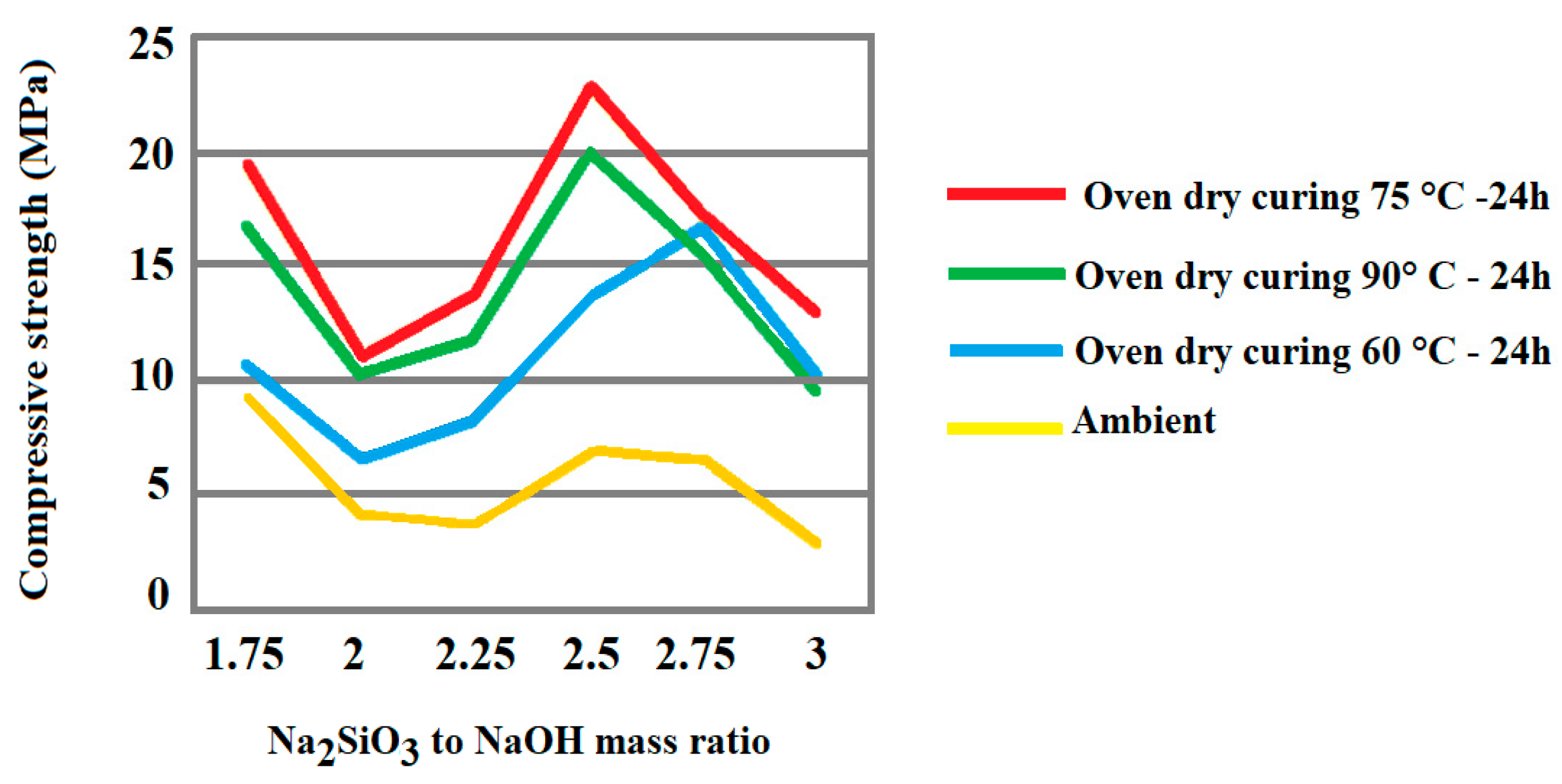
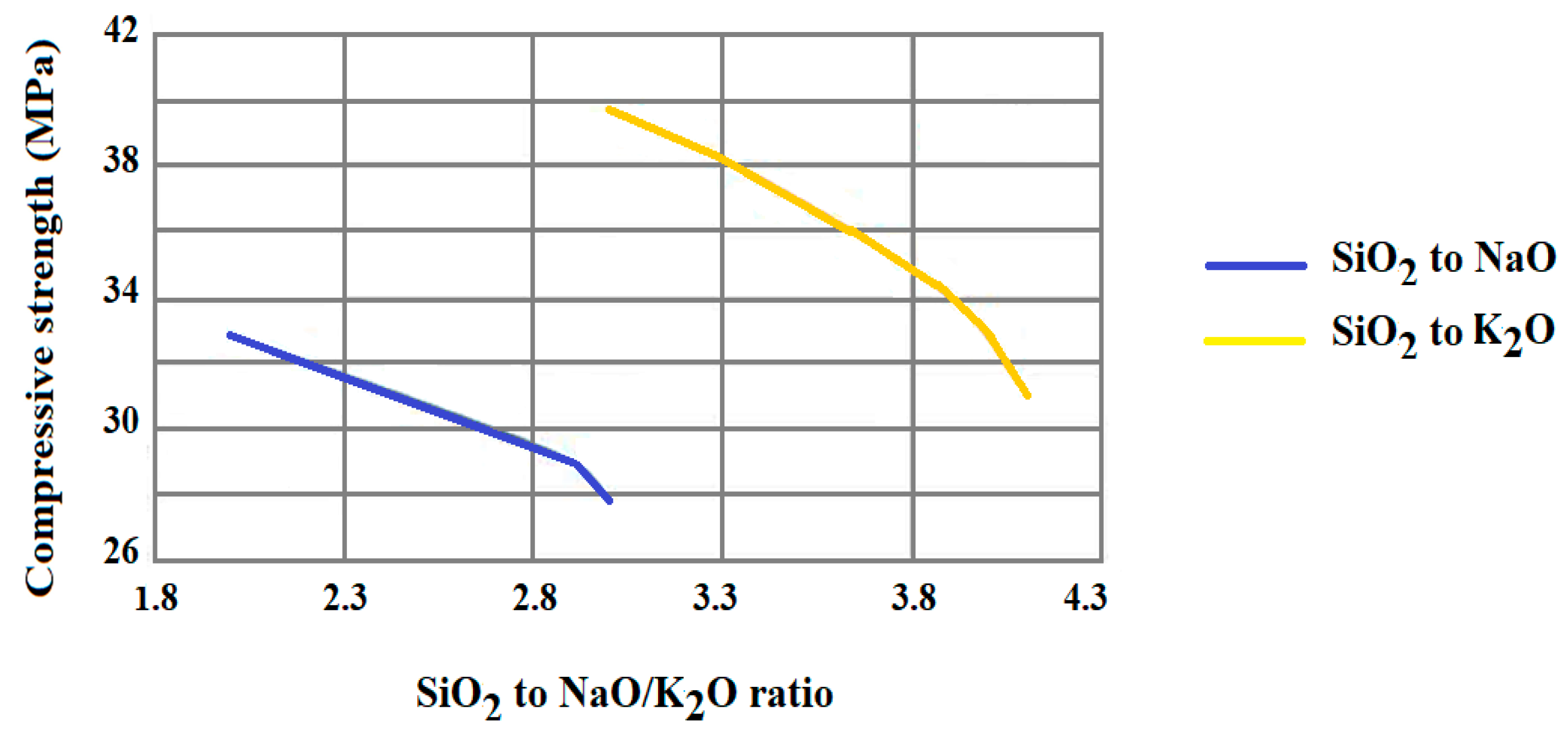

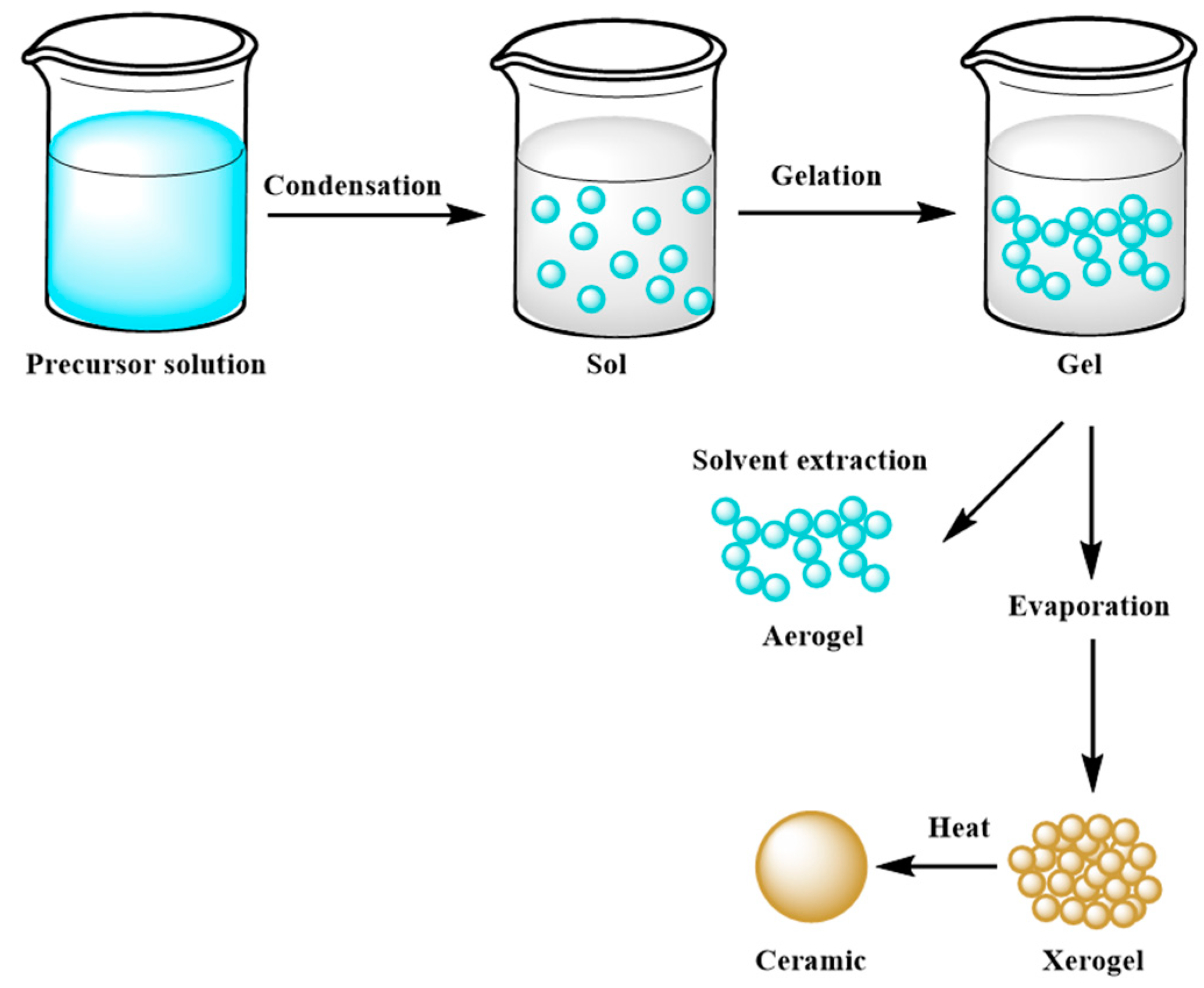
| Inorganic Substrate | Organic Agent | Ref. |
|---|---|---|
| Metakaolin-based geopolymer | (3-Aminopropyl)triethoxysilane (APTES) | [56] |
| Geopolymer concrete waste | Vinyl trimethoxy silane (VTP) + recycled polypropylene (rPP) | [57] |
| Metakaolin-based geopolymer | Polypropylene fiber (PP), polyvinyl alcohol fiber (PVA) | [58] |
| Metakaolin-based geopolymer | Unsaturated orthophtalic polyester resin | [59] |
| Metakaolin-based geopolymer | polyacrylate | [60] |
| Fly ash- based geopolymer | Oligomeric dimethylsiloxane | [54] |
| Kaolin-based geopolymer | Methyl-polysiloxane (MK), methyl-phenyl-polysiloxane (H44), tetraethyl-ortho-silicate (TEOS) and 3-amino-propyl-triethoxysilane (APTES) | [61] |
| Metakaolin-based geopolymer | Polyurethane powders wastes (polyurethane foam and polyisocyanurate foam) | [62] |
| Metakaolin-based geopolymer | Commercial oligomeric dimethylsiloxane mixture and epoxy resin | [28] |
| Fly ash-based geopolymer | Organic molecules deriving from the decomposition of rice husk: D-glucose, native cellulose, phenolic compounds and sucrose | [63] |
| Metakaolin-based geopolymer | Polyethylene glycol (PEG) | [64] |
| Metakaolin-based geopolymer + sepiolite | Methylene blue (MB) and methyl red (MR) | [65] |
| Metakaolin-based geopolymer | Polyethylene (PE) | [66] |
| Metakaolin-based geopolymer | Commercial epoxy resin | [29] |
| Metakaolin-based geopolymer | Epoxy resins formed by N,N-diglycidyl-4-glycidyl-oxyaniline with bis-(2-aminoethyl)amine and N,N-diglycidyl-4-glycidyl-oxyaniline with bis-(2-aminoethyl)amine and 2,4-diaminotoluene) | [67] |
| Metakaolin-based geopolymer | Polyethylene glycol (PEG) | [68] |
| Kaolin-based geopolymer | Epoxide matrix constituted by bisphenol a diglycidyl ether | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacobello, F.; Ielo, I.; Belhamdi, H.; Plutino, M.R. Geopolymers and Functionalization Strategies for the Development of Sustainable Materials in Construction Industry and Cultural Heritage Applications: A Review. Materials 2022, 15, 1725. https://doi.org/10.3390/ma15051725
Giacobello F, Ielo I, Belhamdi H, Plutino MR. Geopolymers and Functionalization Strategies for the Development of Sustainable Materials in Construction Industry and Cultural Heritage Applications: A Review. Materials. 2022; 15(5):1725. https://doi.org/10.3390/ma15051725
Chicago/Turabian StyleGiacobello, Fausta, Ileana Ielo, Hossem Belhamdi, and Maria Rosaria Plutino. 2022. "Geopolymers and Functionalization Strategies for the Development of Sustainable Materials in Construction Industry and Cultural Heritage Applications: A Review" Materials 15, no. 5: 1725. https://doi.org/10.3390/ma15051725
APA StyleGiacobello, F., Ielo, I., Belhamdi, H., & Plutino, M. R. (2022). Geopolymers and Functionalization Strategies for the Development of Sustainable Materials in Construction Industry and Cultural Heritage Applications: A Review. Materials, 15(5), 1725. https://doi.org/10.3390/ma15051725








