Development of Thermo- and pH-Sensitive Liposomal Magnetic Carriers for New Potential Antitumor Thienopyridine Derivatives
Abstract
:1. Introduction
2. Materials and Methods
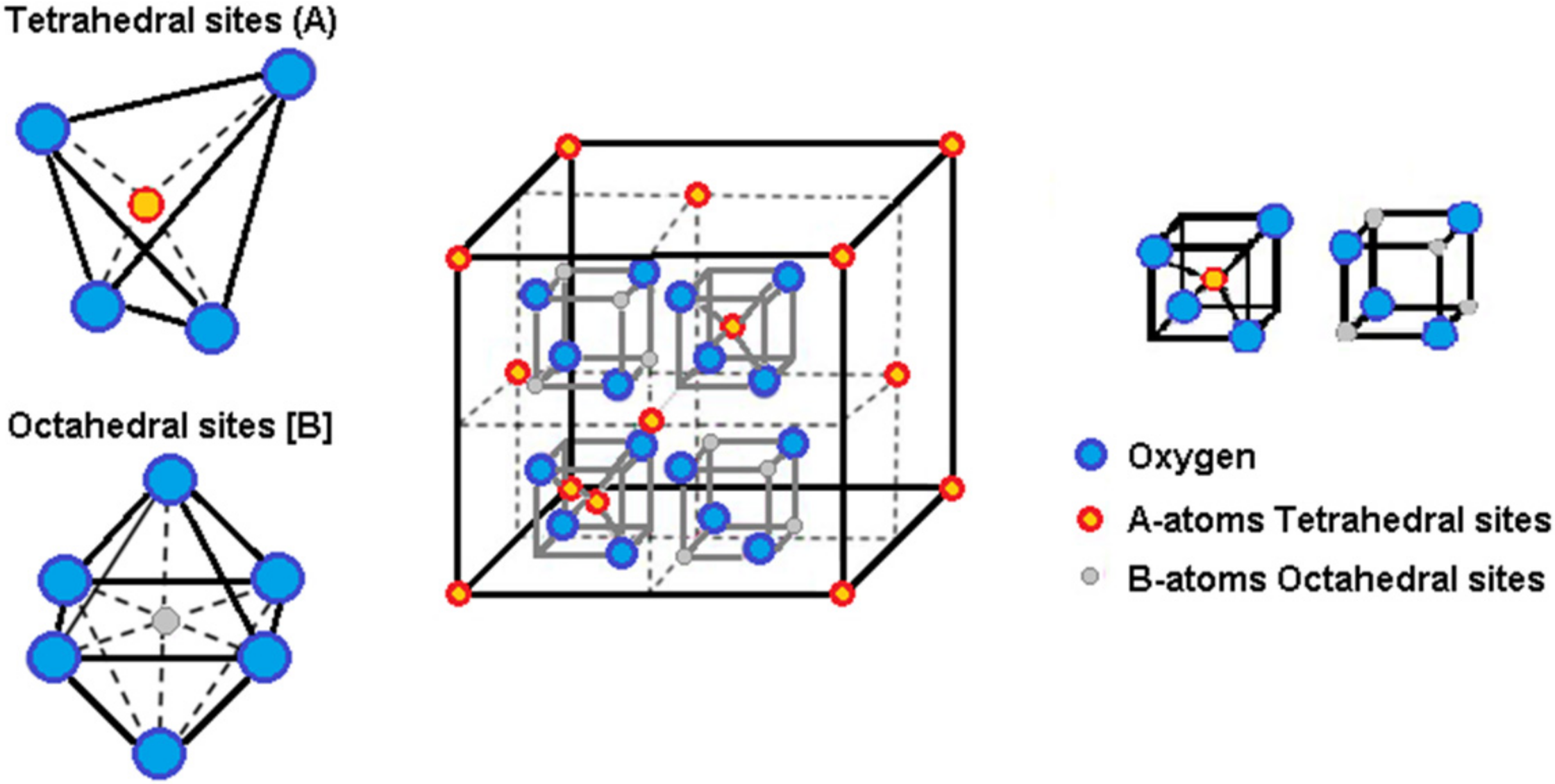
2.1. Preparation of Mixed Calcium/Manganese Ferrite Nanoparticles
2.1.1. Co-Precipitation Method
2.1.2. Sol-Gel Method
2.2. Synthesis of Magnetic Liposomes and Small Unilamellar Vesicles
2.3. Structural Characterization
2.4. Magnetic Measurements
2.5. Spectroscopic Measurements
2.5.1. General Methods
2.5.2. Fluorescence Anisotropy Measurements
2.5.3. Compound Encapsulation Efficiency
3. Results and Discussion
3.1. Nanoparticle Synthesis and Characterization
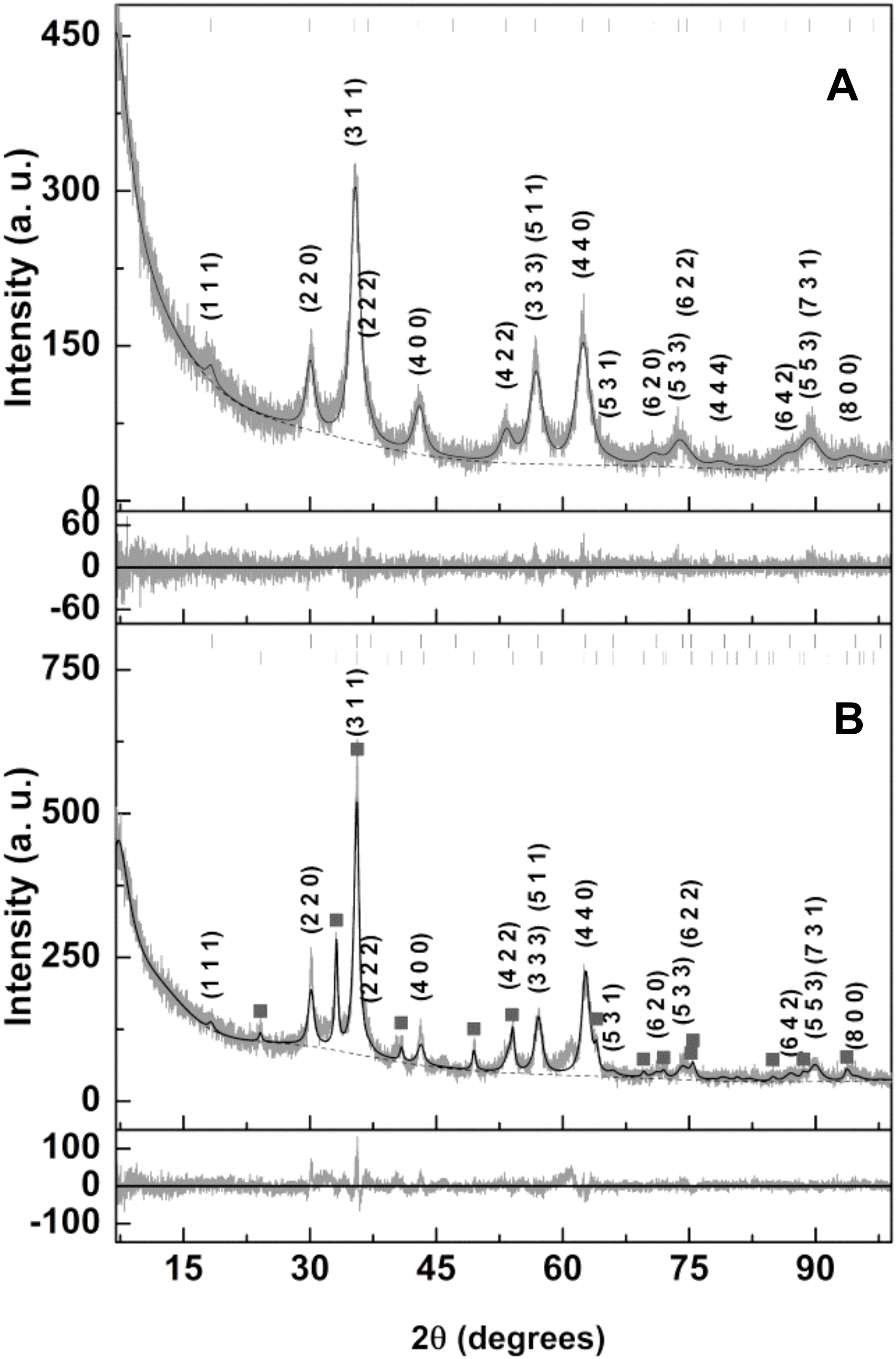
3.1.1. X-ray Analysis
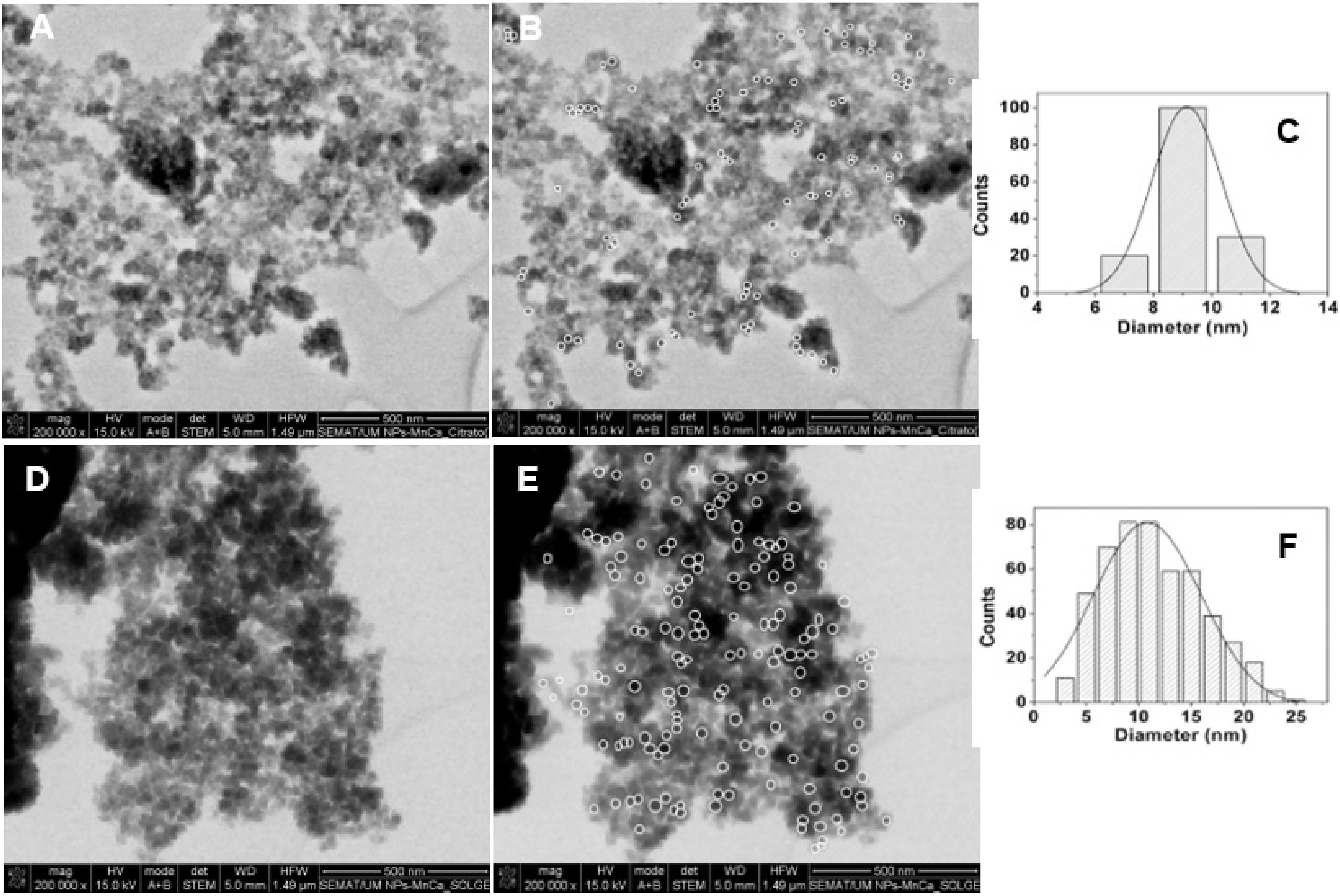
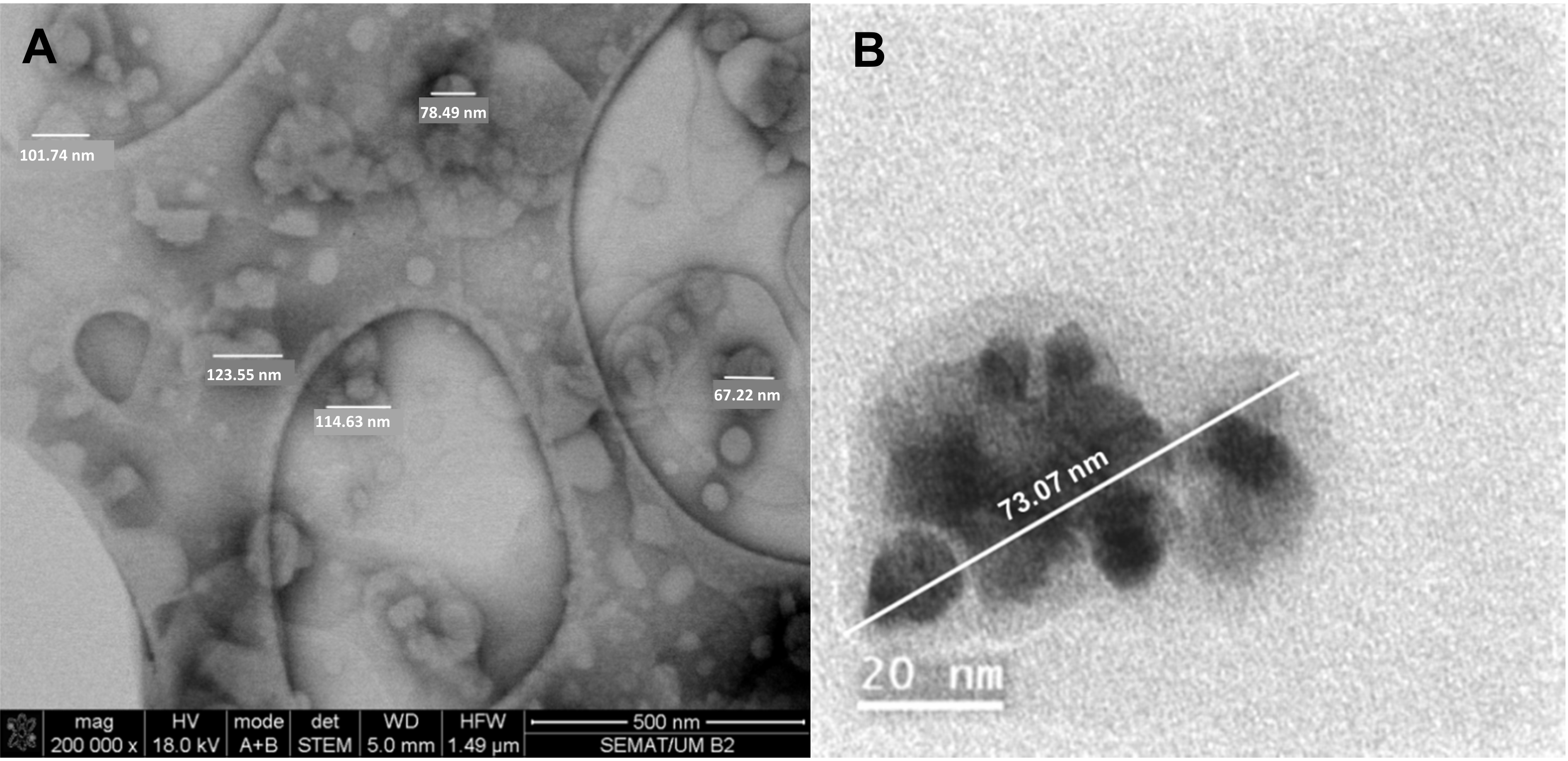
3.1.2. Scanning Electron Microscopy (SEM)
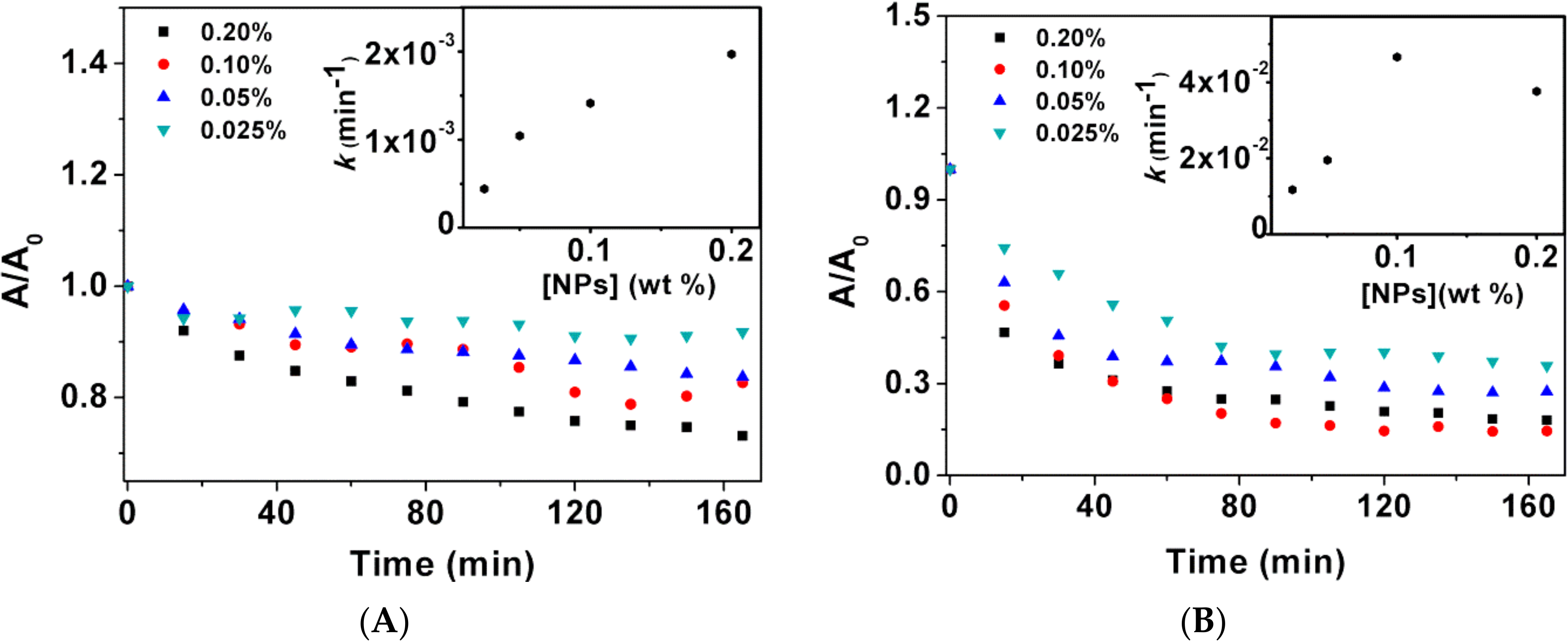
3.1.3. Sedimentation Kinetics
3.1.4. Magnetic Properties
3.2. Magnetoliposome Characterization by DLS and SEM
3.3. Drug-Loaded Magnetic Liposomes
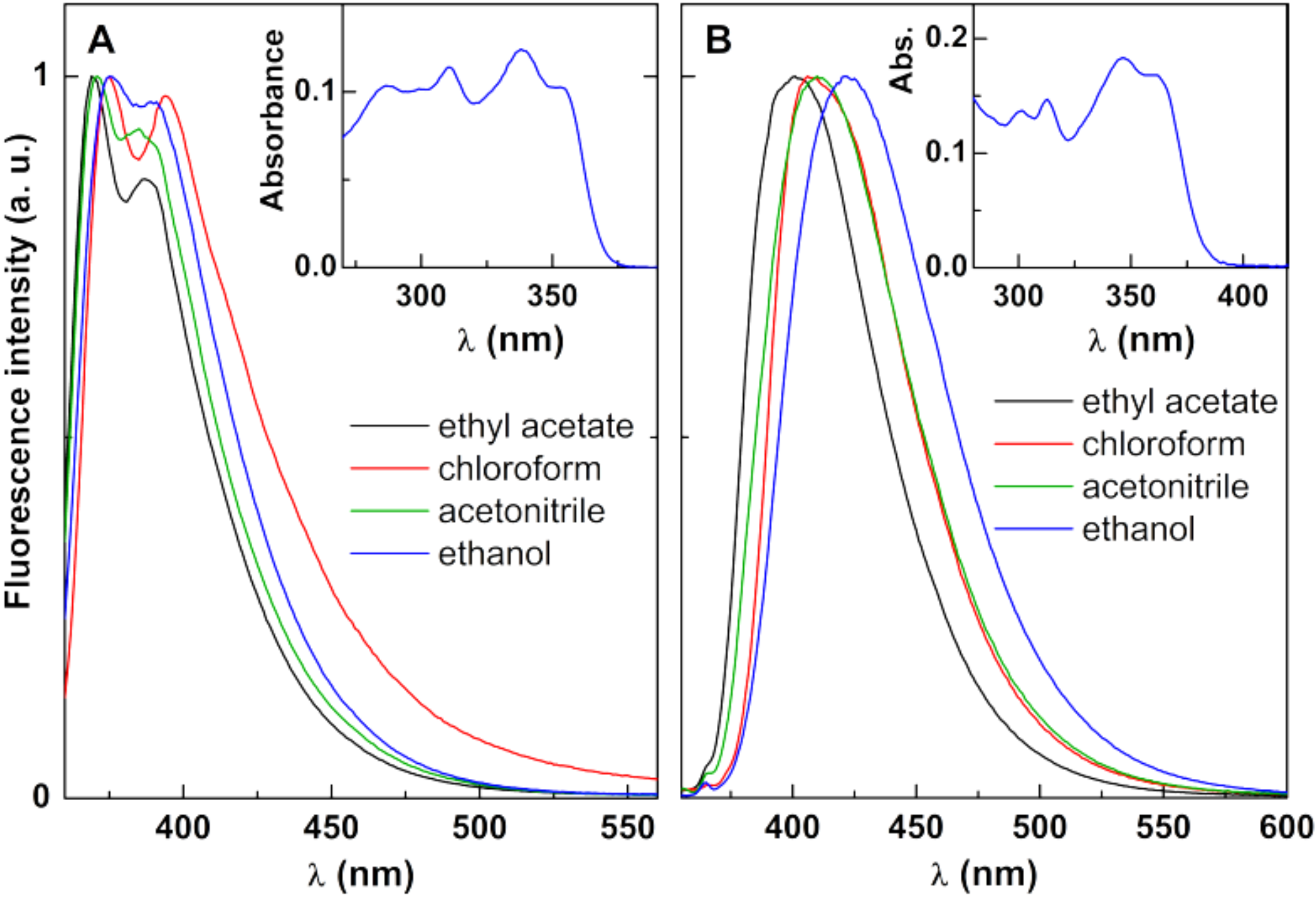
3.3.1. New Antitumor Thienopyridine Derivatives
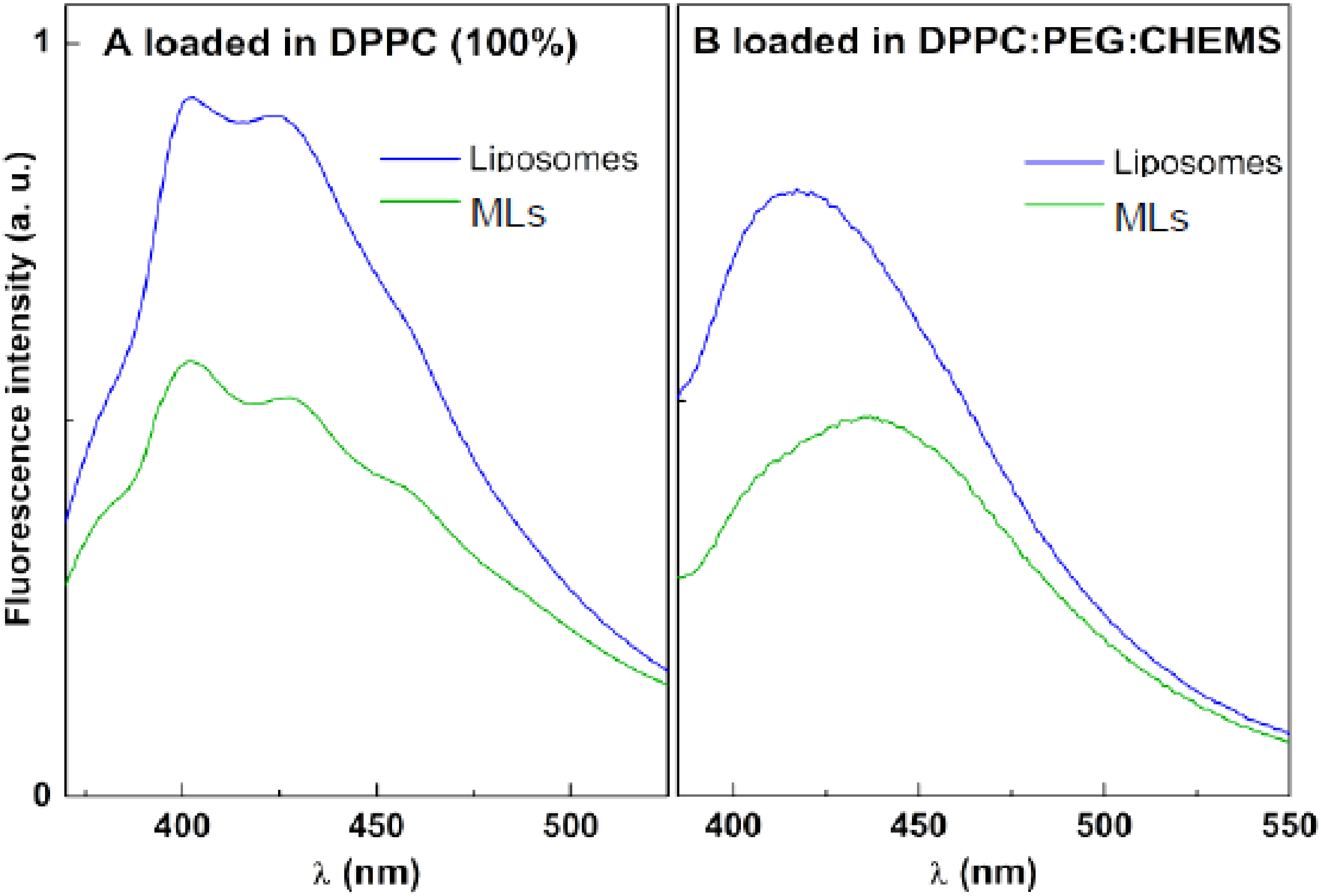
3.3.2. Magnetic Liposomes with Encapsulated Drugs
3.3.3. Förster Resonance Energy Transfer Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-responsive nano-architecture drug-delivery systems to solid tumor micromilieu: Past, present, and future perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzotta, E.; Tavano, L.; Muzzalupo, R. Thermo-Sensitive Vesicles in Controlled Drug Delivery for Chemotherapy. Pharmaceutics 2018, 10, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoee, S.; Karimi, M.R. Dual-drug loaded Janus graphene oxide-based thermoresponsive nanoparticles for targeted therapy. Polymer 2018, 42, 80–98. [Google Scholar] [CrossRef]
- Slam, K.; Haque, M.; Kumar, A.; Hoq, A.; Hyder, F.; Hoque, S.M. Manganese Ferrite Nanoparticles (MnFe2O4): Size Dependence for Hyperthermia and Negative/Positive Contrast Enhancement in MRI. Nanomaterials 2020, 10, 2297. [Google Scholar] [CrossRef]
- Loganathan, A.; Kumar, K. Effects on structural, optical, and magnetic properties of pure and Sr-substituted MgFe2O4 nanoparticles at different calcination temperatures. Appl. Nanosci. 2016, 6, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, B.D.; Rodrigues, A.R.O.; Almeida, B.G.; Amorim, C.O.; Amaral, V.S.; Castanheira, E.M.S.; Coutinho, P.J.G. Stealth Magnetoliposomes Based on Calcium-Substituted Magnesium Ferrite Nanoparticles for Curcumin Transport and Release. Int. J. Mol. Sci. 2020, 21, 3641. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Y.; Zhang, Z.; Fan, F.; Huang, C.; Lu, L.; Wang, C. Dual pH/reduction-responsive hybrid polymeric micelles for targeted chemo-photothermal combination therapy. Acta Biomater. 2018, 75, 371–385. [Google Scholar] [CrossRef]
- Gu, H.; Um, S.; Qiu, G.; Liu, X.; Zhang, L.; Yuan, Y.; Astruc, D. Redox-stimuli-responsive drug delivery systems with supramolecular ferrocenyl-containing polymers for controlled release. Coord. Chem. Rev. 2018, 364, 51–85. [Google Scholar] [CrossRef]
- Hegazy, M.; Zhou, P.; Wu, G.; Wang, L.; Rahoui, N.; Taloub, N.; Huang, Y. Construction of polymer coated core–shell magnetic mesoporous silica nanoparticles with triple responsive drug delivery. Polym. Chem. 2017, 8, 5852–5864. [Google Scholar] [CrossRef]
- Stubbs, M.; McSheehy, P.M.; Griffiths, J.R.; Bashford, C.L. Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today 2000, 6, 15–19. [Google Scholar] [CrossRef]
- Pereira Gomes, I.; Aparecida Duarte, J.; Chaves Maia, A.L.; Rubello, D.; Townsend, D.M.; Branco de Barros, A.L.; Leite, E.A. Thermosensitive Nanosystems Associated with Hyperthermia for Cancer Treatment. Pharmaceuticals 2019, 12, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafez, I.M.; Cullis, P.R. Cholesteryl hemisuccinate exhibits pH sensitive polymorphic phase behavior. Biochim. Biophys. Acta Biomembr. 2000, 1463, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.-X.; Qi, X.-R.; Li, P.; Maitani, Y.; Nagai, T. Cholesteryl hemisuccinate as a membrane stabilizer in dipalmitoylphosphatidylcholine liposomes containing saikosaponin-d. Int. J. Pharm. 2005, 300, 38–47. [Google Scholar] [CrossRef]
- Rodrigues, J.M.; Buisson, P.; Pereira, J.M.; Pinheiro, I.M.; Fernández-Marcelo, T.; Vasconcelos, M.H.; Berteina-Raboin, S.; Queiroz, M.-J.R.P. Synthesis of novel 8-(het)aryl-6H-pyrano[4′,3′:4,5]thieno[3,2-b]pyridines by 6-endo-dig cyclization of Sonogashira products and halolactonizations with Cu salts/NXS. Preliminary antitumor evaluation. Tetrahedron 2019, 75, 1387–1397. [Google Scholar] [CrossRef]
- Queiroz, M.-J.R.P.; Calhelha, R.C.; Vale-Silva, L.; Pinto, E.; Nascimento, M.S.-J. Novel [6-(hetero)arylamino]thieno[3,2-b] pyridines: Synthesis and antitumoral activities. Eur. J. Med. Chem. 2010, 45, 5732–5738. [Google Scholar] [CrossRef]
- Machado, V.A.; Peixoto, D.; Costa, R.; Froufe, H.J.C.; Calhelha, R.C.; Abreu, R.M.V.; Ferreira, I.C.F.R.; Soares, R.; Queiroz, M.-J.R.P. Synthesis, antiangiogenesis evaluation and molecular docking studies of 1-aryl-3-[(thieno[3,2-b]pyridin-7-ylthio)phenyl]ureas: Discovery of a new substitution pattern for type II VEGFR-2 Tyr kinase inhibitors. Bioorg. Med. Chem. 2015, 23, 6497–6509. [Google Scholar] [CrossRef] [Green Version]
- Machado, V.A.; Peixoto, D.; Queiroz, M.-J.R.P.; Soares, R. Antiangiogenic 1-aryl-3-[3-(thieno[3,2-b]pyridin-7-ylthio)phenyl]ureas inhibit MCF-7 and MDA-MB-231 human breast cancer cell lines through PI3K/Akt and MAPK/Erk pathways. J. Cell. Biochem. 2016, 117, 2791–2799. [Google Scholar] [CrossRef]
- Costa, C.N.C.; Hortelão, A.C.L.; Ramos, J.M.F.; Oliveira, A.D.S.; Calhelha, R.C.; Queiroz, M.-J.R.P.; Coutinho, P.J.G.; Castanheira, E.M.S. A new antitumoral heteroarylaminothieno[3,2-b]pyridine derivative: Incorporation in liposomes and interaction with proteins monitored by fluorescence. Photochem. Photobiol. Sci. 2014, 13, 1730–1740. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.R.O.; Almeida, B.A.; Rodrigues, J.M.; Queiroz, M.-J.R.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Coutinho, P.J.G.; et al. Magnetoliposomes as carriers for promising antitumor thieno[3,2-b]pyridin-7-arylamines: Photophysical and biological studies. RSC Adv. 2017, 7, 15352–15361. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.R.O.; Mendes, P.M.F.; Silva, P.M.L.; Machado, V.A.; Almeida, B.G.; Araújo, J.P.; Queiroz, M.-J.R.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Solid and aqueous magnetoliposomes as nanocarriers for a new potential drug active against breast cancer. Coll. Surf. B 2017, 158, 460–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.R.O.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetoliposomes based on nickel/silica core/shell nanoparticles: Synthesis and characterization. Mater. Chem. Phys. 2014, 148, 978–987. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.R.O.; Ramos, J.M.F.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Queiroz, M.-J.R.P.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetoliposomes based on manganese ferrite nanoparticles as nanocarriers for antitumor drugs. RSC Adv. 2016, 6, 17302–17313. [Google Scholar] [CrossRef] [Green Version]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence Principles and Applications; Wiley: Weinheim, Germany, 2002. [Google Scholar]
- Bergmann, J.; Friedel, P.; Kleeberg, R. Commission on Powder Diffraction Newsletter; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1998; pp. 5–8. [Google Scholar]
- Döbelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallog. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Ding, Z.; Zhao, X.; Wu, S.; Li, F.; Yue, M.; Liu, J.P. Microstructure and magnetic properties of MFe2O4 (M = Co, Ni, and Mn) ferrite nanocrystals prepared using colloid mill and hydrothermal method. J. Appl. Phys. 2015, 117, 17A328. [Google Scholar] [CrossRef] [Green Version]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 13, 2836–2846. [Google Scholar] [CrossRef]
- Kim, S.G.; Wang, W.N.; Iwaki, T.; Yabuki, A.; Okuyama, K. Low-Temperature Crystallization of Barium Ferrite Nanoparticles by a Sodium Citrate-Aided Synthetic Process. J. Phys. Chem. C 2007, 111, 10175–10180. [Google Scholar] [CrossRef]
- Foderà, V.; Donald, A. Tracking the heterogeneous distribution of amyloid spherulites and their population balance with free fibrils. Eur. Phys. J. E 2010, 33, 273–282. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Silva, J.F.G.; Hilliou, L.; Moura, C.; Coutinho, P.J.G.; Martins, J.A.; Testa-Anta, M.; Salgueiriño, V.; Correa-Duarte, M.A.; Ferreira, P.M.T.; et al. Impact of Citrate and Lipid-Functionalized Magnetic Nanoparticles in Dehydropeptide Supramolecular Magnetogels: Properties, Design and Drug Release. Nanomaterials 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Shen, C.; Yang, T.; Bao, L.; Tian, J.; Ding, H.; Gao, H.-J. Large-Scale Fe3O4 nanoparticle soluble in water synthesized by a facile method. J. Phys. Chem. C 2008, 112, 11336–11339. [Google Scholar] [CrossRef]
- Bamzai, K.K.; Kour, G.; Kaur, B.; Kulkarni, S.D. Preparation, and Structural and Magnetic Properties of Ca Substituted Magnesium Ferrite with Composition MgCaxFe2−xO4 (x = 0.00, 0.01, 0.03, 0.05, 0.07). J. Mater. 2014, 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Mathew, D.S.; Juang, R.-S. An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 2007, 129, 51–65. [Google Scholar] [CrossRef]
- Grimes, R.W.; Anderson, A.B.; Heuer, A.H. Predictions of Cation Distributions in AB204 Spinels from Normalized Ion Energies. J. Am. Chem. Soc. 1989, 111, 1–7. [Google Scholar] [CrossRef]
- Ahrens, L.H. The use of ionization potentials Part 1. Ionic radii of the elements. Geochim. Cosmochim. Acta 1952, 2, 155–169. [Google Scholar] [CrossRef]
- Wang, Y.; Sui, Y.; Wang, X.; Su, W.; Liu, X. Correlation of structural distortion with magnetic properties in electron-doped Ca0.9r0.1MnO3 perovskites (R = rare-earth). J. Appl. Phys. 2010, 108, 063928. [Google Scholar] [CrossRef]
- Lentz, B. Membrane ‘fluidity’ as detected by diphenylhexatriene probes. Chem. Phys. Lipids 1989, 50, 171–190. [Google Scholar] [CrossRef]
- Chu, C.-J.; Szoka, F.C. pH-sensitive liposomes. J. Liposome Res. 1994, 4, 361–395. [Google Scholar] [CrossRef]
- Massey, J.B. Effect of cholesteryl hemisuccinate on the interfacial properties of phosphatidylcholine bilayers. Biochim. Biophys. Acta 1998, 1415, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Nigam, S.; Barick, K.C.; Bahadur, D. Development of citrate-stabilized Fe3O4 nanoparticles: Conjugation and release of doxorubicin for therapeutic applications. J. Magn. Magn. Mater. 2011, 323, 237–243. [Google Scholar] [CrossRef]
- Rodrigues, A.R.O.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetic liposomes based on nickel ferrite nanoparticles for biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 18011–18021. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.; Dewangan, H.K. Pegylation: An important approach for novel drug delivery system. J. Biomater. Sci. Polym. Ed. 2021, 32, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Wawro, A.M.; Aoki, Y.; Muraoka, T. Enzymatically-cleavable traceless biotin tags for protein PEGylation and purification. Chem. Commun. (Camb.) 2018, 54, 1913–1916. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.-L.; Dastoor, P.C.; Flynn, J.; Palmer, W.; Martin, A.; Smith, D.W.; Woldu, A.; Hua, S. Advantages and Limitations of Current Imaging Techniques for Characterizing Liposome Morphology. Front. Pharmacol. 2018, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, D.M.S.; Cardoso, B.D.; Rodrigues, A.R.O.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Queiroz, M.-J.R.P.; Martinho, O.; Baltazar, F.; Calhelha, R.C.; et al. Magnetoliposomes containing calcium ferrite nanoparticles for applications in breast cancer therapy. Pharmaceutics 2019, 11, 477. [Google Scholar] [CrossRef] [Green Version]
- Janoff, A.S.; Kurtz, C.L.; Jablonski, R.L.; Minchey, S.R.; Boni, L.T.; Gruner, S.M.; Cullis, P.R.; Mayer, L.D.; Hope, M.J. Characterization of cholesterol hemisuccinate and α-tocopherol hemisucccinate vesicles. Biochim. Biophys. Acta 1988, 941, 165–175. [Google Scholar] [CrossRef]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef] [Green Version]


| HCT-15 (nM) | NCI-H460 (nM) | |
|---|---|---|
| Compound A | 5.6 ± 0.6 | >75 |
| Compound B | 10.8 ± 1.1 | 17.0 ± 1.2 |
| Doxorubicin | 353.3 ± 24.2 | 25 ± 0.8 |
| Sample | Ox,y,z (*) | i | Phase Size (nm) | Lattice Constant (nm) | Hematite (wt%) | RP | χ2 | |
|---|---|---|---|---|---|---|---|---|
| A | 0.3816 0.3819 0.3795 0.3780 | 1 0.5 (+) 0.5 (+) 0 (+) | 0 0 1 (+) 1 (+) | 7.6 7.5 7.7 7.7 | 0.8427 0.8427 0.8430 0.8531 | - | 9.19 9.20 9.47 9.48 | 1.26 1.26 1.33 1.33 |
| B | 0.3848 | 1 | 0 | 10.2 | 0.8374 | 27.3 | 9.43 | 1.57 |
| Ms (emu/g) | Mr (emu/g) | Mr/Ms | C (Oe) | |
|---|---|---|---|---|
| Citrate-stabilized Ca0.5Mn0.5Fe2O4 | 53.91 | 0.95 | 0.02 | 13.90 |
| Ca0.5Mn0.5Fe2O4 prepared by sol–gel | 26.68 | 3.88 | 0.15 | 96.77 |
| Formulation | pH | Zeta Potential (mV) |
|---|---|---|
| DPPC (100%) | 7.4 | −1.83 ± 0.65 |
| 5 | −1.75 ± 0.82 | |
| DPPC:CHEMS (80:20) | 7.4 | −26.7 ± 1.10 |
| 5 | −0.73 ± 0.9 | |
| DPPC:CHEMS:DSPE-PEG (80:20:0.4) | 7.4 | −17.0 ± 0.9 |
| 5 | −2.55 ± 0.89 |
| Formulation | Hydrodynamic Size (nm) | PdI | ||
|---|---|---|---|---|
| pH = 7.4 | pH = 5 | pH = 7.4 | pH = 5 | |
| SUVs | 96.5 ± 2.4 | 92.3 ± 12 | 0.27 ± 0.01 | 0.27± 0.01 |
| MLs (DPPC:CHEMS) | 149.9 ± 17 | 203.6 ± 10 | 0.29 ± 0.01 | 0.27 ± 0.04 |
| SUVs + MLs (DPPC:CHEMS) | 171.6 ± 2.2 | 597.3 ± 58 | 0.25 ± 0.01 | 0.29 ± 0.04 |
| MLs (DPPC:CHEMS:DSPE-PEG) | 213.2 ± 1.1 | 225.6 ± 19 | 0.24 ± 0.001 | 0.28 ± 0.002 |
| SUVs + MLs (DPPC:CHEMS:DSPE-PEG) | 336.1 ± 121 | 376.1 ± 66 | 0.24 ± 0.027 | 0.25± 0.050 |
| Solvent | λabs/nm (ε/105 M−1 cm−1) | λem/nm | ΦF | |||
|---|---|---|---|---|---|---|
| Compound A | Compound B | Compound A | Compound B | Compound A | Compound B | |
| Ethyl acetate | 338 (1.37) | 348 (2.07) | 371; 387 | 401 | 0.016 | 0.05 |
| Chloroform | 338 (1.39) | 352 (1.73) | 375; 392 | 406 | 0.020 | 0.08 |
| Acetonitrile | 338 (1.27) | 348 (1.79) | 371; 385 (sh) | 410 | 0.017 | 0.05 |
| Ethanol | 338 (1.24) | 350 (1.76) | 375; 389 (sh) | 421 | 0.018 | 0.10 |
| System | Formulation | Compound A | Compound B |
|---|---|---|---|
| MLs | DPPC | 0.08 (25 °C) 0.06 (55 °C) | 0.03 (25 °C) 0.02 (55 °C) |
| DPPC:CHEMS | 0.14 (25 °C) 0.10 (55 °C) | 0.14 (25 °C) 0.11 (55 °C) | |
| DPPC:CHEMS: DSPE-PEG | 0.11 (25 °C) 0.07 (55 °C) | 0.14 (25 °C) 0.12 (55 °C) | |
| Glycerol | - | 0.33 (25 °C) | 0.30 (25 °C) |
| Nanosystem | Formulation | Compound A | Compound B |
|---|---|---|---|
| MLs | DPPC | 99.1 ± 0.2 | 89.0 ± 3.0 |
| DPPC:CHEMS | 98.6 ± 0.1 | 88.4 ± 2.8 | |
| DPPC:CHEMS:DSPE-PEG | 98.2 ± 0.2 | 91.2 ± 0.3 |
| Formulation | pH | Temperature (°C) | |
|---|---|---|---|
| DPPC:CHEMS | 7.4 | 25 | 10.9 |
| 45 | 22.5 | ||
| 5 | 25 | 23.5 | |
| 45 | 26.6 | ||
| DPPC:CHEMS:DSPE-PEG | 7.4 | 25 | 18.4 |
| 45 | 26.9 | ||
| 5 | 25 | 28.9 | |
| 45 | 35.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, B.C.; Alvarez, C.A.R.; Alves, B.C.; Rodrigues, J.M.; Queiroz, M.J.R.P.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Coutinho, P.J.G.; et al. Development of Thermo- and pH-Sensitive Liposomal Magnetic Carriers for New Potential Antitumor Thienopyridine Derivatives. Materials 2022, 15, 1737. https://doi.org/10.3390/ma15051737
Ribeiro BC, Alvarez CAR, Alves BC, Rodrigues JM, Queiroz MJRP, Almeida BG, Pires A, Pereira AM, Araújo JP, Coutinho PJG, et al. Development of Thermo- and pH-Sensitive Liposomal Magnetic Carriers for New Potential Antitumor Thienopyridine Derivatives. Materials. 2022; 15(5):1737. https://doi.org/10.3390/ma15051737
Chicago/Turabian StyleRibeiro, Beatriz C., Cristina A. R. Alvarez, Bárbara C. Alves, Juliana M. Rodrigues, Maria João R. P. Queiroz, Bernardo G. Almeida, Ana Pires, André M. Pereira, João P. Araújo, Paulo J. G. Coutinho, and et al. 2022. "Development of Thermo- and pH-Sensitive Liposomal Magnetic Carriers for New Potential Antitumor Thienopyridine Derivatives" Materials 15, no. 5: 1737. https://doi.org/10.3390/ma15051737







