Nickel Oxide Films Deposited by Sol-Gel Method: Effect of Annealing Temperature on Structural, Optical, and Electrical Properties
Abstract
:1. Introduction
2. Materials and Methods
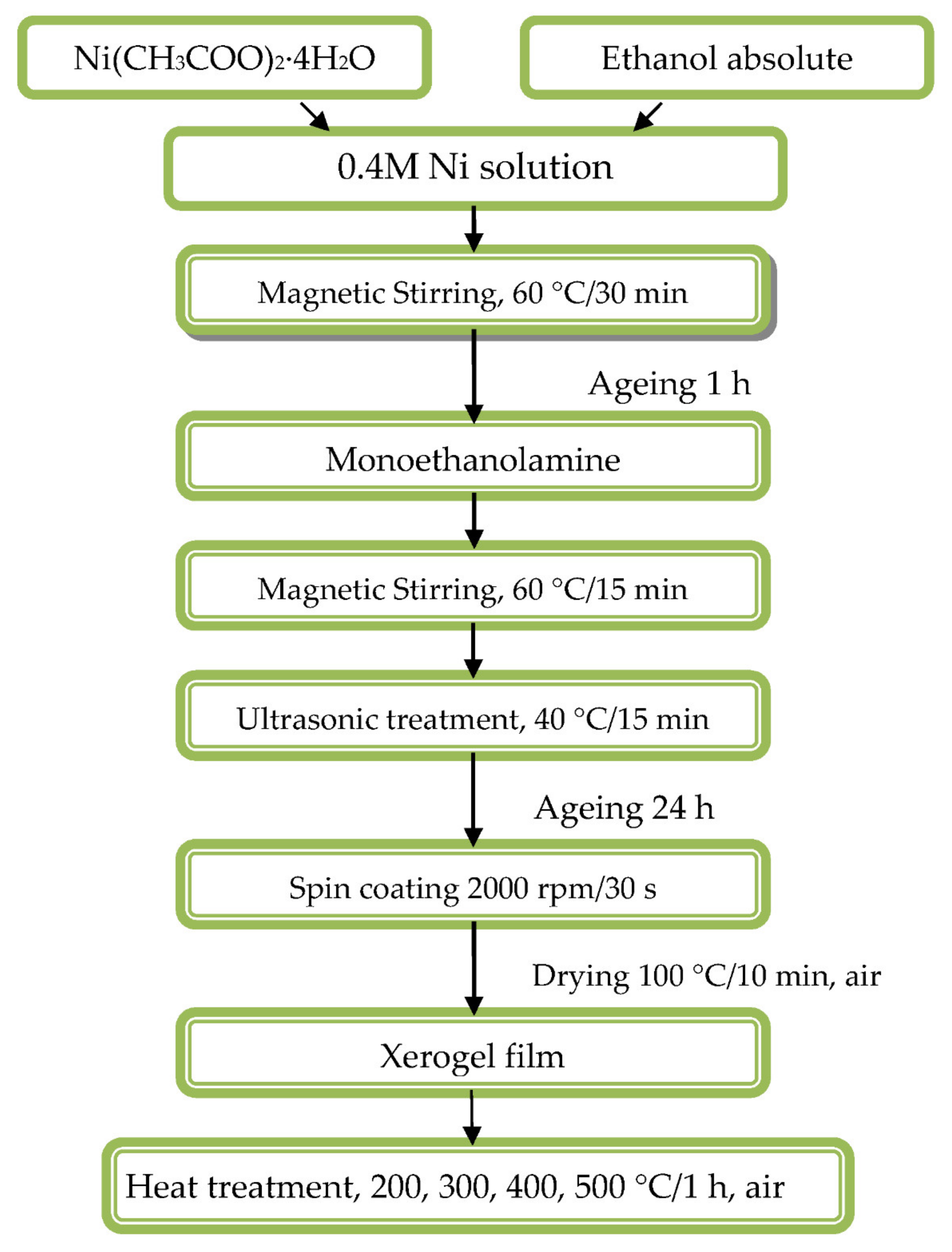
2.1. Thin Films Preparation
2.2. Thin Film Characterization
3. Results and Discussions
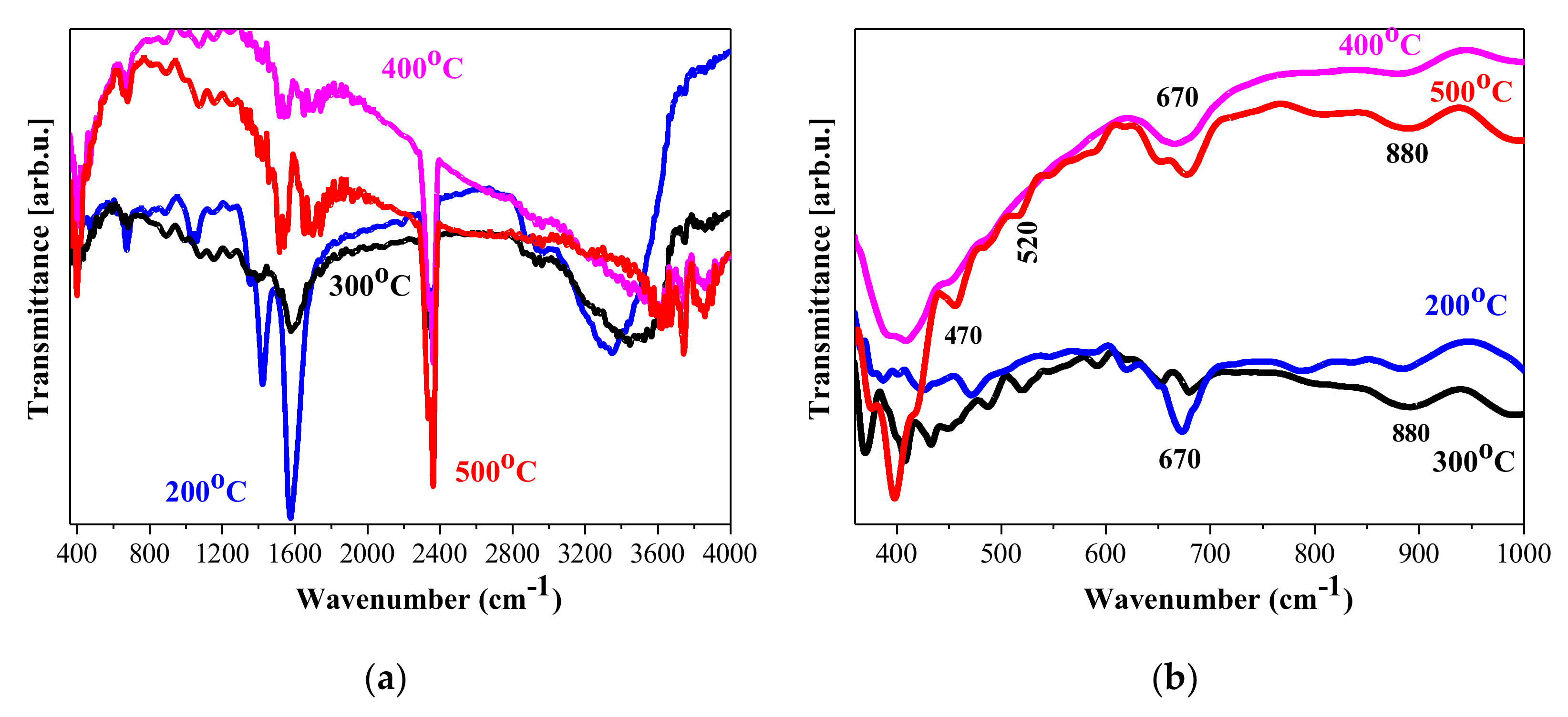
3.1. IR Study
3.2. Chemical States of NiOx Thin Films
3.3. Thickness of the Films
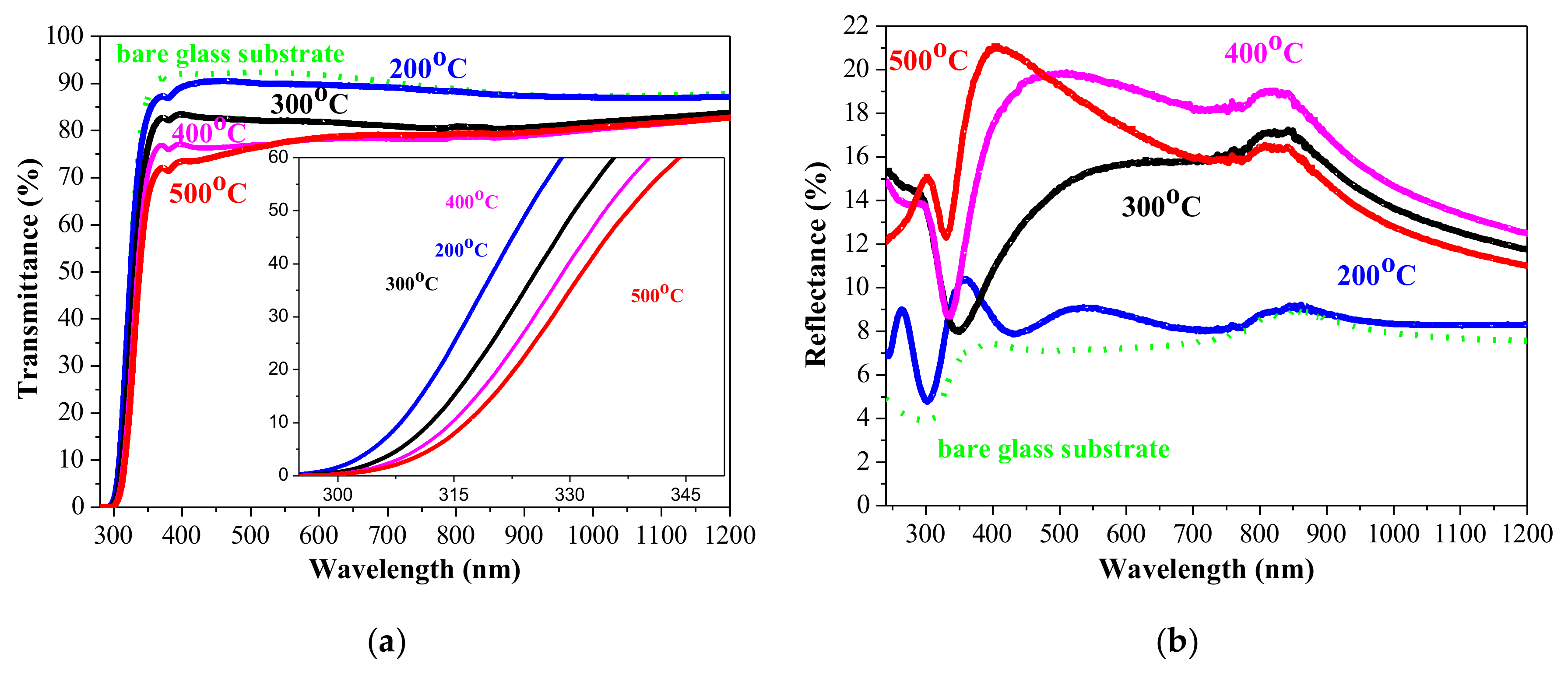
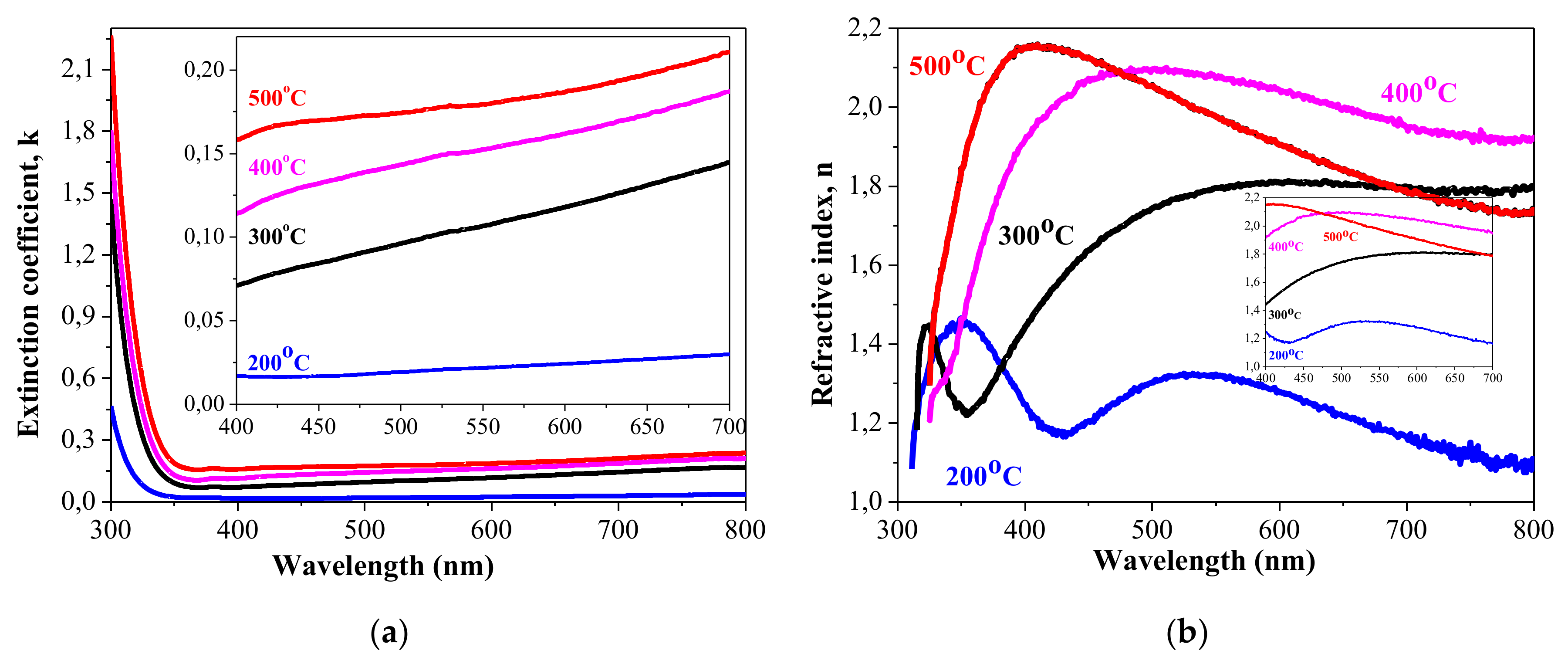
3.4. Optical Properties
3.5. Electrical Characterization
4. Conclusions
- XPS analysis showed that NiOx films annealed at the lowest annealing temperature may consist of Ni hydroxides and the formation of the NiO phase is proved after 400 °C treatment.
- XPS analysis revealed that the higher annealing results in the cubic NiO phase (Ni2+ states) with presence Ni3+ states, which contribute to p-type conductivity of the films.
- FTIR measurements also confirmed conclusions that the sol-gel NiOx film, annealed at 200 °C, consisted of other Ni oxide phases not cubic NiO with presence of hydroxyl and carbon species.
- The transparency of NiOx films is high, with a slight decrease with annealing with an extinction coefficient below 0.03 (400–700 nm) of 200 °C annealed film and below 0.21 for NiOx films, treated at 300–500 °C in the visible spectral range. For NiOx film annealed at 200 °C, the refractive index is found to be in the range of 1.18–1.33. For NiOx film treated at 400 °C, it varies from 1.91–2.10. These values are determined for the spectral range 400–700 nm.
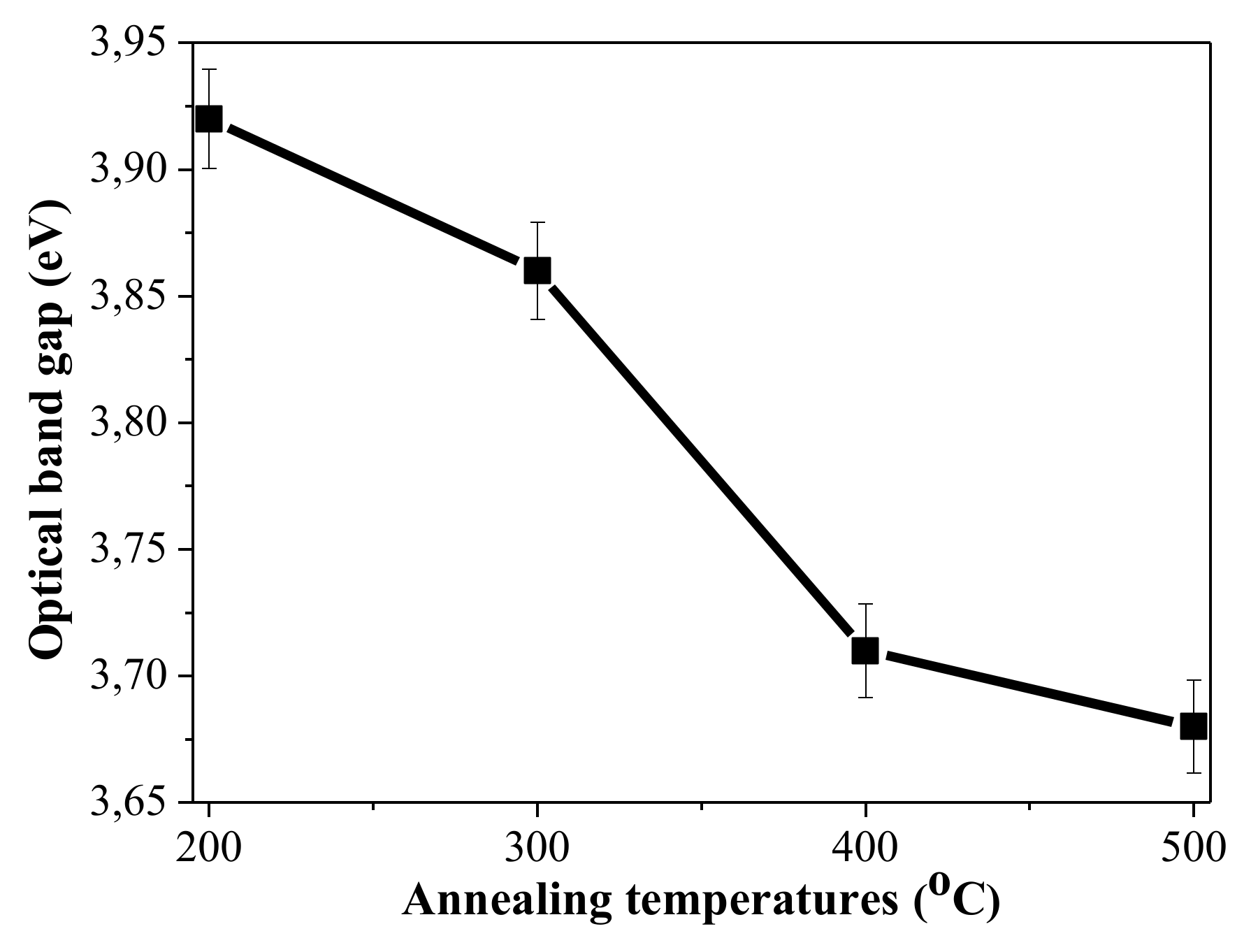
- The optical band gap energy of sol–gel NiOx films decreases from 3.92 eV to 3.68 eV with increase in annealing temperature. These values coincide with the reported values for Ni oxide films.
- NiOx thin films obtained by the sol-gel method are proven to possess p-type electrical conductivity. The 70-nm-thick layers have p-type conductivity and sheet resistance of 690 Ω/sq, which corresponds to a specific resistivity of about 4.8 × 10−3 Ω·cm. It was shown that at temperatures above 300 °C, NiOx thin films with good optical and electrical properties are obtained. Based on the research of the optical and electrical properties of NiOx, it was found that the quality of the NiOx films is comparable to that of films prepared by more complicated and expensive techniques such as rf magnetron sputtering, electron beam evaporation, MBE, etc.
- The advantages of the sol-gel process are low cost, easy fabrication, long-term stability, and that they are suitable for use in large areas. The applied technological approach is compatible with that of deposition of zinc oxide, which implies successful integration of sol-gel processes for the realization of visible-light transparent solar cells.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aftab, M.; Butt, M.Z.; Ali, D.; Bashir, F.; Khan, T.M. Optical and electrical properties of NiO and Cu-doped NiO thin films synthesized by spray pyrolysis. Opt. Mater. 2021, 119, 111369. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Sayed, M.H.; Patil, V.L.; Boshta, M.; Patil, P.S. Gas sensing performance of sprayed NiO thin films toward NO2 gas. J. Alloys Compd. 2021, 885, 160908. [Google Scholar] [CrossRef]
- Kawade, D.; Chichibu, S.F.; Sugiyama, M. Experimental determination of band offsets of NiO-based thin film heterojunctions. J. Appl. Phys. 2014, 116, 163108. [Google Scholar] [CrossRef]
- Jin, S.; Wen, S.; Li, M.; Zhong, H.; Chen, Y.; Wang, X. Effect of the grain size on the electrochromic properties of NiO films. Opt. Mater. 2020, 109, 110280. [Google Scholar] [CrossRef]
- Faria, I.C.; Kleinke, M.; Gorenstein, A.; Fantini, M.C.A.; Tabacniks, M.H. Toward Efficient Electrochromic NiOx Films: A Study of Microstructure, Morphology, and Stoichiometry of Radio Frequency Sputtered Films. J. Electrochem. Soc. 1998, 145, 235–240. [Google Scholar] [CrossRef]
- Mihelcic, M.; Vuk, A.Š.; Jerman, I.; Orel, B.; Švegl, F.; Moulki, H.; Faure, C.; Campet, G.; Rougier, A. Comparison of electrochromic properties of Ni1−xO in lithium and lithium-free aprotic electrolytes: From Ni1−xO pigment coatings to flexible electrochromic devices. Sol. Energy Mater. Sol. Cells 2014, 120, 116–130. [Google Scholar] [CrossRef]
- Guo, X.; Wang, W.; Wen, R.-T. Enhanced electrochromic performance by anodic polarization in nickel oxide films. Crystals 2021, 11, 615. [Google Scholar] [CrossRef]
- Jabeen, S.; Iqbal, J.; Arshad, A.; Williams, J.; Samarin, S.; Rani, M. Heterojunction formation in In2O3–NiO nanocomposites: Towards high specific capacitance. J. Alloys Compd. 2020, 842, 155840. [Google Scholar] [CrossRef]
- Kim, S.K.; Seok, H.J.; Kim, D.H.; Choi, D.H.; Nam, S.J.; Kim, S.C.; Kim, H.K. Comparison of NiOx thin film deposited by spin-coating or thermal evaporation for application as a hole transport layer of perovskite solar cells. RSC Adv. 2020, 10, 43847–43852. [Google Scholar] [CrossRef]
- Kaya, D.; Aydınoğlu, H.S.; Tüzemen, E.Ş.; Ekicibile, A. Investigation of optical, electronic, and magnetic properties of p-type NiO thin film on different substrates. Thin Solid Films 2021, 732, 138800. [Google Scholar] [CrossRef]
- Sahoo, P.; Sharma, A.; Padhan, S.; Thangavel, R. Cu doped NiO thin film photocathodes for enhanced PEC performance. Superlattices Microstruct. 2021, 15, 107050. [Google Scholar] [CrossRef]
- Dawson, J.A.; Guo, Y.; Robertson, J. Energetics of intrinsic defects in NiO and the consequences for its resistive random access memory performance. Appl. Phys. Lett. 2015, 107, 122110. [Google Scholar] [CrossRef]
- Klochko, N.P.; Kopach, V.R.; Tyukhov, I.I.; Zhadan, D.O.; Klepikova, K.S.; Khrypunov, G.S.; Petrushenko, S.I.; Lyubov, V.M.; Kirichenko, M.V.; Dukarov, S.V.; et al. Metal oxide heterojunction (NiO/ZnO) prepared by low temperature solution growth for UV-photodetector and semi-transparent solar cell. Sol. Energy 2018, 164, 149–159. [Google Scholar] [CrossRef]
- Kumar, N.; Nguyen, T.T.; Park, H.-H.; Lee, K.; Kim, S.M.; Kim, J. Role of substrate architecture and modelling on photocurrent and photovoltage in TiO2/NiO transparent photovoltaic. Mater. Res. Bull. 2021, 142, 111421. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Patel, M.; Kim, S.; Mir, R.A.; Yi, J.; Dao, V.-A.; Kim, J. Transparent photovoltaic cells and self-powered photodetectors by TiO2/NiO heterojunction. J. Power Sources 2021, 481, 228865. [Google Scholar] [CrossRef]
- Karsthof, R.; Racke, P.; von Wenckstern, H.; Grundmann, M. Semi-transparent NiO/ZnO UV photovoltaic cells. Phys. Status Solidi A 2016, 213, 30–37. [Google Scholar] [CrossRef]
- Weng, S.; Tamang, A.; Salleo, A.; Fujiwara, H.; Nakamura, M.; Zhang, Y.; Knip, D. Band-gap-engineered transparent perovskite solar modules to combine photovoltaics with photosynthesis. ACS Appl. Mater. Interfaces 2021, 13, 39230–39238. [Google Scholar] [CrossRef]
- Aivalioti, C.; Papadakis, A.; Manidakis, E.; Kayambaki, M.; Androulidaki, M.; Tsagaraki, K.; Pelekanos, N.T.; Stoumpos, C.; Modreanu, M.; Craciun, G. Transparent all-oxide hybrid NiO:N/TiO2 heterostructure for optoelectronic applications. Electronics 2021, 10, 988. [Google Scholar] [CrossRef]
- Bonomo, M. Synthesis and characterization of NiO nanostructures: A review. J. Nanopart. Res. 2018, 20, 222. [Google Scholar] [CrossRef]
- Napari, M.; Huq, T.N.; Hoye, R.L.Z.; MacManus-Driscoll, J.L. Nickel oxide thin films grown by chemical deposition techniques: Potential and challenges in next-generation rigid and flexible device applications. InfoMat 2021, 3, 536–576. [Google Scholar] [CrossRef]
- Chtouki, T.; El Mrabet, M.; Tarbi, A.; Goncharova, I.; Erguig, H. Comprehensive review of the morphological, linear and nonlinear optical characterization of spin-coated NiO thin films for optoelectronic applications. Opt. Mater. 2021, 118, 111294. [Google Scholar] [CrossRef]
- Arunodaya, J.; Sahoo, T. Effect of Li doping on conductivity and band gap of nickel oxide thin film deposited by spin coating technique. Mater. Res. Express 2019, 7, 016405. [Google Scholar]
- Patel, M.; Kim, H.-S.; Kim, J.; Yun, J.-H.; Kim, S.J.; Choi, E.H.; Park, H.H. Excitonic metal oxide heterojunction (NiO/ZnO) solar cells for all-transparent module integration. Sol. Energy Mater. Sol. Cells 2017, 170, 246–253. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, D.; Yang, P.; Yang, L.; Qin, L.; Huang, Y.; Zhao, X. Sol-gel-processed yttrium-doped NiO as hole transport layer in inverted perovskite solar cells for enhanced performance. Appl. Surf. Sci. 2018, 441, 258–264. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films. J. Non Cryst. Solids 2003, 6, 309–319. [Google Scholar] [CrossRef]
- Kalam, A.; Al-Shihri, A.S.; Al-Sehemi, A.G.; Awwad, N.S.; Du, G.; Ahmad, T. Effect of pH on solvothermal synthesis of β-Ni(OH)2 and NiO nano-architectures: Surface area studies, optical properties and adsorption studies. Superlattices Microstruct. 2013, 55, 83–97. [Google Scholar] [CrossRef]
- Kaspar, J.; Bazarjani, M.S.; Schitco, C.; Gurlo, A.; Graczyk-Zajac, M.; Riedel, R. Electrochemical study of NiO nanosheets: Toward the understanding of capacity fading. J. Mater. Sci. 2017, 52, 6498–6505. [Google Scholar] [CrossRef]
- El-Kemary, M.; Nagy, N.; El-Mehasse, I. Nickel oxide nanoparticles: Synthesis and spectral studies of interactions with glucose. Mater. Sci. Semicond. Process. 2013, 16, 1747–1752. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Oluwafemi, O.S.; Joshua, A.O.; Makinde, A.O.; Aderemi, O.; Ogunfowokan, A.O.; Eleruja, M.A.; Ebenso, E.E. Comparative catalytic properties of Ni(OH)2 and NiO nanoparticles towards the degradation of nitrite (NO2-) and Nitric Oxide (NO). Int. J. Electrochem. Sci. 2014, 9, 3008–3021. [Google Scholar]
- Shruthi, B.; Madhu, B.J.; Bheema Raju, V.; Vynatheya, S.; Veena Devi, B.; Jayashree, G.V.; Ravikumar, C.R. Synthesis, spectroscopic analysis and electrochemical performance of modified OI-nickel hydroxide electrode with CuO. J. Sci. Adv. Mater. Dev. 2017, 2, 93–98. [Google Scholar]
- Govindarajan, B.; Palanimuthu, R.; Manikandan, K.M. Influence of Mg doping in magnetic properties of NiO nanoparticles and its electrical applications. J. Mater. Sci. Mater. Electron. 2019, 30, 6519–6527. [Google Scholar] [CrossRef]
- Shaid, M.S.H.M.; Zaini, M.A.A.; Nasri, N.S. Evaluation of methylene blue dye and phenol removal onto modified CO2-activated pyrolysis tyre powder. J. Clean. Prod. 2019, 223, 487–498. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, J.; Ren, H.; Cui, Z.; Sun, D. Influence of OH− and SO42− anions on morphologies of the nanosized nickel hydroxide. J. Phys. Chem. C 2010, 114, 12110–12116. [Google Scholar] [CrossRef]
- Ghalmi, Y.; Habelhames, F.; Sayah, A.; Bahloul, A.; Nessark, B.; Shalabi, M.; Nunzi, J.M. Capacitance performance of NiO thin films synthesized by direct and pulse potentiostatic methods. Ionics 2019, 25, 6025–6033. [Google Scholar] [CrossRef]
- Sharma, P.K.; Singh, M.K.; Sharma, G.D.; Agrawal, A. NiO nanoparticles: Facile route synthesis, characterization and potential towards third generation solar cell. Mater. Today Proc. 2021, 43, 3061–3065. [Google Scholar] [CrossRef]
- Biju, V.; Khadar, M.A. Fourier transform infrared spectroscopy study of nanostructured nickel oxide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2003, 59, 121–134. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. R. Soc. A 2015, 471, 20140792. [Google Scholar]
- Salunkhe, P.; Ali, A.V.M.; Kekuda, D. Structural, spectroscopic and electrical properties of dc magnetron sputtered NiO thin films and an insight into different defect states. Appl. Phys. A 2021, 127, 390. [Google Scholar] [CrossRef]
- Koshtyal, Y.; Nazarov, D.; Ezhov, I.; Mitrofanov, I.; Kim, A.; Rymyantsev, A.; Lyutakov, O.; Popovich, A.; Maximov, M. Atomic Layer Deposition of NiO to Produce Active Material for Thin-Film Lithium-Ion Batteries. Coatings 2019, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Dedova, T.; Asik, I.O.; Danilson, M.; Krunks, M. Nickel oxide films by chemical spray: Effect of deposition temperature and solvent type on structural, optical, and surface properties. Appl. Surf. Sci. 2021, 548, 149118. [Google Scholar] [CrossRef]
- Tran, M.H.; Park, B.J.; Yoon, H.H. A highly active Ni-based anode material for urea electrocatalysis by a modified sol–gel method. J. Colloid Interface Sci. 2020, 578, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Acuña, J.R.A.; Perez, I.; Sosa, V.; Gamboa, F.; Elizalde, J.T.; Farías, R.; Carrillo, D.; Enríquez, J.L.; Burrola, A.; Mani, P. Sputtering power effects on the electrochromic properties of NiO films. Optik 2021, 231, 166509. [Google Scholar] [CrossRef]
- Seo, Y.-H.; Cho, I.H.; Na, S.-I. Investigation of sol-gel and nanoparticle-based NiOx hole transporting layer for high-performance planar perovskite solar cells. J. Alloys Comp. 2019, 797, 1018–1024. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, J.; Chen, M.; Guo, T.; Li, F. Inkjet-printed p-type nickel oxide thin-film transistor. Appl. Surf. Sci. 2018, 441, 295–302. [Google Scholar] [CrossRef]
- Křepelová, A.; Newberg, J.; Huthwelker, T.; Bluhm, H.; Ammann, M. The nature of nitrate at the ice surface studied by XPS and NEXAFS. Phys. Chem. Chem. Phys. 2010, 12, 8870–8880. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-H.; Nam, S.-H.; Gil, Y.E.; Boo, J.-H. The effect of ammonia concentration on the microstructure and electrochemical properties of NiO nanoflakes array prepared by chemical bath deposition. Appl. Surf. Sci. 2020, 532, 147441. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Tong, Y.; Sun, Y.; Fang, X.; Xu, J.; Wang, X. Tuning Ni3+ quantity of NiO via doping of cations with varied valence states: The key role of Ni3+ on the reactivity. Appl. Surf. Sci. 2021, 550, 149316. [Google Scholar] [CrossRef]
- Weidler, N.; Schuch, J.; Knaus, F.; Stenner, P.; Hoch, S.; Maljusch, A.; Schäfer, R.; Kaiser, B.; Jaegermann, W. X-ray Photoelectron Spectroscopic Investigation of Plasma-Enhanced Chemical Vapor Deposited NiOx, NiOx(OH)y, and CoNiOx(OH)y: Influence of the Chemical Composition on the Catalytic Activity for the Oxygen Evolution Reaction. J. Phys. Chem. C 2017, 121, 6455–6465. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, G.; Wei, Y.; Zheng, J.; Xu, C. Enhanced electrochromic performances and cycle stability of NiO-based thin films via Li–Ti co-doping prepared by sol–gel method. Electrochim. Acta 2015, 186, 182–191. [Google Scholar] [CrossRef]
- Chia-Ching, W.; Cheng-Fu, Y. Investigation of the properties of nanostructured Li-doped NiO films using the modified spray pyrolysis method. Nanoscale Res. Lett. 2013, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Salunkhe, P.; Ali, M.A.V.; Kekuda, D. Investigation on tailoring physical properties of nickel oxide thin films grown by dc magnetron sputtering. Mater. Res. Express 2020, 7, 016427. [Google Scholar] [CrossRef]
- Kumar, N.; Lee, H.B.; Hwang, S.; Kang, J.W. Large-area, green solvent spray deposited nickel oxide films for scalable fabrication of triple-cation perovskite solar cells. J. Mater. Chem. A 2020, 8, 3357–3368. [Google Scholar] [CrossRef]
- Ukoba, K.O.; Eloka-Eboka, A.C.; Inambao, F.L. Review of nanostructured NiO thin film deposition using the spray pyrolysis technique. Renew. Sustain. Energy Rev. 2018, 82, 2900–2915. [Google Scholar] [CrossRef]
- Shkir, M.; Ganesh, V.; AlFaify, S.; Yahia, I.S.; Zahran, H.Y. Tailoring the linear and nonlinear optical properties of NiO thin films through Cr3+ doping. J. Mater. Sci. Mater. Electron. 2018, 29, 6446–6457. [Google Scholar] [CrossRef]
- Aydin, H.; Mansour, S.A.; Aydin, C.; Al-Ghamdi, A.A.; Al-Hartomy, O.A.; El-Tantawy, F.; Yakuphanoglu, F. Optical properties of nanostructure boron doped NiO thin films. J. Solgel Sci. Technol. 2012, 64, 728–733. [Google Scholar] [CrossRef]
- Nalage, S.R.; Chougule, M.A.; Sen, S.; Joshi, P.B.; Patil, V.B. Sol–gel synthesis of nickel oxide thin films and their characterization. Thin Solid Films 2012, 520, 4835–4840. [Google Scholar] [CrossRef]
- Usha, K.S.; Sivakumar, R.; Sanjeeviraja, C. Optical constants and dispersion energy parameters of NiO thin films prepared by radio frequency magnetron sputtering technique. J. Appl. Phys. 2013, 114, 123501. [Google Scholar] [CrossRef]
- Emam-Ismail, M.; El-Hagary, M.; El-Sherif, H.M.; El-Naggar, A.M.; El-Nahass, M.M. Spectroscopic ellipsometry and morphological studies of nanocrystalline NiO and NiO/ITO thin films deposited by e-beams technique. Opt. Mater. 2021, 112, 110763. [Google Scholar] [CrossRef]
- Mahmoud, S.A.; Alshomer, S.; Tarawnh, M.A. Structural and optical dispersion characterization of sprayed nickel oxide thin films. J. Modern Phys. 2011, 2, 1178–1186. [Google Scholar] [CrossRef] [Green Version]
- Rashad, M.; Darwish, A.A.A.; Qashou, S.I.; Abd El-Rahman, K.F. Influence of ultraviolet irradiation on physical properties of nano-NiO films for optical applications. Appl. Phys. A 2020, 126, 862. [Google Scholar] [CrossRef]
- Dutta, T.; Gupta, P.; Gupta, A.; Titas, J.N. High work function (p-type NiO1+ x)/Zn0. 95Ga0.05O heterostructures for transparent conducting oxides. J. Phys. D Appl. Phys. 2010, 43, 105301. [Google Scholar] [CrossRef]
- Yip, H.L.; Jen, A.K.-Y. Recent advances in solution-processed interfacialmaterials for efficient and stable polymer solar cells. Energy Environ. Sci. 2012, 5, 5994–6011. [Google Scholar] [CrossRef]
- Greiner, M.T.; Helander, M.G.; Wang, Z.-B.; Tang, W.-M.; Lu, Z.-H. Effects of processing conditions on the work function and energy-level alignment of NiO thin films. J. Phys. Chem. C 2010, 114, 19777–19781. [Google Scholar] [CrossRef]
- Tyagi, M.; Tomar, M.; Gupta, V. Post deposition annealing of NiOx thin films: A transition from n-type to p-type conductivity for short wave length optoelectronic devices. J. Mater. Res. 2013, 28, 723–732. [Google Scholar] [CrossRef]
- Jlassi, M.; Sta, I.; Hajji, M.; Ezzaouia, H. Optical and electrical properties of nickel oxide thin films synthesized by sol–gel spin coating. Mater. Sci. Semicond. Process. 2014, 21, 7–13. [Google Scholar] [CrossRef]
- Menchini, F.; Grilli, M.L.; Dikonimos, T.; Mittiga, A.; Serenelli, L.; Salza, E.; Chierchia, R.; Tucci, M. Application of NiOx thin films as p-type emitter layer in heterojunction solar cells. Phys. Status Solidi C 2016, 13, 1006–1010. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, H.C. Nickel vacancy behavior in the electrical conductance of nonstoichiometric nickel oxide film. J. Appl. Phys. 2012, 112, 034504. [Google Scholar] [CrossRef]
- Jang, W.L.; Lu, Y.M.; Hwang, W.S.; Hsiung, T.L.; Wang, H.P. Point defects in sputtered NiO films. Appl. Phys. Lett. 2009, 94, 062103. [Google Scholar] [CrossRef]

| Component | Atomic % | Mass Conc. % | Ni/O Atomic % |
|---|---|---|---|
| NiOx films, annealed at 200 °C | |||
| O 1s | 74.8 | 48.3 | 0.27 |
| Ni 2p | 20.0 | 47.4 | |
| NiOx films, annealed at 400 °C | |||
| O 1s | 56.4 | 26.1 | 0.76 |
| Ni 2p | 42.9 | 72.9 | |
| Annealing Temperature [°C] | Film Thickness [nm] | Refractive Index NiOx/Si, Reflectometry | Refractive Index NiOx/Glass, from R, T Data |
|---|---|---|---|
| 200 | 213 | 1.55 | 1.24 |
| 300 | 81 | 1.86 | 1.81 |
| 400 | 73 | 1.90 | 2.02 |
| 500 | 62 | 1.90 | 1.86 |
| Optical Band Gap [eV] | Refractive Index | Spectral Range | Deposition Method | Reference |
|---|---|---|---|---|
| 3.572 | 2.15 | Average, 280–900 nm | Spray pyrolisis | [1] |
| 3.63 | - | - | Thermal evaporation | [10] |
| 3.40 | (aqueous solution) | Chemical spray | [40] | |
| 4.00 | (alcoholic solution) | |||
| 3.25–4.00 | 1.871 | 550 nm | Spray pyrolisis | [54] review |
| 3.78–3.85 | ˃2 | 450–800 nm | Sol-gel | [55] |
| 3.73 | 1.82–1.42 | 300–800 nm | Sol-gel | [56] |
| 3.47–3.86 | Decrease with annealing | Sol-gel | [57] | |
| 3.12–3.93 | 2.0–1.4 | 300–1000 nm | Magnetron sputtering | [58] |
| 3.954 | 2.2–1.9 | 400–1000 nm | E-beam technique | [59] |
| 3.14, 3.83 | 2.8–1.8 | 300–1000 nm | Spray pyrolisis | [60] |
| 3.50–3.81 | 2.10–1.45 | 300–1000 nm | Wet method | [61] |
| Annealing Temperature [°C] | Workfunction [eV] |
|---|---|
| 200 | 4.44 |
| 300 | 5.29 |
| 400 | 5.25 |
| 500 | 5.24 |
| Annealing Temperature [°C] | Sheet Resistance [Ω/sq] |
|---|---|
| 200 | - |
| 300 | 689 |
| 400 | 690 |
| 500 | 689 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, T.; Harizanova, A.; Shipochka, M.; Vitanov, P. Nickel Oxide Films Deposited by Sol-Gel Method: Effect of Annealing Temperature on Structural, Optical, and Electrical Properties. Materials 2022, 15, 1742. https://doi.org/10.3390/ma15051742
Ivanova T, Harizanova A, Shipochka M, Vitanov P. Nickel Oxide Films Deposited by Sol-Gel Method: Effect of Annealing Temperature on Structural, Optical, and Electrical Properties. Materials. 2022; 15(5):1742. https://doi.org/10.3390/ma15051742
Chicago/Turabian StyleIvanova, Tatyana, Antoaneta Harizanova, Maria Shipochka, and Petko Vitanov. 2022. "Nickel Oxide Films Deposited by Sol-Gel Method: Effect of Annealing Temperature on Structural, Optical, and Electrical Properties" Materials 15, no. 5: 1742. https://doi.org/10.3390/ma15051742





