Finite Element Analysis of Silver Nanorods, Spheres, Ellipsoids and Core–Shell Structures for Hyperthermia Treatment of Cancer
Abstract
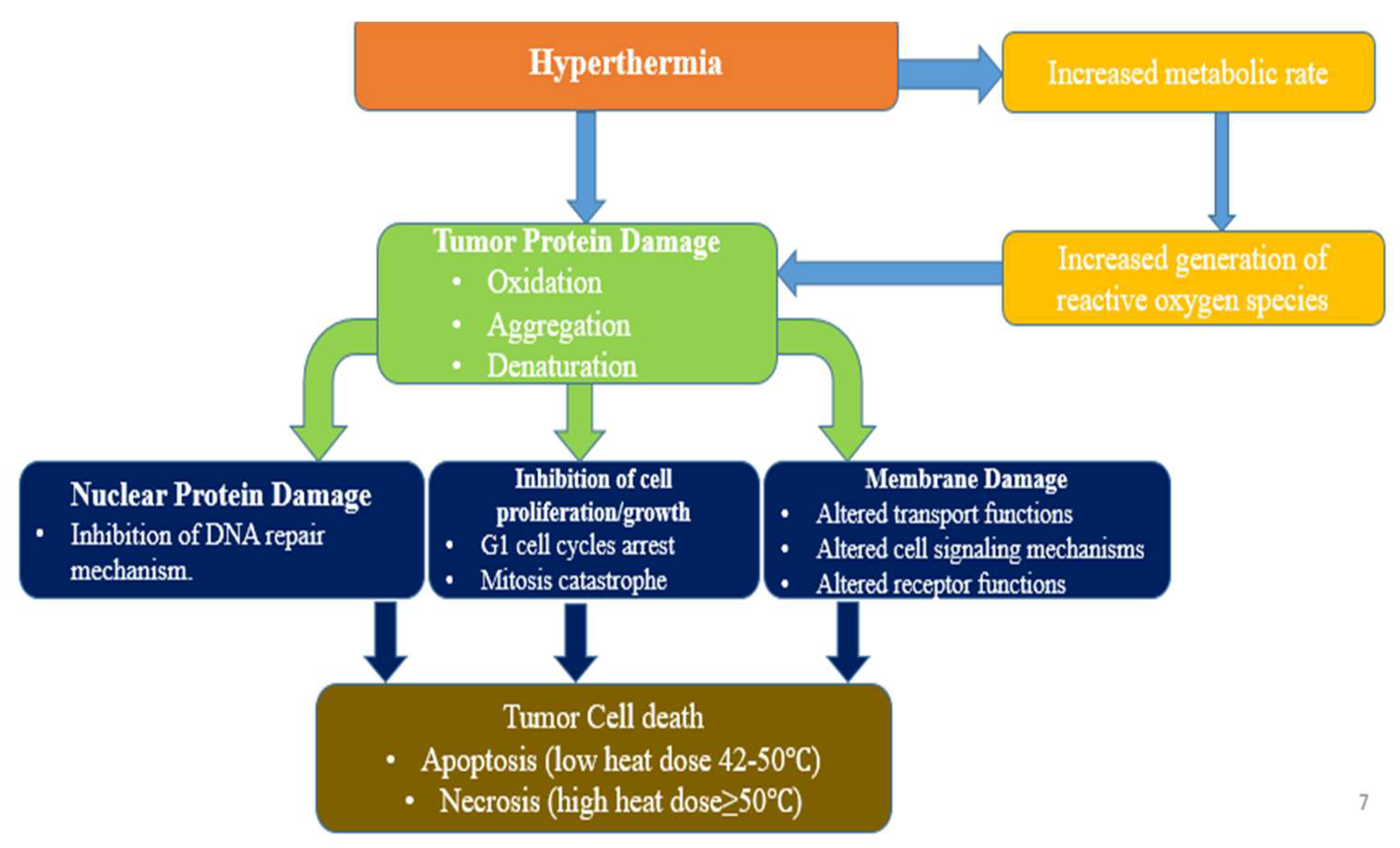
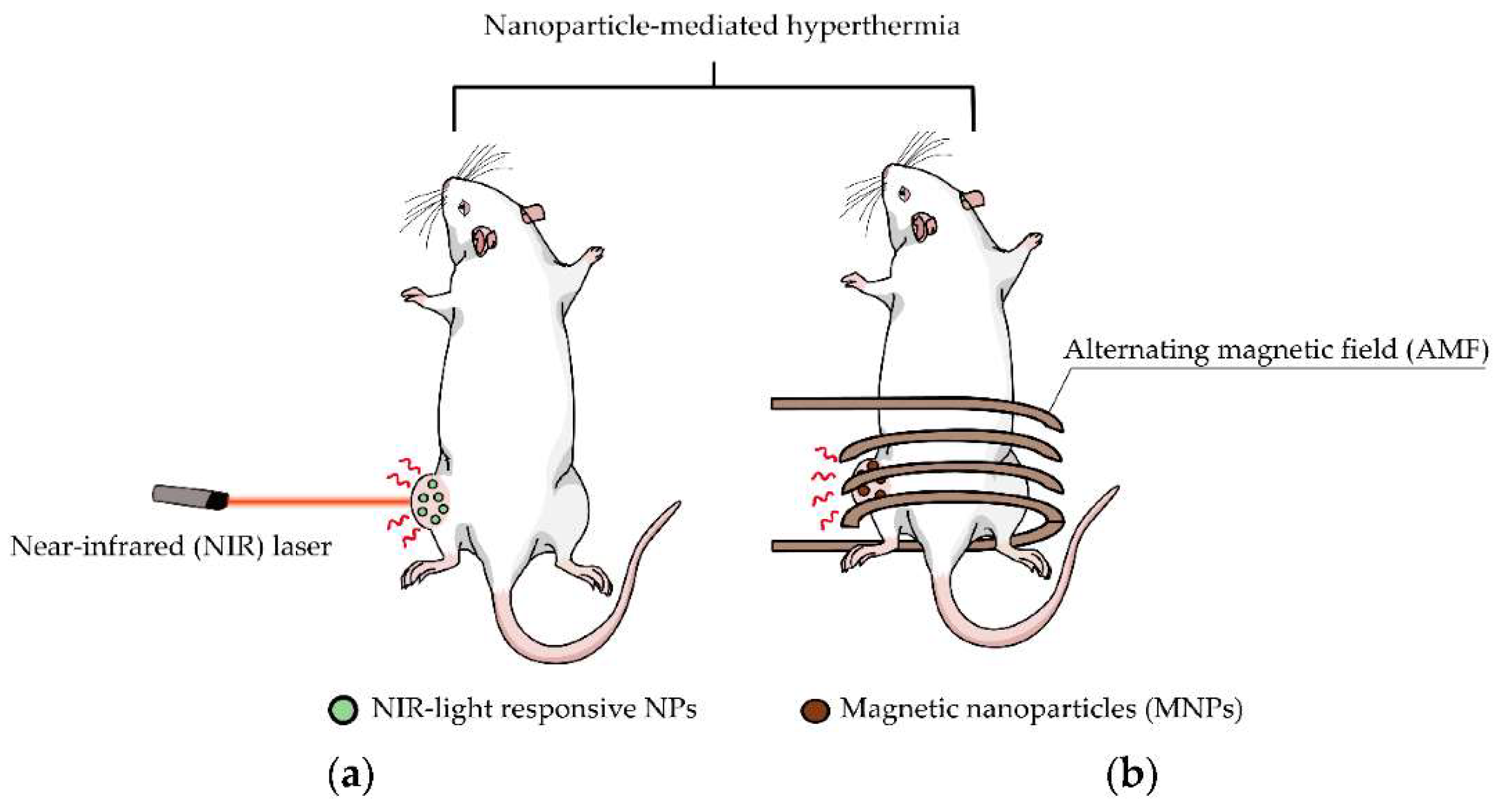
:1. Introduction
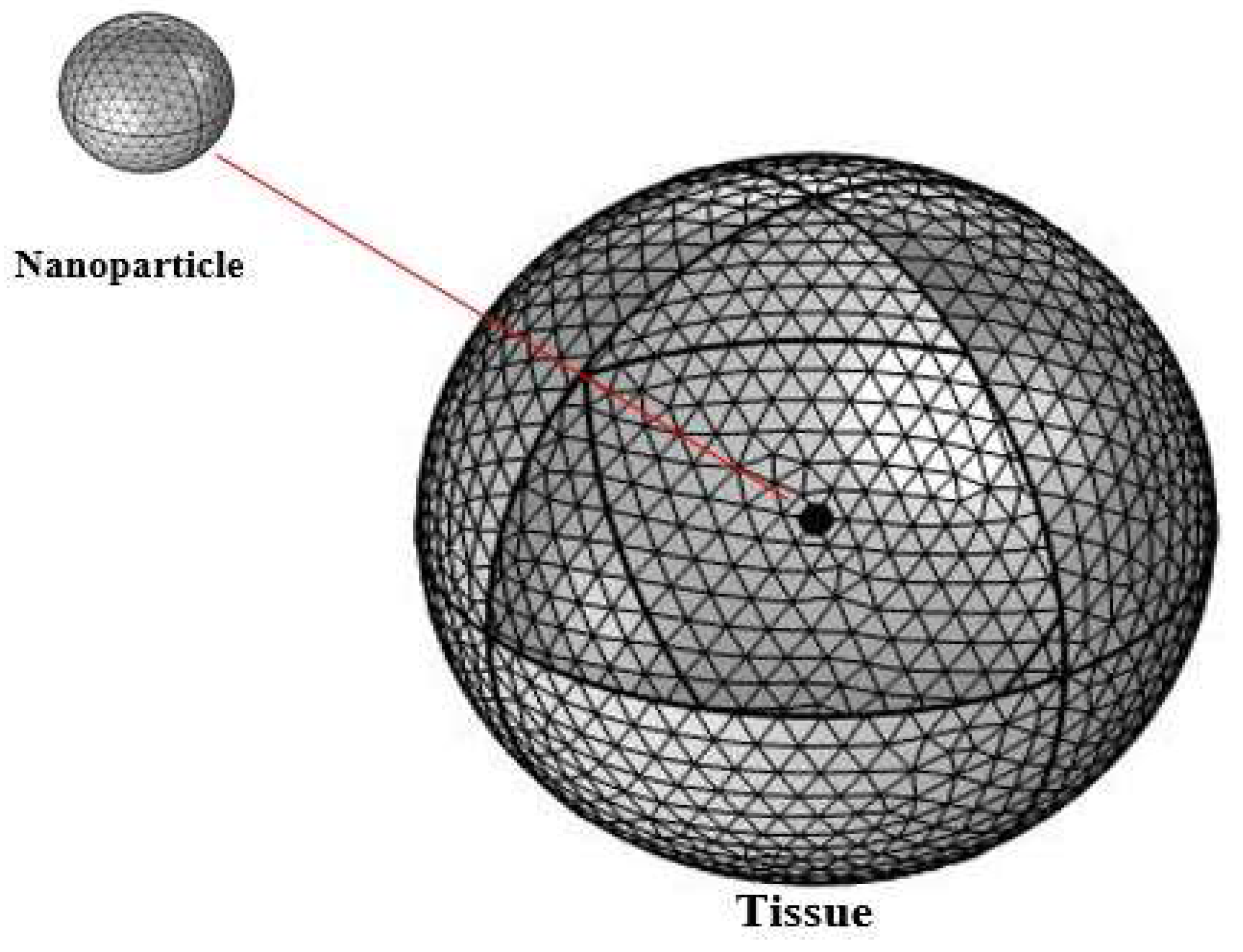
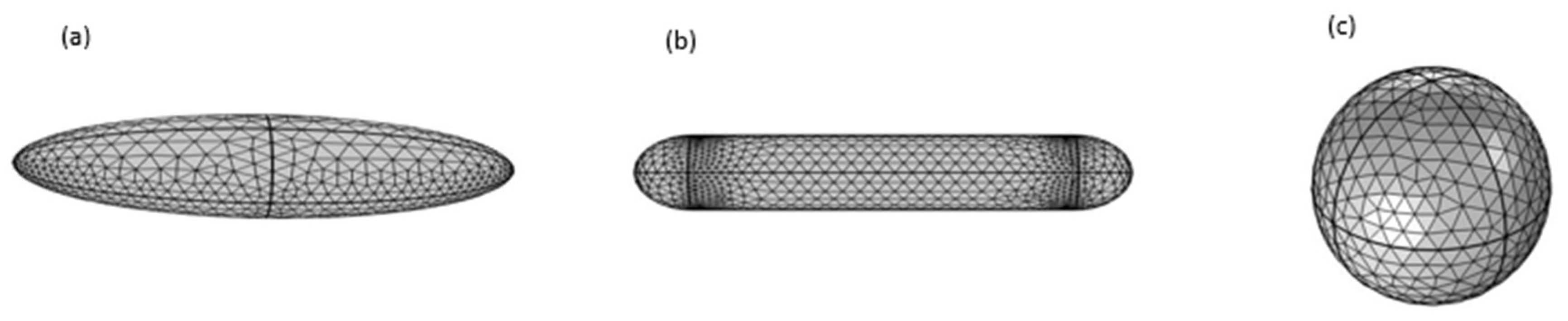
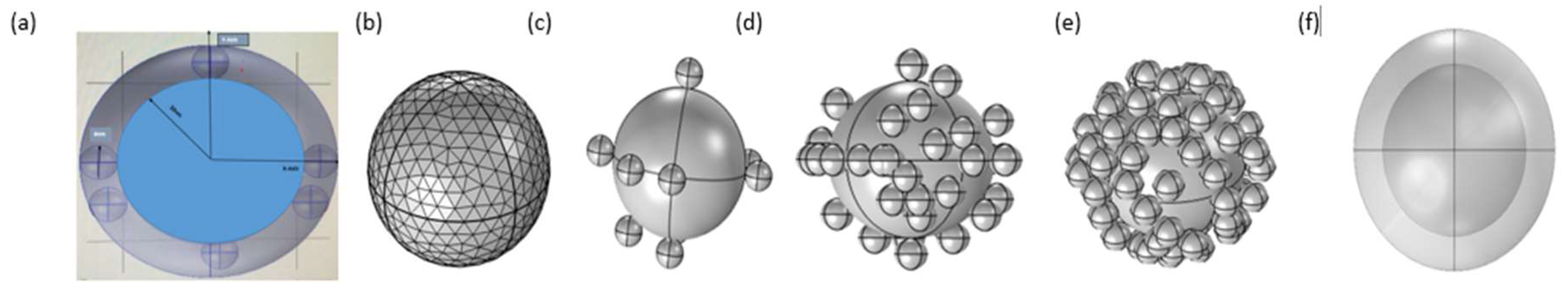
2. Methods
- The tumor cell receives the heat flux from the particle in its entirety, i.e., continuity.
- The temperature of the outer surface of the tissue is maintained at .
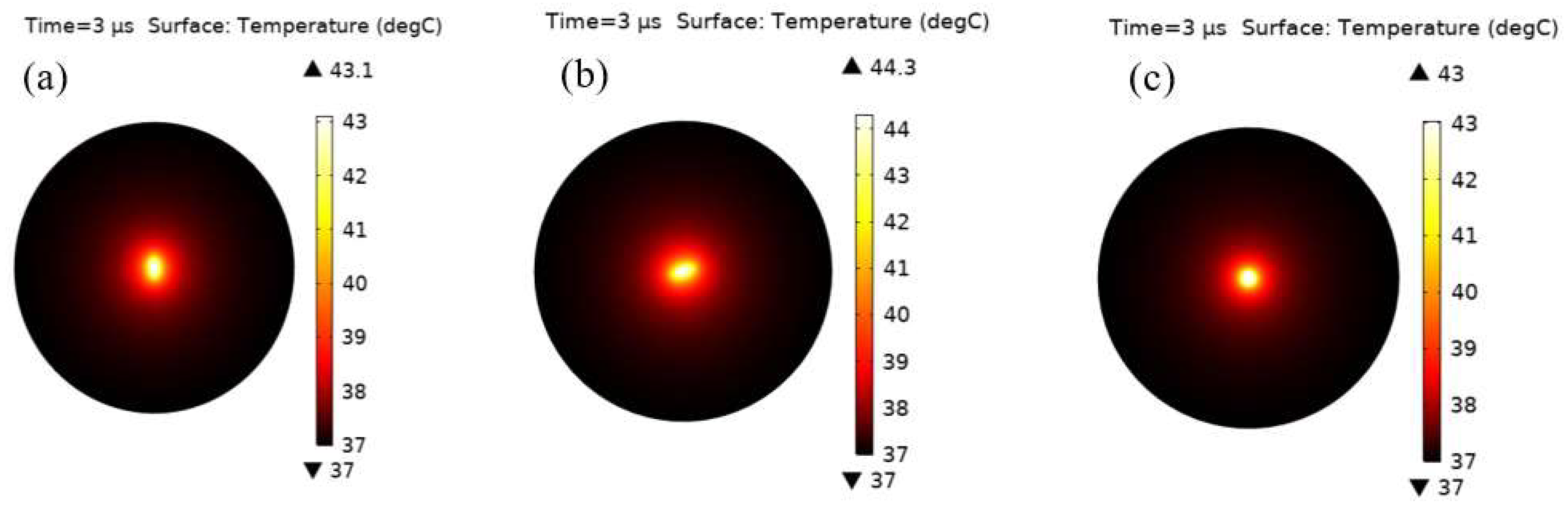
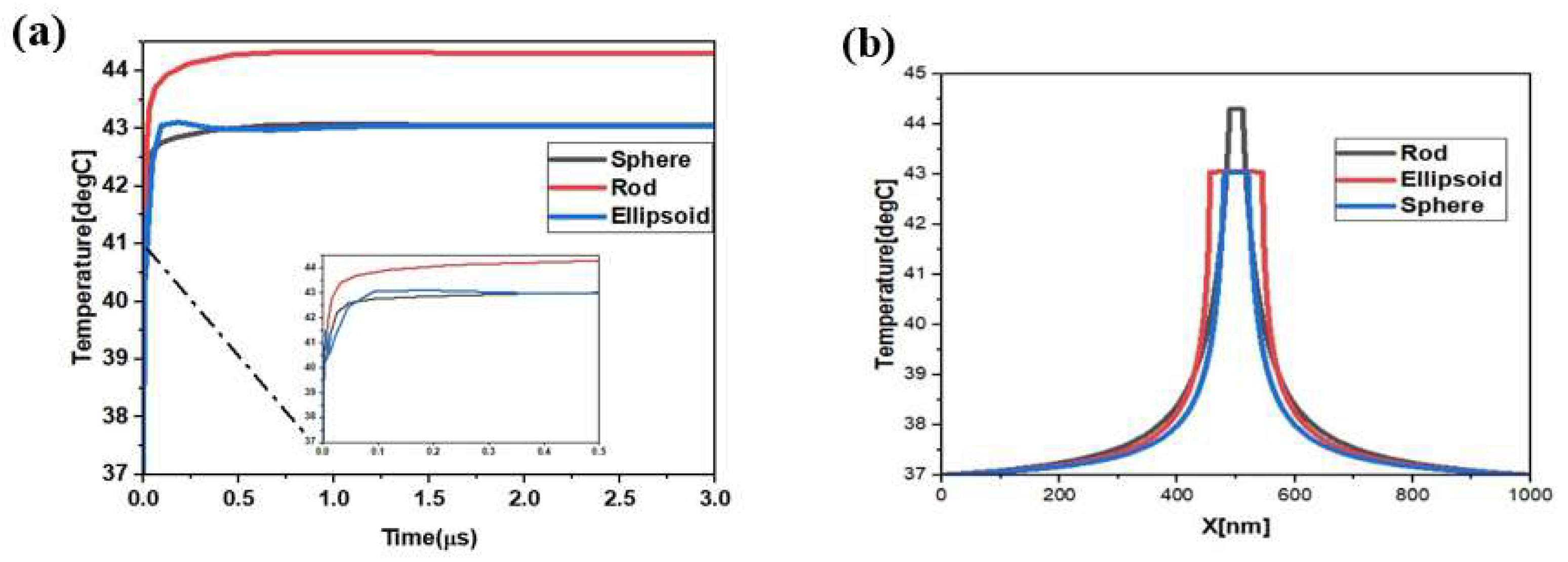
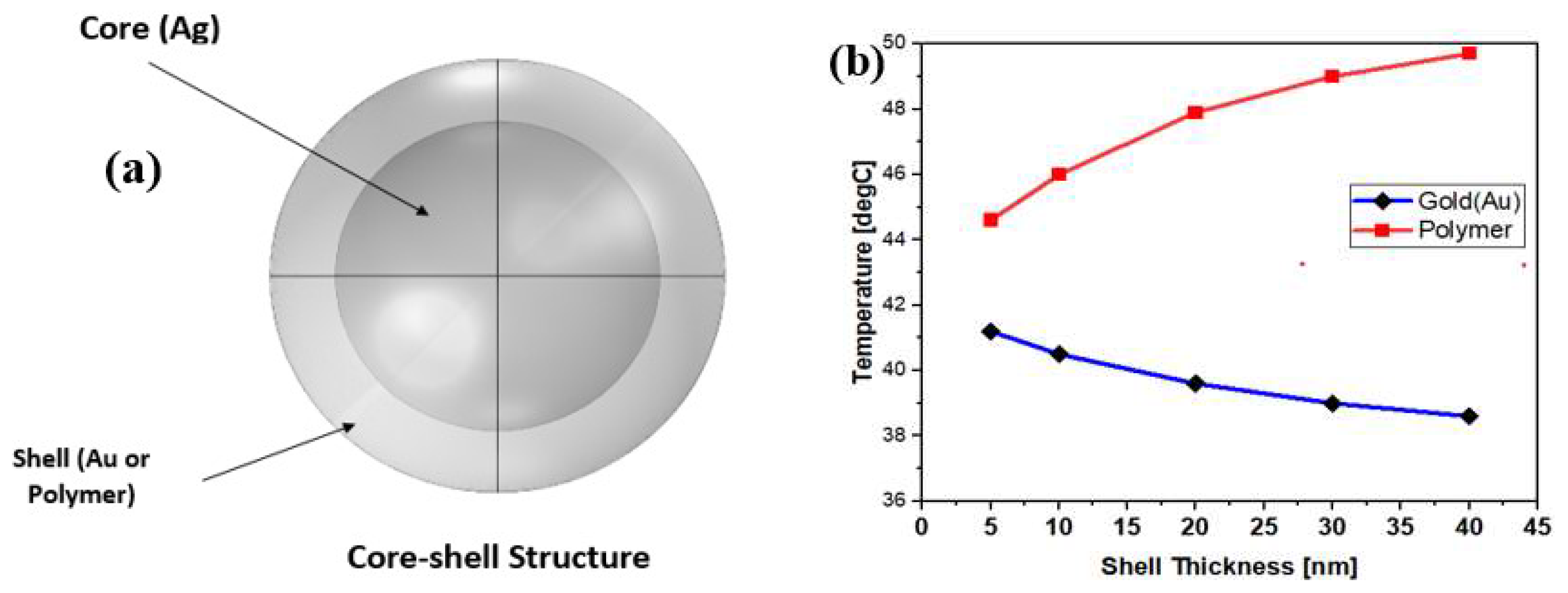
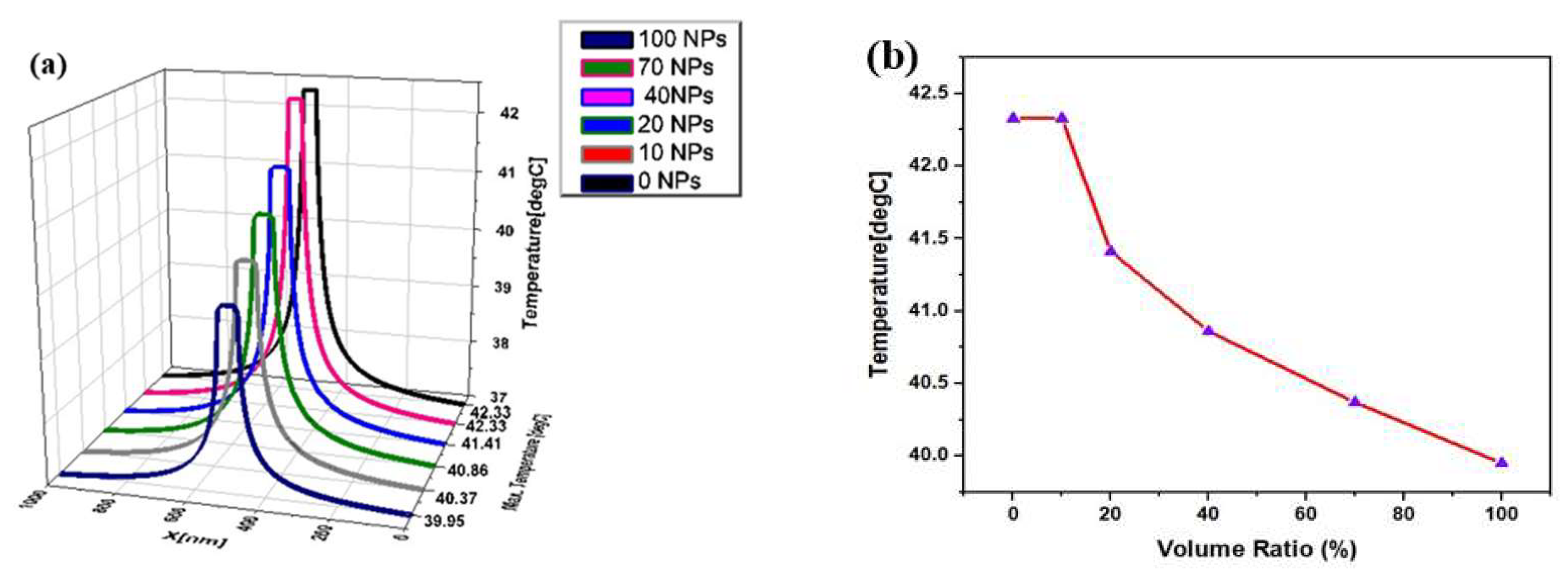
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balmain, A.; Gray, J.W.; Ponder, B.A.J. The genetics and genomics of cancer. Nat. Genet. 2003, 33, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Conde, J.; Doria, G.; Baptista, P. Noble metal nanoparticles applications in cancer. J. Drug Deliv. 2012, 2012, 751075. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Chen, Z.; Shin, D.M. Application of Nanotechnology in Cancer Therapy and Imaging. CA Cancer J. Clin. 2008, 58, 97–110. [Google Scholar] [CrossRef]
- Barreto, J.A.; O’Malley, W.; Kubeil, M.; Graham, B.; Stephan, H.; Spiccia, L. Nanomaterials: Applications in cancer imaging and therapy. Adv. Mater. 2011, 23, H18–H40. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. Magnetic hyperthermia with magnetic nanoparticles: A status review. Curr. Top. Med. Chem. 2014, 14, 572–594. [Google Scholar] [CrossRef]
- Pinheiro, P.S.; Callahan, K.E.; Jones, P.D.; Morris, C.; Ransdell, J.M.; Kwon, D.; Brown, C.P.; Kobetz, E.N. Liver cancer: A leading cause of cancer death in the United States and the role of the 1945–1965 birth cohort by ethnicity. JHEP Rep. 2019, 1, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, P.J. Imaging liver metastases: Current limitations and future prospects. Br. J. Radiol. 2000, 73, 234–241. [Google Scholar] [CrossRef]
- Scheele, J.; Stangl, R.; Altendorf-Hofmann, A. Hepatic metastases from colorectal carcinoma: Impact of surgical resection on the natural history. Br. J. Surg. 1990, 77, 1241–1246. [Google Scholar] [CrossRef]
- Cady, B.; Stone, M.D.; McDermott, W.V.; Jenkins, R.L.; Bothe, A.; Lavin, P.T.; Lovett, E.J.; Steele, G.D. Technical and Biological Factors in Disease-Free Survival After Hepatic Resection for Colorectal Cancer Metastases. Arch. Surg. 1992, 127, 561–569. [Google Scholar] [CrossRef]
- Praetorius, N.P.; Mandal, T.K. Engineered nanoparticles in cancer therapy. Recent Pat. Drug Deliv. Formul. 2007, 1, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life 2010, 1, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Yagawa, Y.; Tanigawa, K.; Kobayashi, Y.; Yamamoto, M. Cancer immunity and therapy using hyperthermia with immunotherapy, radiotherapy, chemotherapy, and surgery. J. Cancer Metastasis Treat. 2017, 3, 218. [Google Scholar] [CrossRef]
- Kobayashi, T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol. J. 2011, 6, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Kharat, P.B.; Somvanshi, S.B.; Khirade, P.P.; Jadhav, K.M. Induction Heating Analysis of Surface-Functionalized Nanoscale CoFe2O4 for Magnetic Fluid Hyperthermia toward Noninvasive Cancer Treatment. ACS Omega 2020, 5, 23378–23384. [Google Scholar] [CrossRef]
- Chang, D.; Lim, M.; Goos, J.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef] [Green Version]
- Somvanshi, S.B.; Kharat, P.B.; Jadhav, K.M. Surface Functionalized Superparamagnetic Zn-Mg Ferrite Nanoparticles for Magnetic Hyperthermia Application Towards Noninvasive Cancer Treatment. Macromol. Symp. 2021, 400, 2100124. [Google Scholar] [CrossRef]
- Silva, A.C.; Oliveira, T.R.; Mamani, J.B.; Malheiros, S.M.F.; Malavolta, L.; Pavon, L.F.; Sibov, T.T.; Amaro, E., Jr.; Tannús, A.; Vidoto, E.L.G.; et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int. J. Nanomed. 2011, 6, 591–603. [Google Scholar] [CrossRef] [Green Version]
- Song, C.W.; Park, H.J.; Lee, C.K.; Griffin, R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int. J. Hyperth. 2005, 21, 761–767. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Moon, M.J.; Surendran, S.P.; Jeong, Y.Y. Biomedical Applications of Hyaluronic Acid-Based Nanomaterials in Hyperthermic Cancer Therapy. Pharmaceutics 2019, 11, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, M.H.; Issels, R.D. Hyperthermia in oncology. Int. J. Hyperth. 2001, 17, 1–18. [Google Scholar] [CrossRef]
- Feldman, A.L.; Libutti, S.K.; Pingpank, J.F.; Bartlett, D.L.; Beresnev, T.H.; Mavroukakis, S.M.; Steinberg, S.M.; Liewehr, D.J.; Kleiner, D.E.; Alexander, H.R. Analysis of Factors Associated with Outcome in Patients With Malignant Peritoneal Mesothelioma Undergoing Surgical Debulking and Intraperitoneal Chemotherapy. J. Clin. Oncol. 2003, 21, 4560–4567. [Google Scholar] [CrossRef] [PubMed]
- Roizin-Towle, L.; Pirro, J.P. The response of human and rodent cells to hyperthermia. Int. J. Radiat. Oncol. 1991, 20, 751–756. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Alvarez, V.A. Advances in Magnetic Noble Metal/Iron-Based Oxide Hybrid Nanoparticles as Biomedical Devices. Bioengineering 2019, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Fiorani, D.; Dormann, J.; Cherkaoui, R.; Tronc, E.; Lucari, F.; D’Orazio, F.; Spinu, L.; Nogues, M.; García-Santiago, A.; Testa, A.M. Collective magnetic state in nanoparticles systems. J. Magn. Magn. Mater. 1999, 196–197, 143–147. [Google Scholar] [CrossRef]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T 1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, e1906539. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Krause, J.; Wlodarczyk, W.; Sander, B.; Vogl, T.; Felix, R. Effects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo. Int. J. Hyperth. 1997, 13, 587–605. [Google Scholar] [CrossRef]
- Dombrovsky, L.A.; Timchenko, V.; Jackson, M.; Yeoh, G.H. A combined transient thermal model for laser hyperthermia of tumors with embedded gold nanoshells. Int. J. Heat Mass Transf. 2011, 54, 5459–5469. [Google Scholar] [CrossRef]
- Pavel, M.; Stancu, A. Ferromagnetic Nanoparticles Dose Based on Tumor Size in Magnetic Fluid Hyperthermia Cancer Therapy. IEEE Trans. Magn. 2009, 45, 5251–5254. [Google Scholar] [CrossRef]
- Henrich, F.; Rahn, H.; Odenbach, S. Heat transition during magnetic heating treatment: Study with tissue models and simulation. J. Magn. Magn. Mater. 2015, 380, 353–359. [Google Scholar] [CrossRef]
- Rengan, A.K.; Bukhari, A.; Pradhan, A.; Malhotra, R.; Banerjee, R.; Srivastava, R.; De, A. In Vivo Analysis of Biodegradable Liposome Gold Nanoparticles as Efficient Agents for Photothermal Therapy of Cancer. Nano Lett. 2015, 15, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Cheng, J.; Liu, W.; Chen, X. Numerical Analysis of Electromagnetically Induced Heating and Bioheat Transfer for Magnetic Fluid Hyperthermia. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar] [CrossRef]
- Lara HH Garza-Trevin, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Sun, R.W.-Y.; Chen, R.; Hui, C.-K.; Ho, C.-M.; Luk, J.M.; Lau, G.K.K.; Che, C.-M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Jakubowska, M.; Młożniak, A.; Horaczek, M.; Rudka, D.; Free, C.; Carey, J.D. Enhanced Electrical Conductivity of Silver Nanoparticles for High Frequency Electronic Applications. ACS Appl. Mater. Interfaces 2012, 4, 7007–7010. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Cameron, S.J.; Hosseinian, F.; Willmore, W.G. A current overview of the biological and cellular effects of nanosilver. Int. J. Mol. Sci. 2018, 19, 2030. [Google Scholar] [CrossRef] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- COMSOL. COMSOL Multiphysics Heat Transfer Module, User Manual; COMSOL Inc.: Burlington, MA, USA, 2008. [Google Scholar]
- Charny, C.K. Mathematical Models of Bioheat Transfer. In Advances in Heat Transfer; Elsevier BV: Amsterdam, The Netherlands, 1992; Volume 22, pp. 19–155. [Google Scholar]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef]
- Ng, E.-K.; Sudharsan, N. Effect of blood flow, tumour and cold stress in a female breast: A novel time-accurate computer simulation. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2001, 215, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Taloub, S.; Hobar, F.; Astefanoaei, I.; Dumitru, I.; Caltun, O.F. FEM Investigation of coated magnetic nanoparticles for hyperthermia. Nanosci. Nanotechnol. 2016, 6, 55–61. [Google Scholar]
- Saw, C.B.; Loper, A.; Komanduri, K.; Combine, T.; Huq, S.; Scicutella, C. Determination of CT-to-density conversion relationship for image-based treatment planning systems. Med Dosim. 2005, 30, 145–148. [Google Scholar] [CrossRef]
- Govorov, A.O.; Zhang, W.; Skeini, T.; Richardson, H.; Lee, J.; Kotov, N.A. Gold nanoparticle ensembles as heaters and actuators: Melting and collective plasmon resonances. Nanoscale Res. Lett. 2006, 1, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Tippa, S.; Narahari, M.; Pendyala, R. Unsteady natural convection flow of nanofluids past a semi-infinite isothermal vertical plate. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1787, p. 020014. [Google Scholar]
- Kim, D.-H.; Nikles, D.E.; Brazel, C.S. Synthesis and Characterization of Multifunctional Chitosan-MnFe2O4 Nanoparticles for Magnetic Hyperthermia and Drug Delivery. Materials 2010, 3, 4051–4065. [Google Scholar] [CrossRef] [Green Version]

| Study Type | Particle Type and Size | Coating | Results and Drawbacks | Reference |
|---|---|---|---|---|
| Magnetic Hyperthermia (Experimental) | Dextran | Post-treatment tumor development is slowed. Induces inadvertent MF infiltration. | Jordan et al. (1997) [30] | |
| Photo-thermal hyperthermia (theoretical) | Silica nano shell of 20 nm radius | Au coated | Suitable to obtain thermal regime. There is inconsideration of the role of blood perfusion rate and metabolic heat. | Dombrovsky et al. (2011) [31] |
| Theoretical model | of 0.9 mm | The appropriate dose of nanoparticles for hyperthermia. The blood vessels close to the tumor reduce the temperature achieved in the tissue, whereas there are no large blood vessels around the tumor. | Pavel M. et al. (2009) [32] | |
| Theoretical + experimental | of 10 nm | The highest achievable temperature depends on surface-to-volume ratio. It is possible to describe patient-specific models. | Henrich F., Rahn H. and Odenbachs. (2015) [33] | |
| Experimental Photothermal hyperthermia | Lipos AuNPs of 5–8 nm | Gold coated | Hybrid NPs (biodegradable) for treatment. | Rengan et al. (2015) [34] |
| Theoretical | of 18 nm | They found that steady stable temperature achieved after 200 s was at the center of the tumor. The temperature curve declines with increasing distance from the centre at different exposure times. | Wu, L., Cheng, J., Liu, W., & Chen, X. (2015) [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daud, M.U.; Abbas, G.; Afzaal, M.; Naz, M.Y.; Fatima, N.G.; Ghuffar, A.; Irfan, M.; Mahnashi, M.H.; Legutko, S.; Petrů, J.; et al. Finite Element Analysis of Silver Nanorods, Spheres, Ellipsoids and Core–Shell Structures for Hyperthermia Treatment of Cancer. Materials 2022, 15, 1786. https://doi.org/10.3390/ma15051786
Daud MU, Abbas G, Afzaal M, Naz MY, Fatima NG, Ghuffar A, Irfan M, Mahnashi MH, Legutko S, Petrů J, et al. Finite Element Analysis of Silver Nanorods, Spheres, Ellipsoids and Core–Shell Structures for Hyperthermia Treatment of Cancer. Materials. 2022; 15(5):1786. https://doi.org/10.3390/ma15051786
Chicago/Turabian StyleDaud, Muhammad Usama, Ghulam Abbas, Muhammad Afzaal, Muhammad Yasin Naz, Nazma Goher Fatima, Abdul Ghuffar, Muhammad Irfan, Mater H. Mahnashi, Stanislaw Legutko, Jana Petrů, and et al. 2022. "Finite Element Analysis of Silver Nanorods, Spheres, Ellipsoids and Core–Shell Structures for Hyperthermia Treatment of Cancer" Materials 15, no. 5: 1786. https://doi.org/10.3390/ma15051786
APA StyleDaud, M. U., Abbas, G., Afzaal, M., Naz, M. Y., Fatima, N. G., Ghuffar, A., Irfan, M., Mahnashi, M. H., Legutko, S., Petrů, J., Kratochvíl, J., & Niazi, U. M. (2022). Finite Element Analysis of Silver Nanorods, Spheres, Ellipsoids and Core–Shell Structures for Hyperthermia Treatment of Cancer. Materials, 15(5), 1786. https://doi.org/10.3390/ma15051786









