Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors
Abstract
:1. Introduction
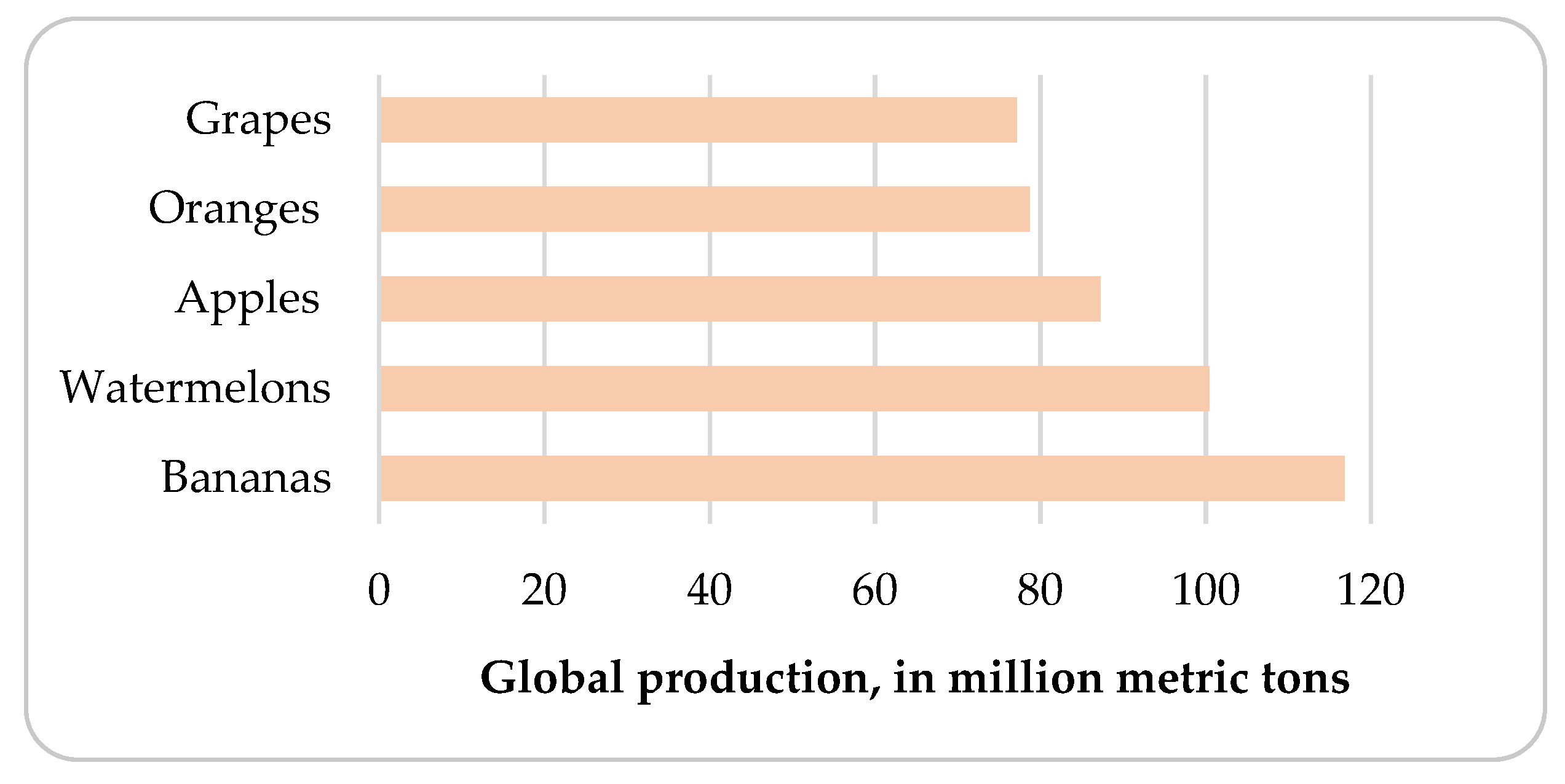
Apple and Apple Pomace Production in Poland and the World
2. The Recovery from Apple Pomace Dried and Powder
2.1. The Pretreatment of Apple Pomace
2.1.1. Biofuels
Bioethanol
Biogas
Biochar
2.1.2. Sodium-Ion Batteries
2.1.3. Biopolymers
3. The Recovery from Apple Pomace Extraction for the Building and Construction Sectors
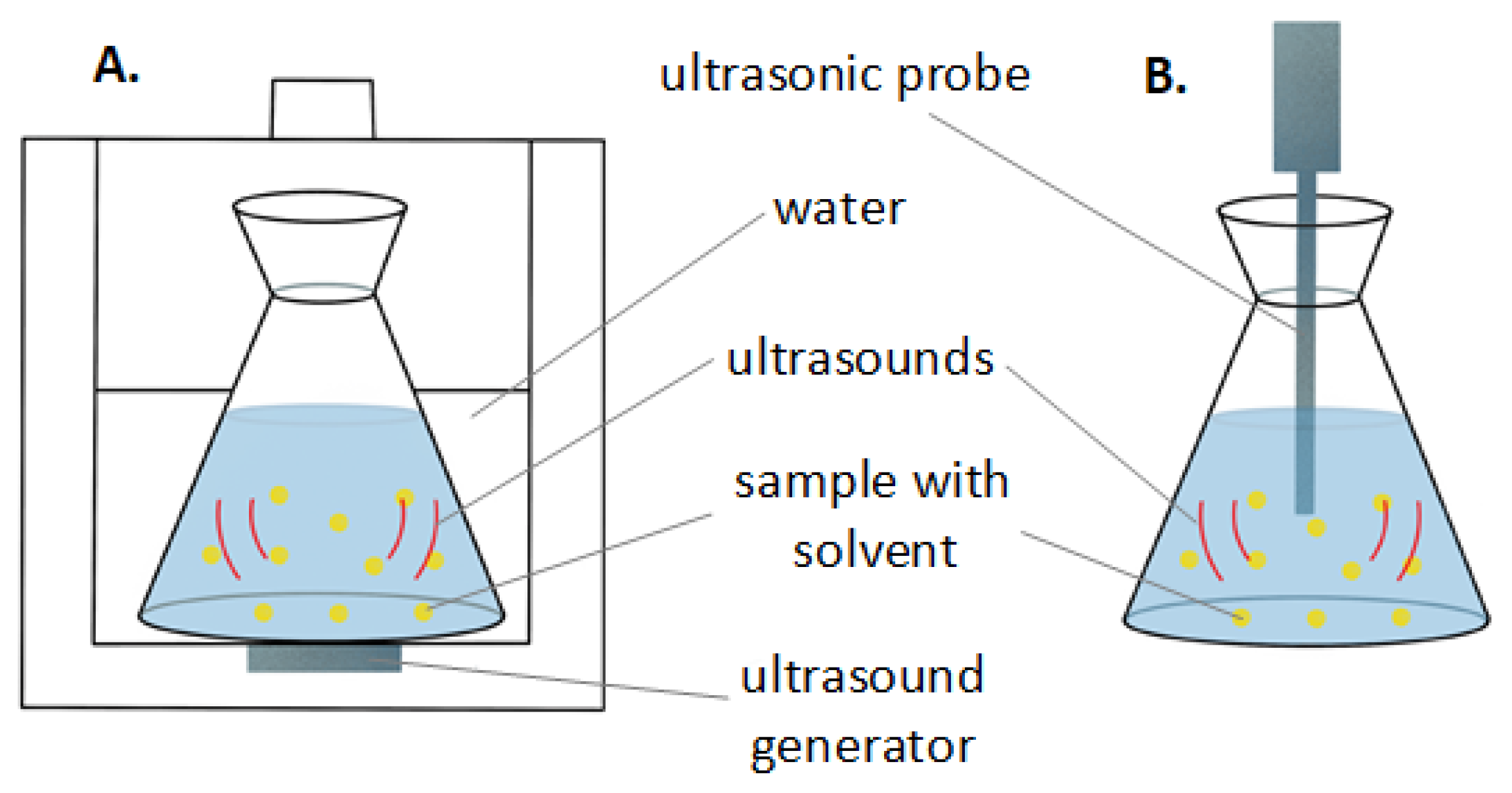
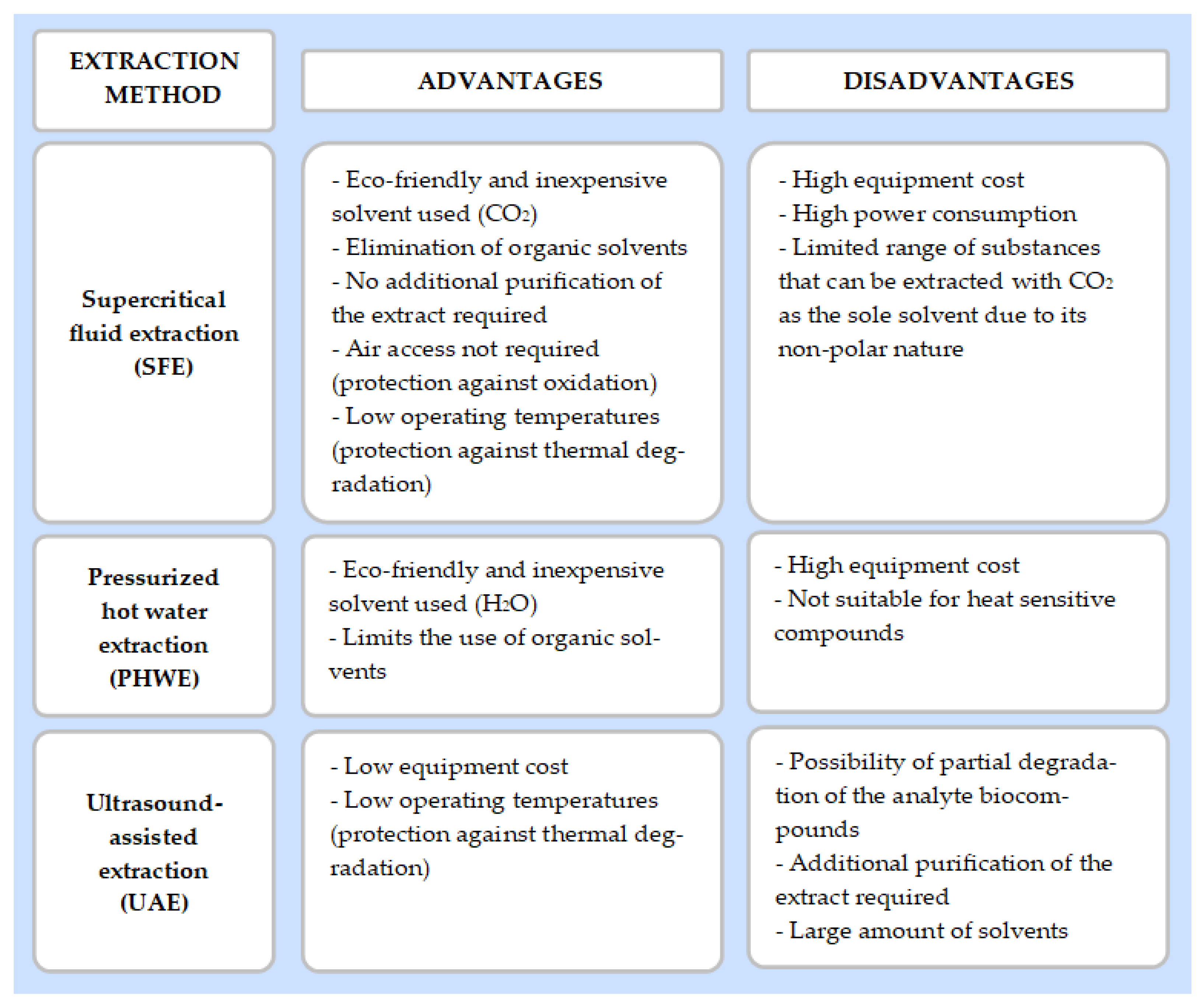
3.1. Green Extraction Techniques
3.1.1. Green Corrosion Inhibitors Active Compounds
3.1.2. Green Wood Protectors’ Active Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guerrero, L.A.; Maas, G.; Hogland, W. Solid waste management challenges for cities in developing countries. Waste Manag. 2013, 33, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, E.; Williams, I.; Shaw, P.; Ongondo, F. A Tale of Two Cities: The Emergence of Urban Waste Systems in a Developed and a Developing City. Recycling 2016, 1, 254–270. [Google Scholar] [CrossRef] [Green Version]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Shalini, R.; Gupta, D.K. Utilization of pomace from apple processing industries: A review. J. Food Sci. Technol. 2010, 47, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trends Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Hijosa-Valsero, M.; González-García, I.; Paniagua-García, A.I.; Hernández, D.; Garita-Cambronero, J.; Díez-Antolínez, R.; García-González, M.C. Valorization of apple pomaces for biofuel production: A biorefinery approach. Biomass Bioenergy 2020, 142, 105785. [Google Scholar] [CrossRef]
- Olech, E.; Sikora, J.; Kuboń, M. Biogas production from corn silage and apple pomace. J. Res. Appl. Agric. Eng. 2017, 62, 155–157. [Google Scholar]
- Xu, R.; Ferrante, L.; Hall, K.; Briens, C.; Berruti, F. Thermal self-sustainability of biochar production by pyrolysis. J. Anal. Appl. Pyrolysis 2011, 91, 55–66. [Google Scholar] [CrossRef]
- Wu, L.; Buchholz, D.; Vaalma, C.; Giffin, G.A.; Passerini, S. Apple-Biowaste-Derived Hard Carbon as a Powerful Anode Material for Na-Ion Batteries. ChemElectroChem 2016, 3, 292–298. [Google Scholar] [CrossRef]
- Koryś, K.A.; Latawiec, A.E.; Grotkiewicz, K.; Kuboń, M. The Review of Biomass Potential for Agricultural Biogas Production in Poland. Sustainability 2019, 11, 6515. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Kumar, V.; Jia, L.; Sarsaiya, S.; Kumar, D.; Juneja, A.; Zhang, Z.; Sindhu, R.; Binod, P.; Bhatia, S.K.; et al. Biopolymer poly-hydroxyalkanoates (PHA) production from apple industrial waste residues: A review. Chemosphere 2021, 284, 131427. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.; Landberg, M.; Bátori, V.; Åkesson, D.; Taherzadeh, M.J.; Zamani, A. Development of bio-based films and 3D objects from apple pomace. Polymers 2019, 11, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Śliwa, K.; Malinowska, M.A.; Sikora, E.; Ogonowski, J. Comparison of extraction methods used for separation of active compounds from waste generated during the production of apple juice. Przem. Chem. 2015, 94, 357–360. [Google Scholar]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruit: World Production by Type 2019 Statista. Available online: https://www.statista.com/statistics/264001/worldwide-production-of-fruit-by-variety/ (accessed on 22 March 2021).
- Rozkrut, D. (Ed.) Statistical Yearbook of Agriculture 2019; Zakład Wydawnictw Statystycznych: Warsaw, Poland, 2020. [Google Scholar]
- PKO Bank Polski. Available online: https://www.pkobp.pl/centrum-analiz/analizy-sektorowe/#subcategory=165947&page=2 (accessed on 27 March 2021).
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of apple pomace for bioactive molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Vukušić, J.L.; Millenautzki, T.; Cieplik, R.; Obst, V.; Saaid, A.M.; Clavijo, L.; Zlatanovic, S.; Hof, J.; Mösche, M.; Barbe, S. Reshaping Apple Juice Production into a Zero Discharge Biorefinery Process. Waste Biomass Valorization 2021, 12, 3617–3627. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Fang, Z.; Wang, X.; Bu, H. Enhanced bio-hydrogen production by anaerobic fermentation of apple pomace with enzyme hydrolysis. Int. J. Hydrogen Energy 2010, 35, 8303–8309. [Google Scholar] [CrossRef]
- Martău, G.A.; Teleky, B.E.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Apple Pomace as a Sustainable Substrate in Sourdough Fermentation. Front. Microbiol. 2021, 12, 3850. [Google Scholar] [CrossRef]
- FAO. World Fertilizer Trends and Outlook to 2018. Available online: http://www.fao.org/3/a-i4324e.pd (accessed on 12 January 2022).
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding Food Loss and Waste—Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeger, S.R.; Machín, L.; Aschemann-Witzel, J.; Antúnez, L.; Harker, F.R.; Ares, G. Buy, eat or discard? A case study with apples to explore fruit quality perception and food waste. Food Qual. Prefer. 2018, 69, 10–20. [Google Scholar] [CrossRef]
- Matei, E.; Râpă, M.; Predescu, A.M.; Țurcanu, A.A.; Vidu, R.; Predescu, C.; Bobirica, C.; Bobirica, L.; Orbeci, C. Valorization of Agri-Food Wastes as Sustainable Eco-Materials for Wastewater Treatment: Current State and New Perspectives. Materials 2021, 14, 4581. [Google Scholar] [CrossRef] [PubMed]
- Abiad, M.G.; Meho, L.I. Food loss and food waste research in the Arab world: A systematic review. Food Secur. 2018, 10, 311–322. [Google Scholar] [CrossRef]
- Santeramo, F.G. Exploring the link among food loss, waste and food security: What the research should focus on? Agric. Food Secur. 2021, 10, 26. [Google Scholar] [CrossRef]
- Marks-Bielska, R.; Bielski, S.; Pik, K.; Kurowska, K. The Importance of Renewable Energy Sources in Poland’s Energy Mix. Energies 2020, 13, 4624. [Google Scholar] [CrossRef]
- Richards, B.K.; Herndon, F.G.; Jewell, W.J.; Cummings, R.J.; White, T.E. In situ methane enrichment in methanogenic energy crop digesters. Biomass Bioenergy 1994, 6, 275–282. [Google Scholar] [CrossRef]
- Bozym, M.; Florczak, I.; Zdanowska, P.; Wojdalski, J.; Klimkiewicz, M. An analysis of metal concentrations in food wastes for biogas production. Renew. Energy 2015, 77, 467–472. [Google Scholar] [CrossRef]
- Magyar, M.; da Costa Sousa, L.; Jin, M.; Sarks, C.; Balan, V. Conversion of apple pomace waste to ethanol at industrial relevant conditions. Appl. Microbiol. Biotechnol. 2016, 100, 7349–7358. [Google Scholar] [CrossRef] [PubMed]
- Riggio, V.; Comino, E.; Rosso, M. Energy production from anaerobic co-digestion processing of cow slurry, olive pomace and apple pulp. Renew. Energy 2015, 83, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Karimi, K.; Chisti, Y. Bioethanol Production and Technologies. In Encyclopedia of Sustainable Technologies; Elsevier: London, UK, 2017; Volume 3, pp. 273–284. [Google Scholar]
- Rodrussamee, N.; Lertwattanasakul, N.; Hirata, K.; Suprayogi; Limtong, S.; Kosaka, T.; Yamada, M. Growth and ethanol fermentation ability on hexose and pentose sugars and glucose effect under various conditions in thermotolerant yeast Kluyveromyces marxianus. Appl. Microbiol. Biotechnol. 2011, 90, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Demiray, E.; Kut, A.; Ertuğrul Karatay, S.; Dönmez, G. Usage of soluble soy protein on enzymatically hydrolysis of apple pomace for cost-efficient bioethanol production. Fuel 2021, 289, 119785. [Google Scholar] [CrossRef]
- Kut, A.; Demiray, E.; Ertuğrul Karatay, S.; Dönmez, G. Second generation bioethanol production from hemicellulolytic hydrolyzate of apple pomace by Pichia stipitis. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 42, 1–12. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sudharsana, T.; Jayamuthunagai, J.; Praveenkumar, R.; Chozhavendhan, S.; Iyyappan, J. Biogas production–A review on composition, fuel properties, feed stock and principles of anaerobic digestion. Renew. Sustain. Energy Rev. 2018, 90, 570–582. [Google Scholar] [CrossRef]
- Kim, S.; Sung, T.; Kim, K.C. Performance and Greenhouse Gas Reduction Analysis of Biogas-Fueled Solid-Oxide Fuel Cells for a Sewage Sludge and Food Waste Treatment Facility. Energies 2018, 11, 600. [Google Scholar] [CrossRef] [Green Version]
- Claes, A.; Melchi, L.; Uludag-Demirer, S.; Demirer, G.N. Supplementation of Carbon-Based Conductive Materials and Trace Metals to Improve Biogas Production from Apple Pomace. Sustainability 2021, 13, 9488. [Google Scholar] [CrossRef]
- Saletnik, B.; Bajcar, M.; Saletnik, A.; Zaguła, G.; Puchalski, C. Effect of the Pyrolysis Process Applied to Waste Branches Biomass from Fruit Trees on the Calorific Value of the Biochar and Dust Explosivity. Energies 2021, 14, 4898. [Google Scholar] [CrossRef]
- Kumar, G.; Eswari, A.P.; Kavitha, S.; Kumar, M.D.; Kannah, R.Y.; How, L.C.; Muthukaruppan, G.; Banu, J.R. Thermochemical conversion routes of hydrogen production from organic biomass: Processes, challenges and limitations. Biomass Convers. Biorefinery 2020, 2, 1–26. [Google Scholar] [CrossRef]
- Kosakowski, W.; Bryszewska, M.A.; Dziugan, P. Biochars from Post-Production Biomass and Waste from Wood Management: Analysis of Carbonization Products. Materials 2020, 13, 4971. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.R.B.; Salinas Gutiérrez, J.M.; Meléndez Zaragoza, M.J.; López Ortiz, A.; Collins-Martínez, V. Optimal slow pyrolysis of apple pomace reaction conditions for the generation of a feedstock gas for hydrogen production. Int. J. Hydrogen Energy 2016, 41, 23232–23237. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, Y.; Dang, J.; Zhao, J.; Chen, S. Magnetic apple pomace biochar: Simple preparation, characterization, and application for enriching Ag(I) in effluents. Sci. Total Environ. 2019, 668, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Geng, C.; Buchholz, D.; Passerini, S. Research Update: Hard carbon with closed pores from pectin-free apple pomace waste for Na-ion batteries. APL Mater. 2018, 6, 047501. [Google Scholar] [CrossRef] [Green Version]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. Complete Utilization of Waste Pomegranate Peels to Produce a Hydrocolloid, Punicalagin Rich Phenolics, and a Hard Carbon Electrode. ACS Sustain. Chem. Eng. 2018, 6, 16363–16374. [Google Scholar] [CrossRef]
- Nita, C.; Zhang, B.; Dentzer, J.; Matei Ghimbeu, C. Hard carbon derived from coconut shells, walnut shells, and corn silk biomass waste exhibiting high capacity for Na-ion batteries. J. Energy Chem. 2021, 58, 207–218. [Google Scholar] [CrossRef]
- Arie, A.A.; Kristianto, H.; Demir, E.; Cakan, R.D. Activated porous carbons derived from the Indonesian snake fruit peel as anode materials for sodium ion batteries. Mater. Chem. Phys. 2018, 217, 254–261. [Google Scholar] [CrossRef]
- Muruganantham, R.; Wang, F.-M.; Yuwono, R.A.; Sabugaa, M.; Liu, W.-R. Biomass Feedstock of Waste Mango-Peel-Derived Porous Hard Carbon for Sustainable High-Performance Lithium-Ion Energy Storage Devices. Energy Fuels 2021, 35, 10878–10889. [Google Scholar] [CrossRef]
- Meenatchi, T.; Priyanka, V.; Subadevi, R.; Liu, W.-R.; Huang, C.-H.; Sivakumar, M. Probe on hard carbon electrode derived from orange peel for energy storage application. Carbon Lett. 2021, 31, 1033–1039. [Google Scholar] [CrossRef]
- Górka, J.; Vix-Guterl, C.; Matei Ghimbeu, C. Recent Progress in Design of Biomass-Derived Hard Carbons for Sodium Ion Batteries. C 2016, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Saavedra Rios, C.D.M.; Simonin, L.; De Geyer, A.; Ghimbeu, C.M.; Dupont, C. Unraveling the properties of biomass-derived hard carbons upon thermal treatment for a practical application in na-ion batteries. Energies 2020, 13, 3513. [Google Scholar] [CrossRef]
- Moriwake, H.; Kuwabara, A.; Fisher, C.A.J.; Ikuhara, Y. Why is sodium-intercalated graphite unstable? RSC Adv. 2017, 7, 36550–36554. [Google Scholar] [CrossRef] [Green Version]
- Stevens, D.A.; Dahn, J.R. The Mechanisms of Lithium and Sodium Insertion in Carbon Materials. J. Electrochem. Soc. 2001, 148, A803–A811. [Google Scholar] [CrossRef]
- Saurel, D.; Orayech, B.; Xiao, B.; Carriazo, D.; Li, X.; Rojo, T. From Charge Storage Mechanism to Performance: A Roadmap toward High Specific Energy Sodium-Ion Batteries through Carbon Anode Optimization. Adv. Energy Mater. 2018, 8, 1703268. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Hekmatfar, M.; Diemant, T.; Behm, R.J.; Buchholz, D.; Passerini, S. Pectin, Hemicellulose, or Lignin? Impact of the Biowaste Source on the Performance of Hard Carbons for Sodium-Ion Batteries. ChemSusChem 2017, 10, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Use of Pectin from Agro-Waste Residues as a Natural-Based Biopolymer for Food Packaging Applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Production of Bio-Plastic Materials from Apple Pomace: A New Application for the Waste Material. Available online: https://www.diva-portal.org/smash/get/diva2:1327184/FULLTEXT01.pdf/ (accessed on 19 December 2021).
- Rani, G.U.; Sharma, S. Biopolymers, Bioplastics and Biodegradability: An Introduction; Elsevier: Amsterdam, The Netherlands, 2021; pp. 2–10. [Google Scholar]
- Crutchik, D.; Franchi, O.; Caminos, L.; Jeison, D.; Belmonte, M.; Pedrouso, A.; Val del Rio, A.; Mosquera-Corral, A.; Campos, J.L. Polyhydroxyalkanoates (PHAs) Production: A Feasible Economic Option for the Treatment of Sewage Sludge in Municipal Wastewater Treatment Plants? Water 2020, 12, 1118. [Google Scholar] [CrossRef]
- Ten, E.; Jiang, L.; Zhang, J.; Wolcott, M.P. Mechanical performance of polyhydroxyalkanoate (PHA)-based biocomposites. In Biocomposites; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2015; pp. 39–52. [Google Scholar]
- Pereira, J.R.; Araújo, D.; Freitas, P.; Marques, A.C.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Fortunato, E.; Reis, M.A.M.; Freitas, F. Production of medium-chain-length polyhydroxyalkanoates by Pseudomonas chlororaphis subsp. aurantiaca: Cultivation on fruit pulp waste and polymer characterization. Int. J. Biol. Macromol. 2021, 167, 85–92. [Google Scholar] [CrossRef]
- Rebocho, A.T.; Pereira, J.R.; Freitas, F.; Neves, L.A.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Reis, M.A.M. Production of medium-chain length polyhydroxyalkanoates by Pseudomonas citronellolis grown in apple pulp waste. Appl. Food Biotechnol. 2019, 6, 71–82. [Google Scholar]
- Łata, B.; Tomala, K. Apple peel as a contributor to whole fruit quantity of potentially healthful bioactive compounds. Cultivar and year implication. J. Agric. Food Chem. 2007, 55, 10795–10802. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska-Trypuć, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Ferreira, J.A.; Sirohi, R.; Sarsaiya, S.; Khoshnevisan, B.; Baladi, S.; Sindhu, R.; Binod, P.; Pandey, A.; Juneja, A.; et al. A critical review on the development stage of biorefinery systems towards the management of apple processing-derived waste. Renew. Sustain. Energy Rev. 2021, 143, 110972. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oklu, N.K.; Matsinha, L.C.; Makhubela, B.C. Bio-Solvents: Synthesis, Industrial Production and Applications. In Solvents, Ionic Liquids and Solvent Effects; BoD–Books on Demand: Norderstedt, Germany, 2020; pp. 1–24. [Google Scholar]
- König, G.; Reetz, M.T.; Thiel, W. 1-Butanol as a Solvent for Efficient Extraction of Polar Compounds from Aqueous Medium: Theoretical and Practical Aspects. J. Phys. Chem. B 2018, 122, 6975–6988. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26. [Google Scholar] [CrossRef]
- Wong, V.; Wyllie, S.G.; Cornwell, C.P.; Tronson, D. Supercritical fluid extraction (SFE) of monoterpenes from the leaves of Melaleuca alternifolia (Tea Tree). Molecules 2001, 6, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Girotra, P.; Singh, S.K.; Nagpal, K. Supercritical fluid technology: A promising approach in pharmaceutical research. Pharm. Dev. Technol. 2013, 18, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Szakiel, A.; Pączkowski, C.; Marszałek, K.; Skąpska, S.; Kowalska, H.; Jędrzejczak, R. Extraction of Triterpenic Acids and Phytosterols from Apple Pomace with Supercritical Carbon Dioxide: Impact of Process Parameters, Modelling of Kinetics, and Scaling-Up Study. Molecules 2018, 23, 2790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- De la Peña Armada, R.; Bronze, M.R.; Matias, A.; Mateos-Aparicio, I. Triterpene-Rich Supercritical CO2 Extracts from Apple By-product Protect Human Keratinocytes Against ROS. Food Bioprocess Technol. 2021, 14, 909–919. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Abrahamsson, V.; Turner, C. Extraction and Neoformation of Antioxidant Compounds by Pressurized Hot Water Extraction from Apple Byproducts. J. Agric. Food Chem. 2013, 61, 5500–5510. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Al Jitan, S.; Alkhoori, S.A.; Yousef, L.F. Phenolic Acids from Plants: Extraction and Application to Human Health. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 389–417. [Google Scholar]
- Wang, L.; Boussetta, N.; Lebovka, N.; Vorobiev, E. Effects of ultrasound treatment and concentration of ethanol on selectivity of phenolic extraction from apple pomace. Int. J. Food Sci. Technol. 2018, 53, 2104–2109. [Google Scholar] [CrossRef]
- Malinowska, M.; Śliwa, K.; Sikora, E.; Ogonowski, J.; Oszmiański, J.; Kolniak-Ostek, J. Ultrasound-assisted and micelle-mediated extraction as a method to isolate valuable active compounds from apple pomace. J. Food Process. Preserv. 2018, 42, e13720. [Google Scholar] [CrossRef]
- Zhang, Z.; Poojary, M.M.; Choudhary, A.; Rai, D.K.; Tiwari, B.K. Comparison of selected clean and green extraction technologies for biomolecules from apple pomace. Electrophoresis 2018, 39, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of Phenolic Compounds from Fresh Apple Pomace by Different Non-Conventional Techniques. Molecules 2021, 26, 4272. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ma, Y.; Liu, Y.; Yang, Z.; Zhang, L. Ultrasonic-assisted extraction and antioxidant activity of flavonoids from Adinandra nitida leaves. Trop. J. Pharm. Res. 2013, 12, 1045–1051. [Google Scholar] [CrossRef] [Green Version]
- Altemimi, A.; Watson, D.G.; Choudhary, R.; Dasari, M.R.; Lightfoot, D.A. Ultrasound Assisted Extraction of Phenolic Compounds from Peaches and Pumpkins. PLoS ONE 2016, 11, e0148758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingret, D.; Fabiano-Tixier, A.S.; Bourvellec, C.L.; Renard, C.M.G.C.; Chemat, F. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Khan, Z.; Troquet, J.; Vachelard, C. Sample preparation and analytical techniques for determination of polyaromatic hydrocarbons in soils. Int. J. Environ. Sci. Technol. 2005, 2, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds from Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Raja, P.B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.C.; Kakooei, S.; Rahim, A.A. Reviews on Corrosion Inhibitors: A Short View. Chem. Eng. Commun. 2016, 203, 1145–1156. [Google Scholar] [CrossRef]
- Vorobyova, V.; Skiba, M. Peach Pomace Extract as Efficient Sustainable Inhibitor for Carbon Steel Against Chloride-Induced Corrosion. J. Bio Tribo-Corros. 2021, 7, 11. [Google Scholar] [CrossRef]
- Dewangan, A.K.; Dewangan, Y.; Verma, D.K.; Verma, C. Synthetic Environment-Friendly Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Ahmed, S.K.; Ali, W.B.; Khadom, A.A. Synthesis and investigations of heterocyclic compounds as corrosion inhibitors for mild steel in hydrochloric acid. Int. J. Ind. Chem. 2019, 10, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Thakur, A.; Kumar, A. Sustainable Inhibitors for Corrosion Mitigation in Aggressive Corrosive Media: A Comprehensive Study. J. Bio Tribo-Corros. 2021, 7, 67. [Google Scholar] [CrossRef]
- Honarvar Nazari, M.; Shihab, M.S.; Havens, E.A.; Shi, X. Mechanism of corrosion protection in chloride solution by an apple-based green inhibitor: Experimental and theoretical studies. J. Infrastruct. Preserv. Resil. 2020, 1, 7. [Google Scholar] [CrossRef]
- Ikhmal, W.M.K.W.M.; Yasmin, M.Y.N.; Maria, M.F.F.; Syaizwadi, S.M.; Rafizah, W.A.W.; Sabri, M.G.M.; Zahid, B.M. Evaluating the performance of andrographis paniculata leaves extract as additive for corrosion protection of stainless steel 316l in seawater. Int. J. Corros. Scale Inhib. 2020, 9, 118–133. [Google Scholar]
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Organic Corrosion Inhibitors; Itors, Principles and Recent Applications, Mahmood Aliofkhazraei; IntechOpen: London, UK, 2017; pp. 1–32. [Google Scholar]
- Vera, R.; Figueredo, F.; Díaz-Gómez, A.; Molinari, A. Evaluation of Fuji Apple Peel Extract as a Corrosion Inhibitor for Carbon Steel in a Saline Medium. Int. J. Electrochem. Sci. 2018, 13, 4139–4159. [Google Scholar] [CrossRef]
- Vorobyova, V.I.; Skiba, M.I.; Shakun, A.S.; Nahirniak, S.V. Relationship between the inhibition and antioxidant properties of the plant and biomass wastes extracts-A Review. Int. J. Corros. Scale Inhib 2019, 8, 150–178. [Google Scholar]
- Abdallah, M.; Fawzy, A.; Hawsawi, H.; Hameed, R.S.A.; Al-Juaid, S.S. Estimation of Water-Soluble Polymers (Poloxamer and Pectin) as Corrosion Inhibitors for Carbon Steel in Acidic Medium. Int. J. Electrochem. Sci. 2020, 15, 8129–8144. [Google Scholar] [CrossRef]
- Fiori-Bimbi, M.V.; Alvarez, P.E.; Vaca, H.; Gervasi, C.A. Corrosion inhibition of mild steel in HCL solution by pectin. Corros. Sci. 2015, 92, 192–199. [Google Scholar] [CrossRef]
- Prabakaran, M.; Ramesh, S.; Periasamy, V.; Sreedhar, B. The corrosion inhibition performance of pectin with propyl phosphonic acid and Zn2+ for corrosion control of carbon steel in aqueous solution. Res. Chem. Intermed. 2015, 41, 4649–4671. [Google Scholar] [CrossRef]
- Huang, L.; Yang, K.P.; Zhao, Q.; Li, H.J.; Wang, J.Y.; Wu, Y.C. Corrosion resistance and antibacterial activity of procyanidin B2 as a novel environment-friendly inhibitor for Q235 steel in 1 M HCl solution. Bioelectrochemistry 2022, 143, 107969. [Google Scholar] [CrossRef]
- Dehghani, A.; Mostafatabar, A.H.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Detailed-level computer modeling explorations complemented with comprehensive experimental studies of Quercetin as a highly effective inhibitor for acid-induced steel corrosion. J. Mol. Liq. 2020, 309, 113035. [Google Scholar] [CrossRef]
- Abdelhafez, A.; Awad, Y.; El-Azeem, S.A.; Kim, M.; Ham, K.-J.; Lim, K.; Yang, J.-E.; Ok, Y. Leaching of Chromium, Copper and Arsenic in Soils and Rapid Identification of CCA-Treated Woods Using Modified PAN Stain. Korean J. Soil Sci. Fert 2010, 43, 60–67. [Google Scholar]
- Schiopu, N.; Tiruta-Barna, L. Wood Preservatives; Woodhead Publishing: Cambridge, UK, 2012; pp. 138–165. [Google Scholar]
- Woźniak, M.; Mania, P.; Roszyk, E.; Ratajczak, I. Bending Strength of Wood Treated with Propolis Extract and Silicon Compounds. Materials 2021, 14, 819. [Google Scholar] [CrossRef]
- WHO. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/332193/9789240005662-eng.pdf?ua=1 (accessed on 21 January 2022).
- Morais, S.; Fonseca, H.M.A.C.; Oliveira, S.M.R.; Oliveira, H.; Gupta, V.K.; Sharma, B.; de Lourdes Pereira, M. Environmental and Health Hazards of Chromated Copper Arsenate-Treated Wood: A Review. Int. J. Environ. Res. Public Health 2021, 18, 5518. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Gołębiewska, E.; Świderski, G.; Męczyńska-Wielgosz, S.; Lewandowska, H.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Świsłocka, R.; Samsonowicz, M.; et al. Plant-Derived and Dietary Hydroxybenzoic Acids—A Comprehensive Study of Structural, Anti-/Pro-Oxidant, Lipophilic, Antimicrobial, and Cytotoxic Activity in MDA-MB-231 and MCF-7 Cell Lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.; Yamamoto, R. Natural resistance of Alaska-cedar, redwood, and teak to Formosan subterranean termites. For. Prod. J. 1994, 44, 41–45. [Google Scholar]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J.; Tsunoda, K. Effects of heartwood extractive fractions of Thuja plicata and Chamaecyparis nootkatensis on wood degradation by termites or fungi. J. Wood Sci. 2006, 52, 147–153. [Google Scholar] [CrossRef]
- Borges, C.C.; Tonoli, G.H.D.; Cruz, T.M.; Duarte, P.J.; Junqueira, T.A. Nanoparticles-based wood preservatives: The next generation of wood protection? CERNE 2018, 24, 397–407. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Santana, A.L.B.D.; Maranhão, C.A.; Oliveira, L.S.; Bieber, L. Phenolic Extractives and Natural Resistance of Wood. In Biodegradation-Life of Science; IntechOpen: London, UK, 2013; pp. 349–370. [Google Scholar]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. In Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020; pp. 517–532. [Google Scholar]
- Böttger, A.; Vothknecht, U.; Bolle, C.; Wolf, A. Plant Secondary Metabolites and Their General Function in Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–17. [Google Scholar]
- Surendran, A.; Siddiqui, Y.; Ahmad, K.; Fernanda, R. Deciphering the physicochemical and microscopical changes in ganoderma boninense-infected oil palm woodblocks under the influence of phenolic compounds. Plants 2021, 10, 1797. [Google Scholar] [CrossRef] [PubMed]
- Serine, N.; Ashitani, T.; Murayama, T.; Shibutani, S.; Hattori, S.; Takahashi, K. Bioactivity of latifolin and its derivatives against termites and fungi. J. Agric. Food Chem. 2009, 57, 5707–5712. [Google Scholar]
- Little, N.S.; Schultz, T.P.; Nicholas, D.D. Termite-resistant heartwood. Effect of antioxidants on termite feeding deterrence and mortality. Holzforschung 2010, 64, 395–398. [Google Scholar] [CrossRef]
- Boué, S.M.; Raina, A.K. Effects of plant flavonoids on fecundity, survival, and feeding of the Formosan subterranean termite. J. Chem. Ecol. 2003, 29, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ohmura, W.; Doi, S.; Aoyama, M. Termite feeding deterrent from Japanese larch wood. Bioresour. Technol. 2004, 95, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Efhamisisi, D.; Thevenon, M.F.; Hamzeh, Y.; Pizzi, A.; Karimi, A.; Pourtahmasi, K. Tannin-boron complex as a preservative for 3-ply beech plywoods designed for humid conditions. Holzforschung 2020, 71, 249–258. [Google Scholar] [CrossRef]


| Product | Pyrolysis Type | Pyrolysis Parameters | Products Obtained | Net Caloric Values | Applications | Ref. |
|---|---|---|---|---|---|---|
| Apple pomace | Rapid | The temperature in the reactor was gradually increased to 850 °C over 30 min. Pyrolysis was continued for 60 min at a constant temperature. The experiments were carried out in triplicate with seven gas collection points (450, 515, 585, 650, 715, 785 and 850 °C). | Gas fraction: 47.5% Oil fraction: 14.1% Water: 12.6% Biochar: 25.9% | 30.948 ± 168 kJ/kg (biochar) 19.775,2 ± 125,8 kJ/kg (biomass) | Biochar and gaseous products with sufficiently high combustion heat and net calorific values. Can be applied as additives in other fuels | [43] |
| Flavoured spirits production waste (FSW) (lime, grapefruit and lemon) | Gas fraction: 42.0% Oil fraction: 21.7% Water: 9.7% Biochar: 26.7% | 26.598 ± 75 kJ/kg (biochar) 14.904,3 ± 553,8 kJ/kg (biomass) | ||||
| Beetroot pulp | Gas fraction: 32.9% Oil fraction: 24.6% Water: 12.3% Biochar: 30.2% | 25.572 ± 139 kJ/kg (biochar) 15.169,2 ± 25,8 kJ/kg (biomass) | ||||
| Apple pomace | Slow | Temperature: 300–450 °C; heating rate: 5–20 °C/min; residence time: 60 min | CO, CO2, CH4 | 7.639,18 kJ/kg (AP) | Solid product yield is maximum in slow pyrolysis | [44] |
| Apple pomace | Not defined | Temperature: 600 °C; (pyrolysis followed by immersion ageing in Fe(II)/Fe(III) aqueous solution for obtaining AP-based magnetic biochar) | Magnetic AP biochar | Not defined | Magnetic AP biochar that can be used for enriching Ag(I) in effluents | [45] |
| Apple pomace | Not defined | Pyrolysis was carried out in a pilot bubbling fluidised bed pyrolyser operating under a range of temperature from 300 to 600 °C and vapour residence times ranging from 2 to 5 s. | Major gases: H2, CO, CO2, CH4; biochar; bio-oil | ≈4–6 kJ/g (biomass) | A promising material for biochar production | [9] |
| Grape residues (GS—grape skins; GSS—grape skins and seeds) | ≈0.1–4.1 kJ/g (biomass) ≈0.2–3.5 kJ/g (biomass) |
| Material for Research: Apple Variety, Sample Preparation | Extraction Method/Parameters | Analytical/Identification Method | Extract Composition/Identified Compounds | Antioxidant Activity | Ref. |
|---|---|---|---|---|---|
| Apple pomace separated from seeds and stems; a variety of apples not defined: (a) Fresh, only enzymatically stabilised; (b) Enzymatically stabilised, freeze-dried (−18 °C for 4h; 35 °C, 1.01 mbar for 20 h; 40 °C, 0.05 mbar for 6 h); (c) Enzymatically stabilised, oven-dried (50 °C for 4 days). | SFE; solvent: CO2; temp.: 45 and 55 °C; pressure: 20 and 30 MPa; extraction time: 120 min | Total phenolic content (Folin–Ciocalteu) | For 55 °C, 30 MPa: (a) 3.91 ± 0.27 mg GAE/g of extract; (b) 6.41 ± 0.19 mg GAE/g of extract; (c) 5.65 ± 0.14 mg GAE/g of extract | DPPH: (a) 1.93 ± 0.12 mg TEA/g of extract; (b) 3.24 ± 0.11 mg TEA/g of extract; (c) 2.72 ± 0.19 mg TEA/g of extract | [76] |
| SFE; solvent: CO2 and ethanol (5%); temp.: 45 and 55 °C; pressure: 20 and 30 MPa; extraction time: 120 min | For 55 °C, 30 MPa: (a) 6.13 ± 0.16 mg GAE/g of extract; (b) 8.87 ± 0.17 mg GAE/g of extract; (c) 7.31 ± 0.18 mg GAE/g of extract | DPPH: (a) 2.67 ± 0.14 mg TEA/g of extract; (b) 5.99 ± 0.11 mg TEA/g of extract; (c) 4.73 ± 0.11 mg TEA/g of extract | [76] | ||
| Soxhlet; solvent: ethanol; temp.: boiling temp. of ethanol; extraction time: 6 h | (a) 4.01 ± 0.06 mg GAE/g of extract; (b) 4.13 ± 0.90 mg GAE/g of extract; (c) 3.31 ± 0.12 mg GAE/g of extract | DPPH: (a) 1.96 ± 0.10 mg TEA/g of extract; (b) 2.05 ± 0.21 mg TEA/g of extract; (c) 1.38 ± 0.29 mg TEA/g of extract | [76] | ||
| Boiling water maceration; solvent: water; temp.: 100 °C; extraction time: 37 min; 0.01 g/mL (solid-to-solvent ratio) | (a) 2.41 ± 0.01 mg GAE/g of extract; (b) 2.37 ± 0.01 mg GAE/g of extract; (c) 1.08 ± 0.11 mg GAE/g of extract | DPPH: (a) 1.17 ± 0.01 mg TEA/g of extract; (b) 1.14 ± 0.01 mg TEA/g of extract; (c) 0.92 ± 0.01 mg TEA/g of extract | [76] | ||
| Apple pomace constituted by seed, stalks, peel and a small proportion of pulp; “Golden Delicious” variety; apple pomace was freeze-dried at −45 °C and then milled to a fine powder | SFE; solvent: CO2; temp.: 37, 46 and 55 °C; pressure: 300, 425 and 550 bar; extraction time: 100 min | UHPLC (Ultrahigh-performance liquid chromatography) | Main compounds: betulinic acid, oleanolic acid, ursolic acid, uvaol, erythrodiol, lupeol | ORAC: 609.17 ± 96.11 μmol; TE/g extract (46 °C, 425 bar); HORAC: 104.83 ± 8.82 μmol; CAE/g extract (46 °C, 425 bar) | [76] |
| Soxhlet; solvent: n-hexane; temp.: 70 °C; extraction time: 6 h | Main compounds: betulinic acid, oleanolic acid, ursolic acid, uvaol, erythrodiol, lupeol | ORAC: 565.95 ± 60.66 μmol; TE/g extract; HORAC: 193.20 ± 17.49 μmol; CAE/g extract | [76] | ||
| Apple pomace composed of seeds, cores, stems, skin and parenchyma; obtained from Kiviks Musteri in Kivik, Sweden | PHWE; solvent: n-hexane; temp.: 25, 50, 112, 175 and 200 °C; extraction time: 3, 5, 10, 15 and 17 min; extractions were performed in 11 mL extraction cells, containing 5 g of fresh sample | Total phenols concentration calculated by RSM | 1.8 µmol/g of dry AP (170 °C, 3 min) | n.t. | [80] |
| Apple pomace; “Champion” variety; (a) conventional and (b) ecological crops; fresh apple pomace was stored at 4 °C for 24 h | UAE; solvent: water; temp.: 20 °C; extraction time: 30 min; solid/liquid ratio of 1:20 (g/mL); US bath (50 Hz, 300 W) | Total phenolic content (Folin–Ciocalteu) | (a) 14.33 ± 0.26 mg/l; (b) 31.28 ± 0.29 mg/l | n.t. | [84] |
| UAE; solvent: ethanol; temp.: 20 °C; extraction time: 30 min; solid/liquid ratio of 1:20 (g/mL); US bath (50 Hz, 300 W) | (a) 28.46 ± 0.28 mg/l; (b) 44.34 ± 0.44 mg/l | n.t. | [84] | ||
| Apple pomace separated from seeds and petioles; “Red Delicious” variety; blended | UAE; solvent: ethanol and water in different ratios ((a) 50:50, (b) 70:30, and (c) 30:70, v/v); temp.: 60 °C; extraction time: 60 min; solid/liquid ratio of 1:10 (g/mL) | Total phenolic content (Folin–Ciocalteu) | (a) 1062.9 ± 59.80 µg GAE/g of fresh AP; (b) ≈ 900 µg GAE/g of fresh AP; (c) ≈ 800 µg GAE/g of fresh AP | n.t. | [86] |
| Apple pomace obtained from Val-de-Vire Bioactives (Conde-sur-Vire, France); kept in the dark | UAE; solvent: water; temp.: 40 °C; extraction time: 40 min; solid/liquid ratio 150 g/mL; US bath (25 kHz, 150 W) | Total phenolic content (Folin–Ciocalteu) | Predicted/calculated value: 555 mg of catechin equivalent/100 g of dry AP | n.t. | [89] |
| Source | The Most Frequently Occurring Active Compounds | Metal and Electrolyte | Ref. |
|---|---|---|---|
| Fuji apple peel | 3,5,2′-Trihydroxy-7,8,4′-trimethoxyflavone 5- glucosyl-(1->2)-galactoside, 5-Methoxy-6″,6″-dimethyl-3′,4′- methylenedioxypyrano(2″,3″,7,8)flavone | Carbon steel, 0.1 M NaCl | [100] |
| Apple pomace | 1-Linoleoyl-sn-glycero-3-phosphocholine | Carbon steel, 3.5% NaCl | [97] |
| Pectin | - | Carbon steel, 1.0 M HCl | [102] |
| Pectin | - | Mild steel, 1.0 M HCl | [103] |
| Pectin | - | Carbon steel, H2O | [104] |
| Procyanidin B2 | - | Carbon steel, 1.0 M HCl | [105] |
| Quercetin | - | Mild steel, 1.0 M HCl | [106] |
| Active Ingredient | Toxicity Class | Lethal Dose (LD50) * (mg/kg) | Main Use |
|---|---|---|---|
| Azaconazole | II. Moderately hazardous | 308 | Fungicide |
| Copper hydroxide | II. Moderately hazardous | 1000 | Fungicide |
| Copper oxychloride | II. Moderately hazardous | 1440 | Fungicide |
| Copper sulphate | II. Moderately hazardous | 300 | Fungicide |
| Chlorpyrifos | II. Moderately hazardous | 135 | Insecticide |
| Fipronil | II. Moderately hazardous | 92 | Insecticide |
| Thiamethoxam | II. Moderately hazardous | 871 | Insecticide |
| Disodium tetraborate (Borax) | III. Slightly hazardous | 4500 | Fungicide |
| Fenpropimorph | III. Slightly hazardous | 3515 | Fungicide |
| Tebuconazole | III. Slightly hazardous | 1700 | Fungicide |
| Dichlofluanid | U. Unlikely to present acute hazard in normal use | >5000 | Fungicide |
| Fenoxycarb | U. Unlikely to present acute hazard in normal use | >10,000 | Fungicide |
| Active Compounds | Wood Protected | Protection against | Results | Ref. |
|---|---|---|---|---|
| Benzoic acid Salicylic acid Syringic acid Vanillic acid | Oil palm (Elaeis guineensis Jacq.) | Ganoderma boninense | Controlled BSR disease. | [119] |
| Latifolin 2’-O-methyllatifolin Latifolin dimethyl ether Latifolin diacetate | Studies on paper discs | Trametes versicolor Fomitopsis palustris Reticulitermes speratus (Kolbe) | A significant activity of Latifolin against tested termites and fungi. | [120] |
| Quercetin Morin Catechin Tannic acid | Pinus sp. | Reticulitermes flavipes | A significant activity of catechin and tannic acid against tested termites. | [121] |
| Condensed tannin | European beach (Fagus sylvatica L.) | Trametes versicolor Reticulitermes flavipes | Increased resistance against tested termites and fungi. | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788. https://doi.org/10.3390/ma15051788
Gołębiewska E, Kalinowska M, Yildiz G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials. 2022; 15(5):1788. https://doi.org/10.3390/ma15051788
Chicago/Turabian StyleGołębiewska, Ewelina, Monika Kalinowska, and Güray Yildiz. 2022. "Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors" Materials 15, no. 5: 1788. https://doi.org/10.3390/ma15051788
APA StyleGołębiewska, E., Kalinowska, M., & Yildiz, G. (2022). Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials, 15(5), 1788. https://doi.org/10.3390/ma15051788







