Microstructure and Properties of Additively Manufactured AlCoCr0.75Cu0.5FeNi Multicomponent Alloy: Controlling Magnetic Properties by Laser Powder Bed Fusion via Spinodal Decomposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens Preparation
2.2. Characterization Methods
3. Results
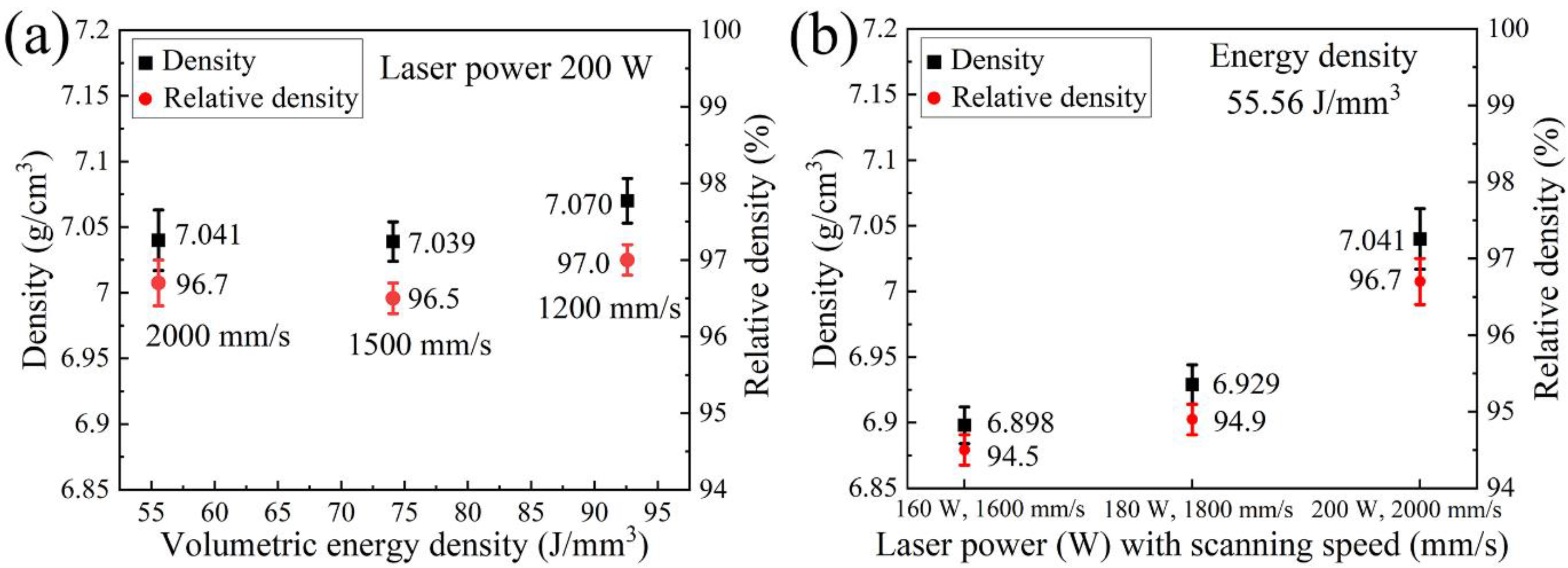
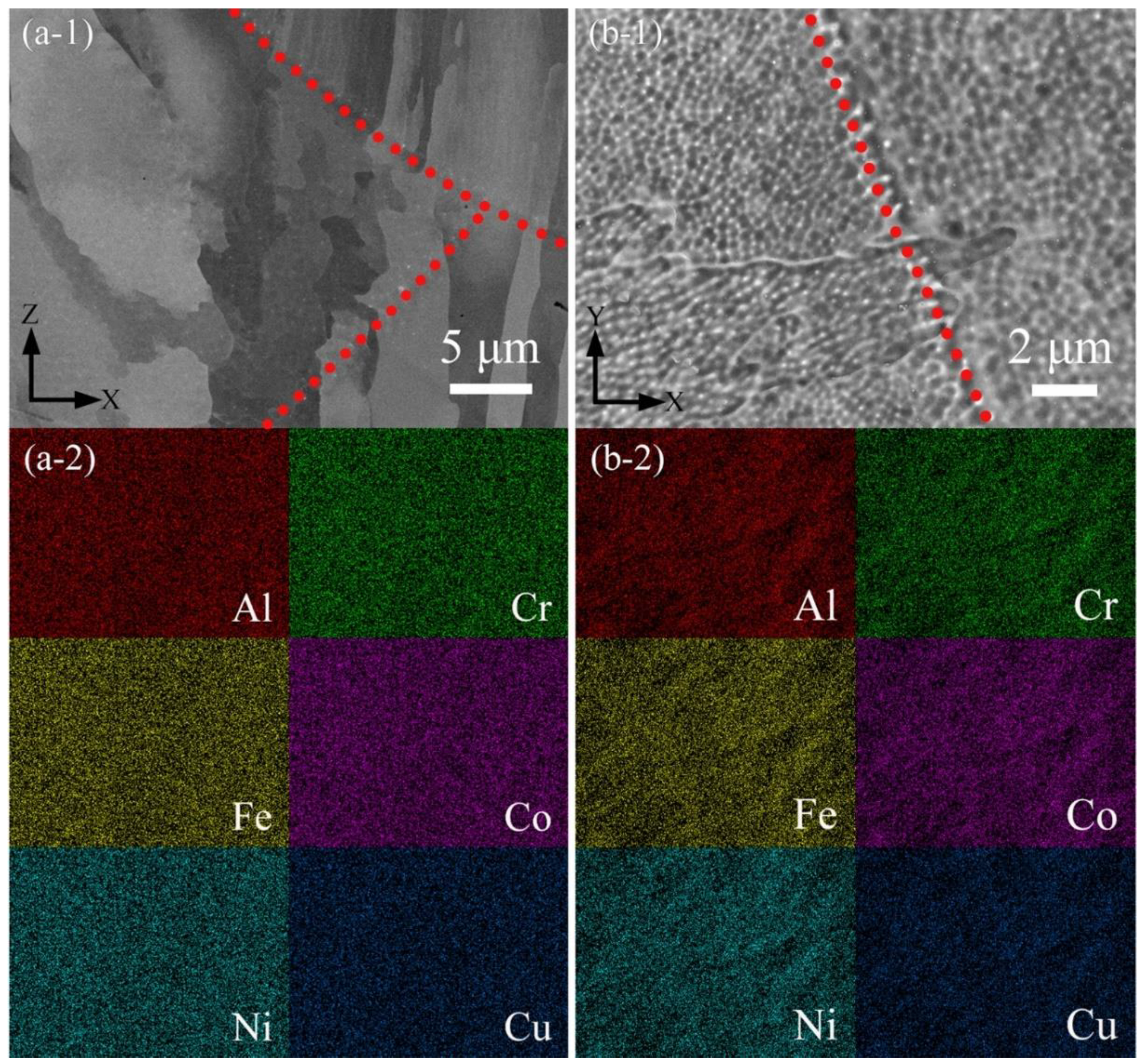
3.1. Density and Chemical Composition
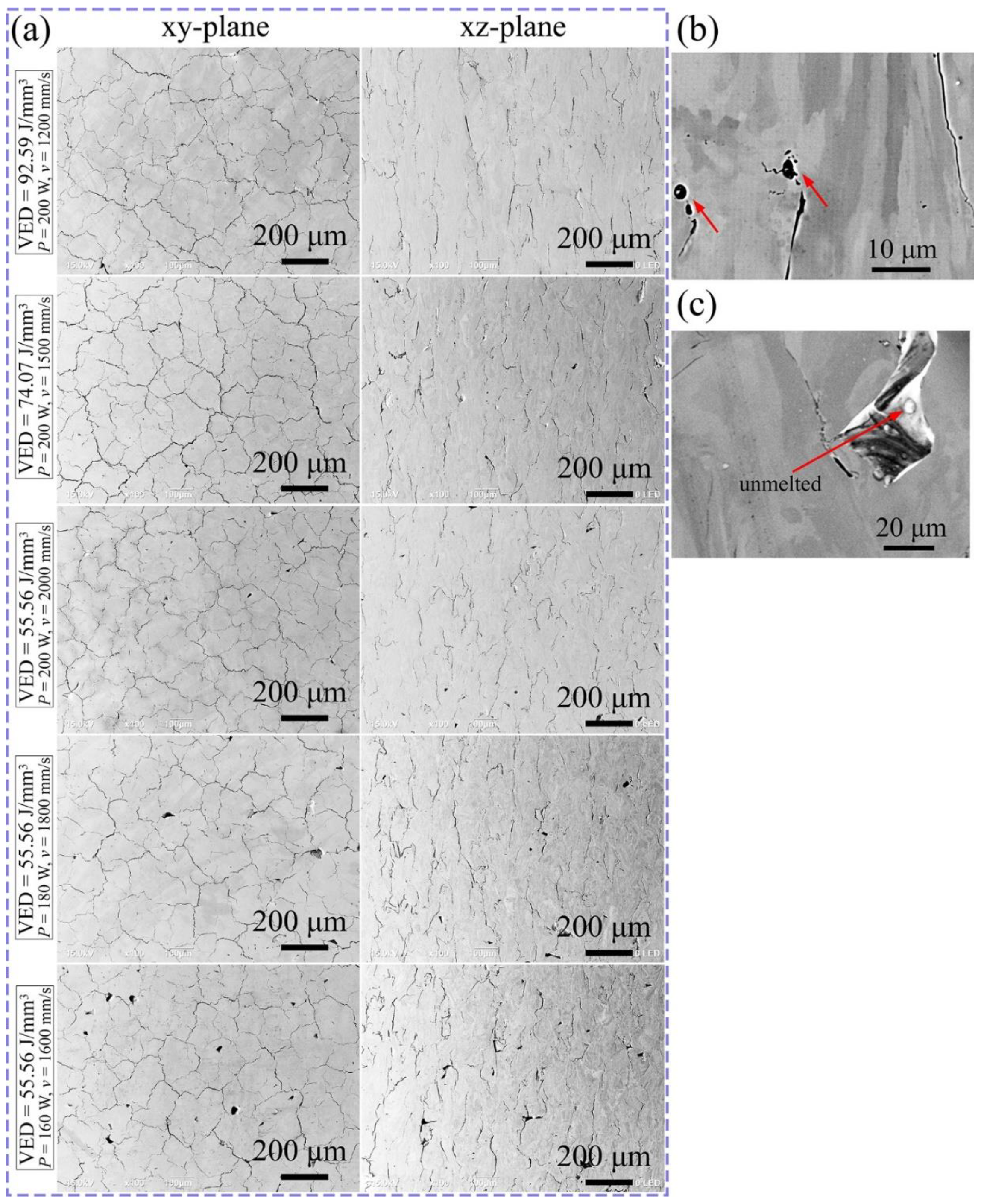
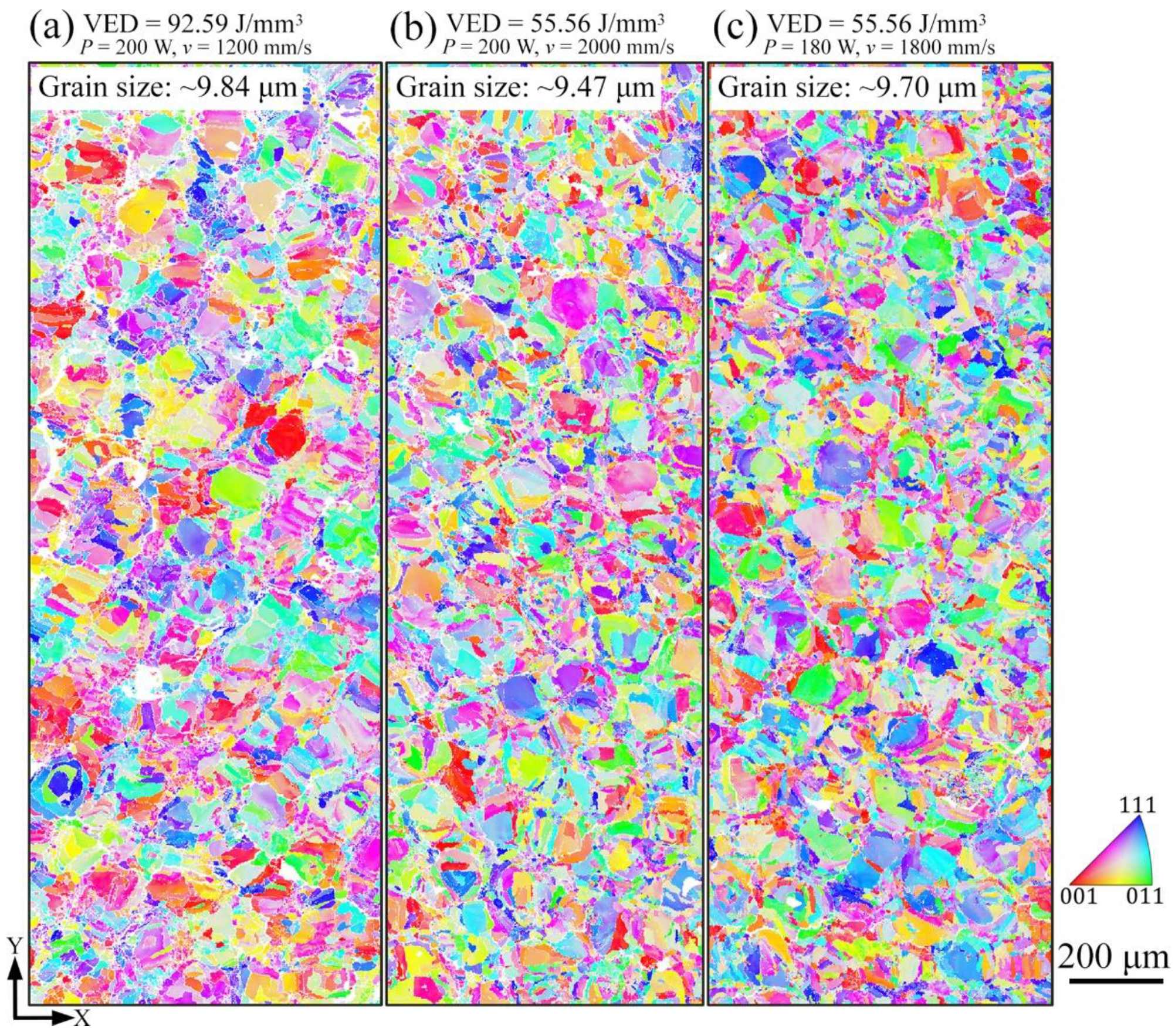
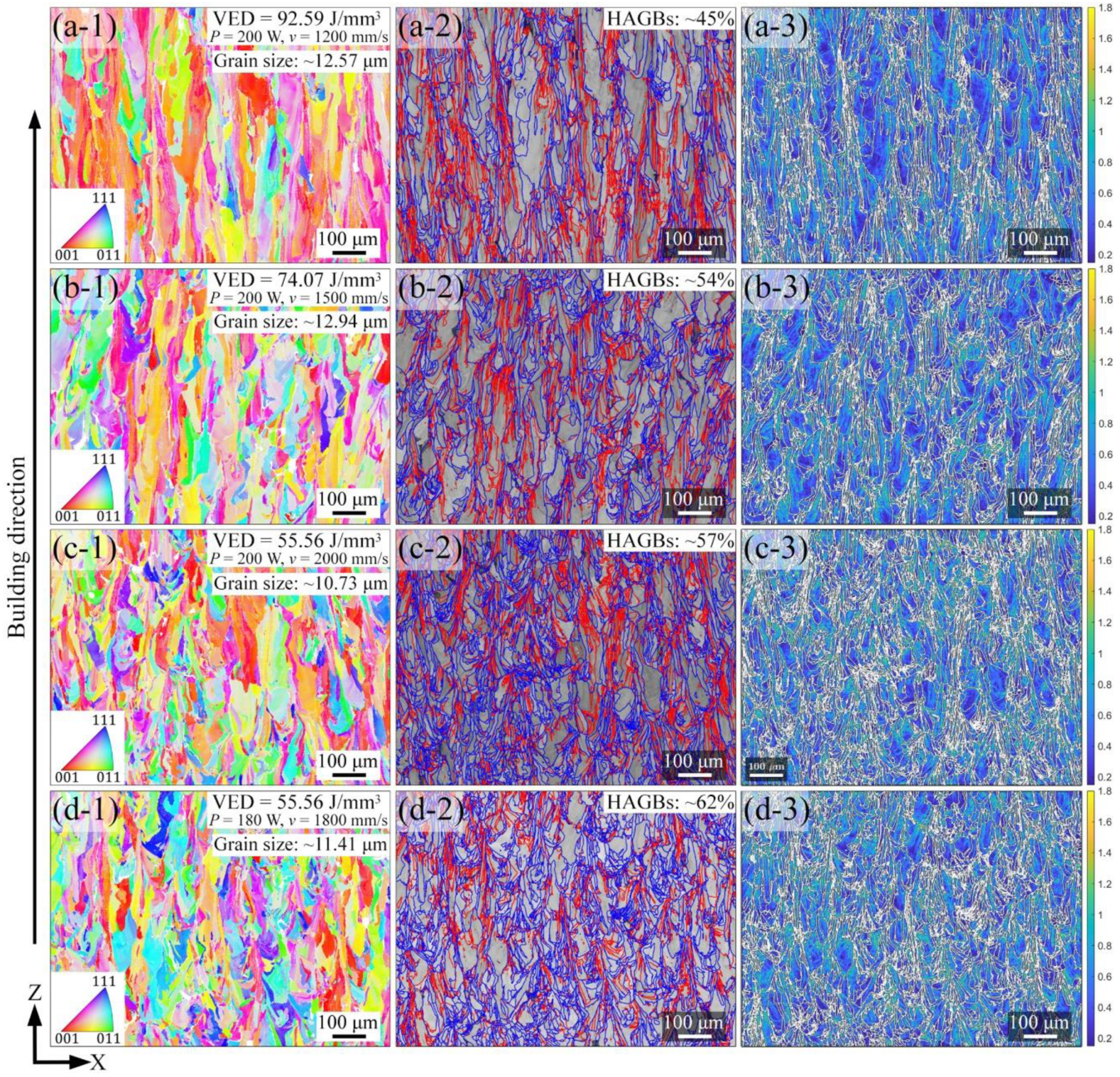
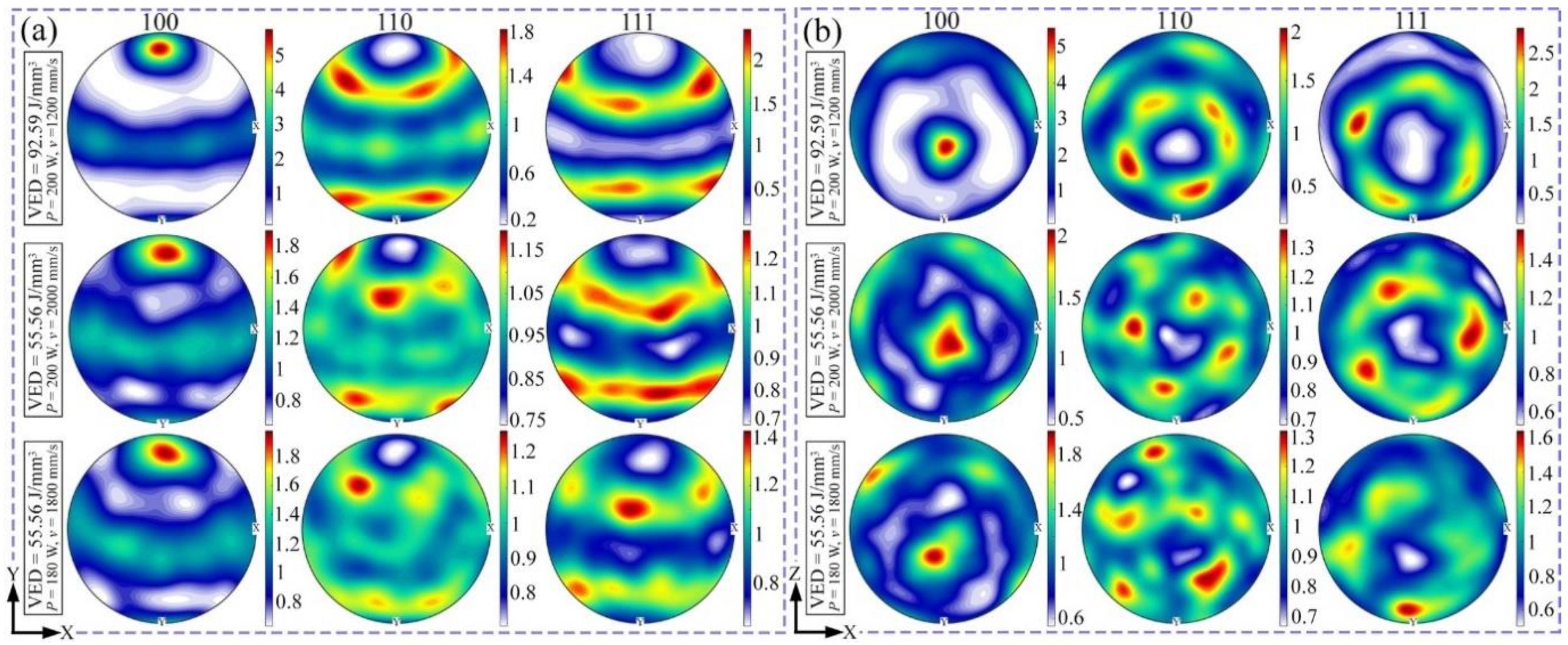
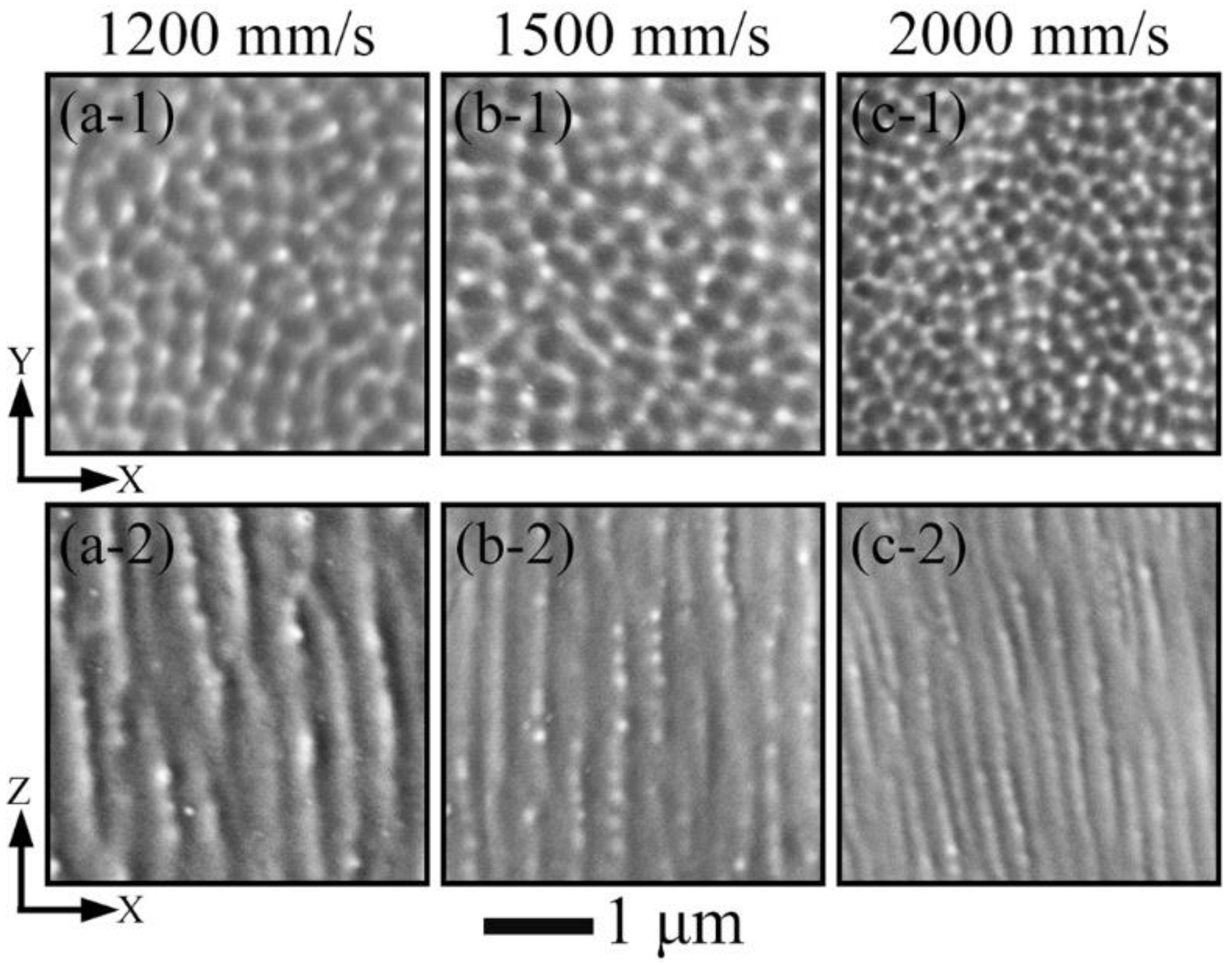
3.2. Phase and Microstructure
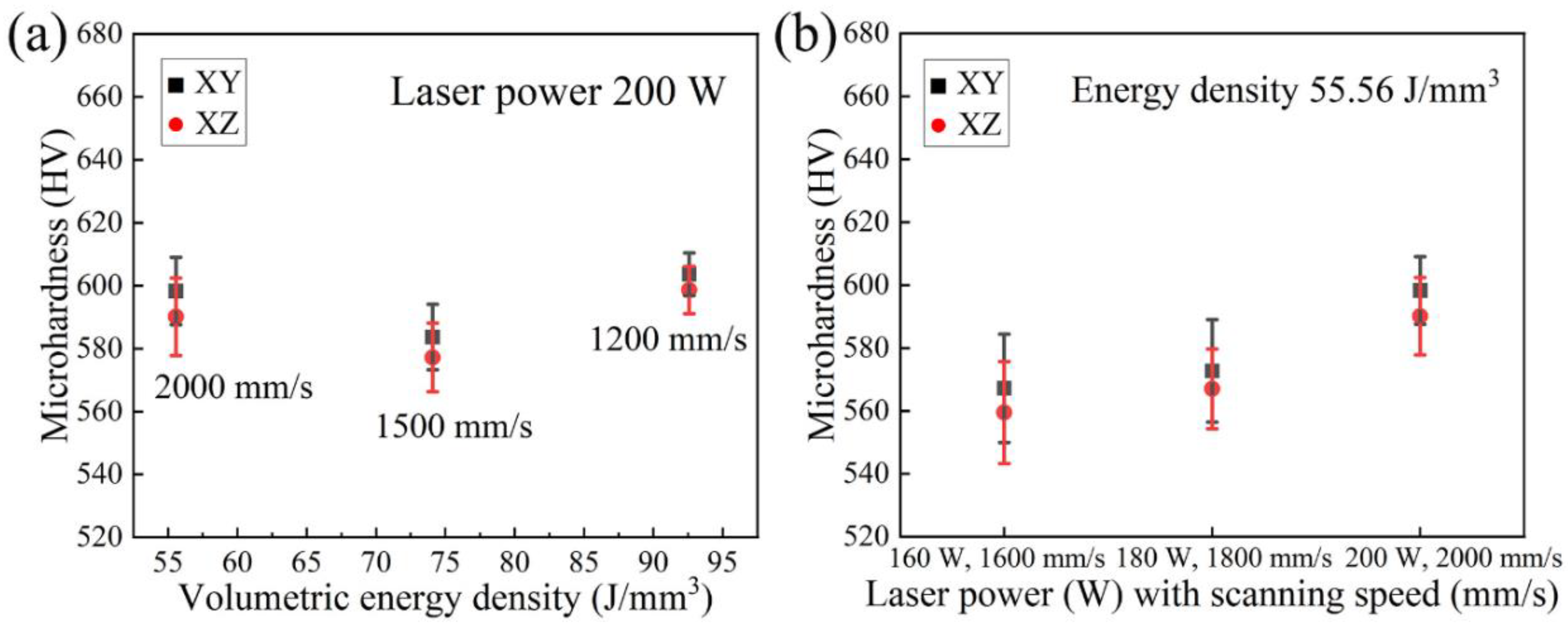
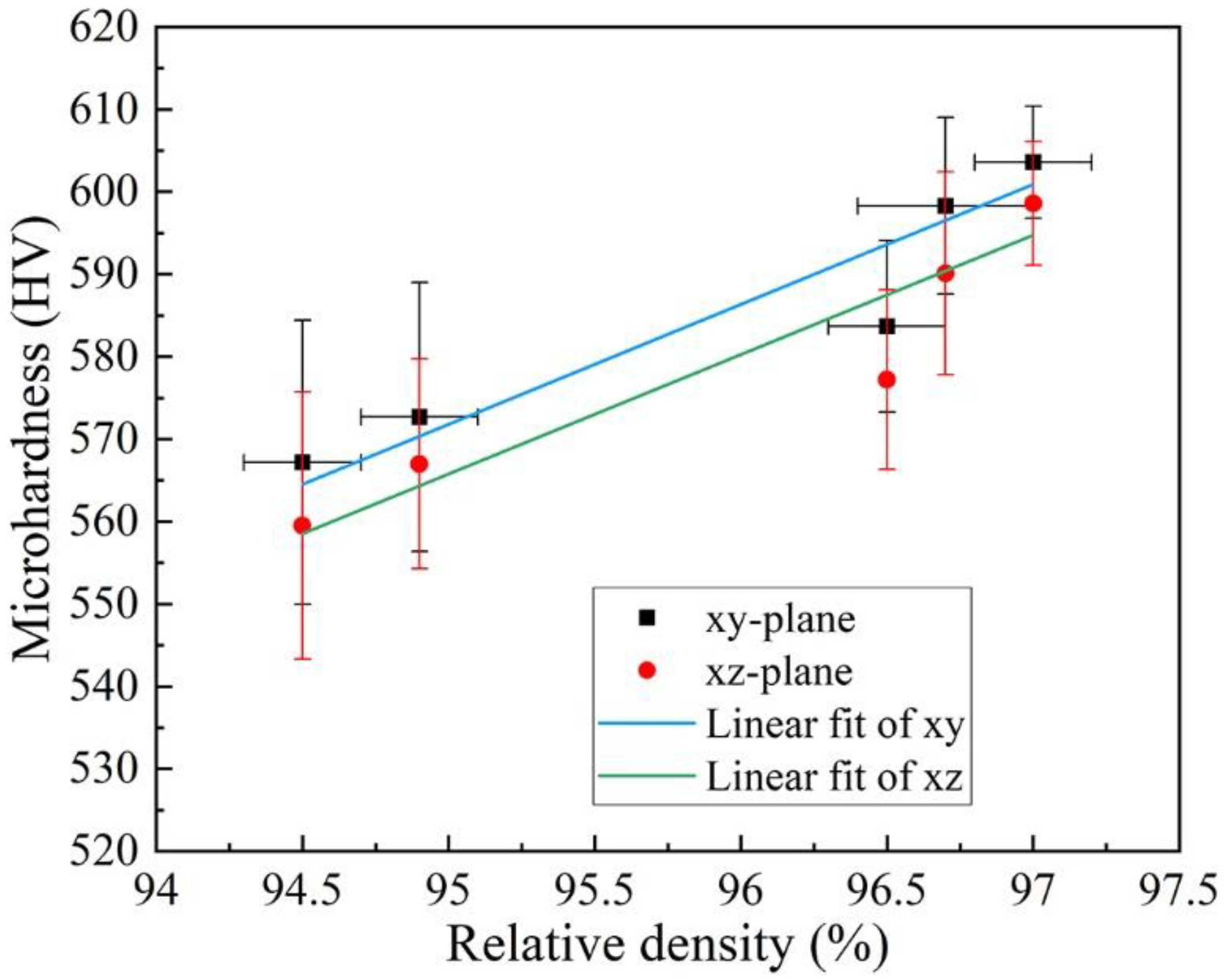
3.3. Microhardness
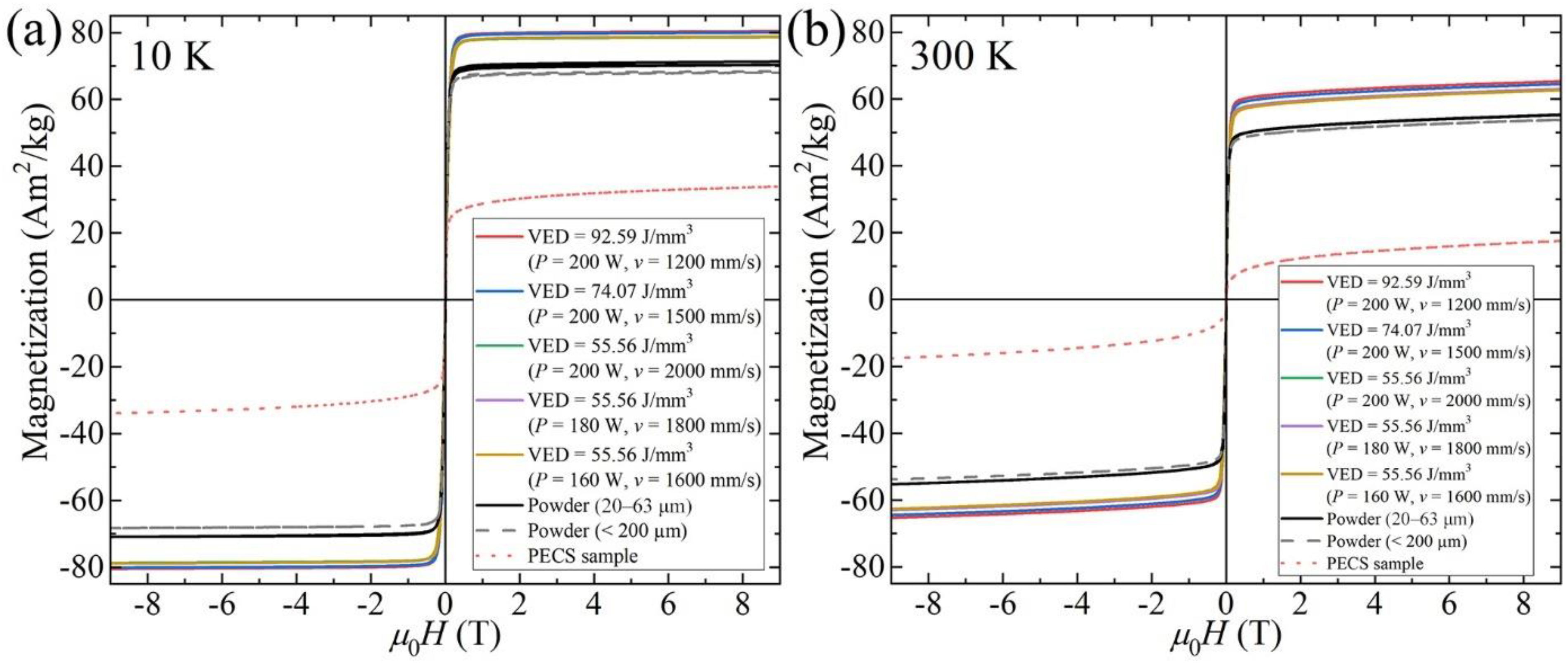
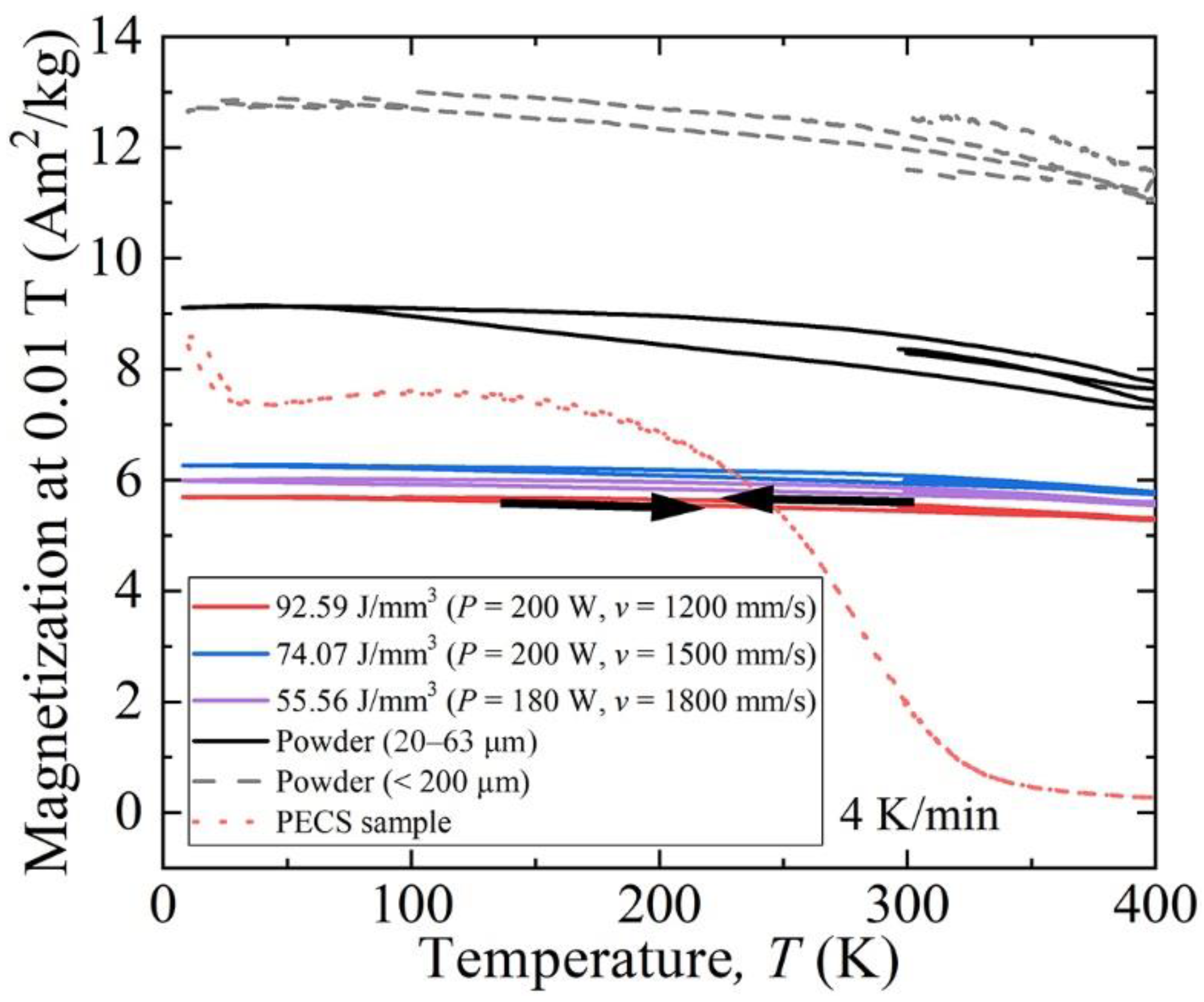
3.4. Magnetic Properties
4. Discussion
4.1. Effect of Process Parameters on General Microstructure
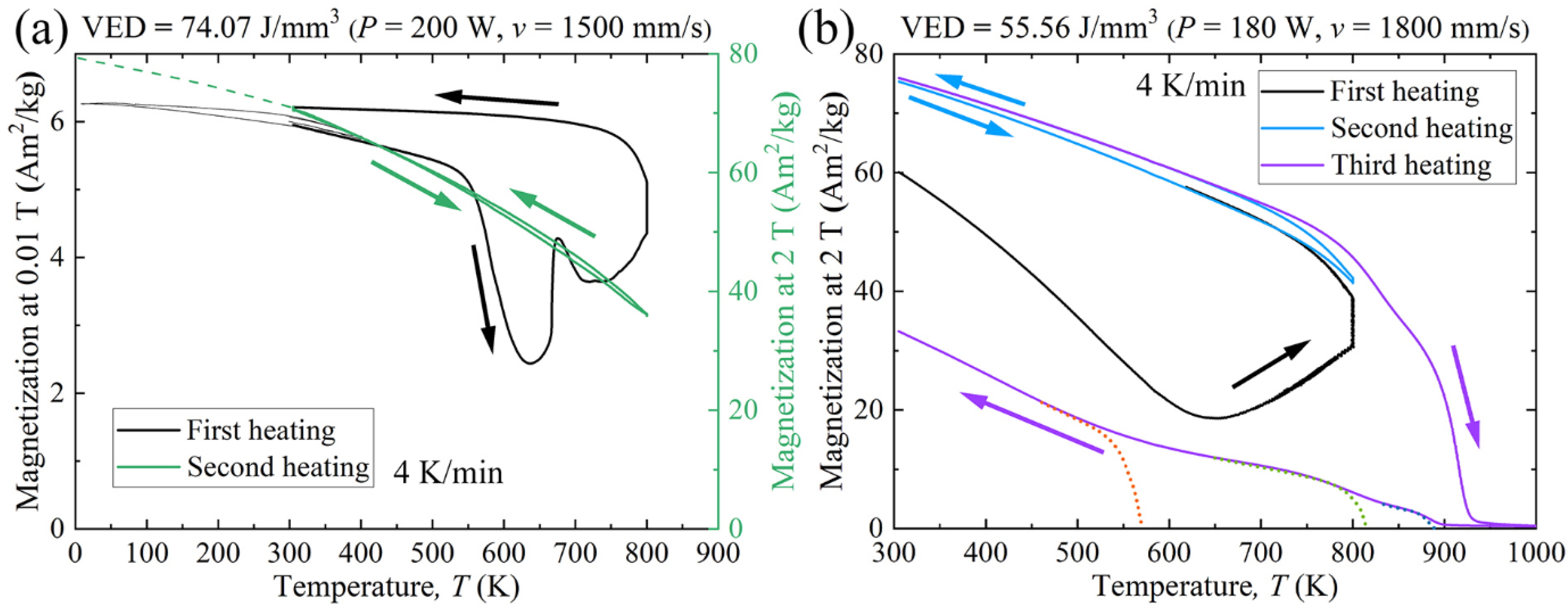
4.2. Effect of Microstructure Evolution on Magnetic Properties
5. Conclusions
- Two sets of process parameters have been employed, namely, the different VEDs corresponding to the alteration of scanning speed under the same laser power, and the same VED referring to different laser power and scanning speed. The observations demonstrate that these three parameters contribute to the different aspects during the process, and they should all be optimized to achieve the best results.
- All LPBF specimens are found to consist of BCC phase, while the reference PECS sample contains both FCC and BCC phase. The phase structure in LPBF specimens estimated by XRD reveals that the A2 structure formation is suppressed by the increasing cooling rate. Smaller cellular structures are formed by employing a higher scanning speed under the same laser power. This contributes to the high microhardness of 604.6 ± 6.8 HV0.05, despite the cracks and other defects. It is noticeably higher than 409.5 ± 4.2 HV1 of the reference PECS sample.
- Raw powder, LPBF specimens, and PECS sample all exhibit soft magnetic behavior. The highest Ms of 65.3 Am2/kg is reached in as-built alloy. The saturation magnetization is related to the spinodal decomposition into A2 and B2 phase structure, and the degree of decomposition is controlled by the cooling rate, which can be adjusted by means of altering the scanning speed in the LPBF process. As the scanning speed declines, corresponding to a decreased cooling rate, a higher degree of decomposition is conducted, leading to the formation of a larger fraction of A2 structure. It contributes to the enhancement of Ms comparable to the formerly reported value of the as-annealed sample, yet without the need for time-consuming post treatment. In addition, the decomposition is furthered via annealing at 800 K for 10 min, resulting in the increase of Ms, approximately by more than 15%.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable High-Entropy Dual-Phase Alloys Overcome the Strength–Ductility Trade-Off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-K.; Yeh, J.-W.; Shun, T.-T.; Chen, S.-K. Multi-Principal-Element Alloys with Improved Oxidation and Wear Resistance for Thermal Spray Coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Nene, S.S.; Frank, M.; Liu, K.; Sinha, S.; Mishra, R.S.; McWilliams, B.A.; Cho, K.C. Corrosion-Resistant High Entropy Alloy with High Strength and Ductility. Scr. Mater. 2019, 166, 168–172. [Google Scholar] [CrossRef]
- Kumar, N.A.P.K.; Li, C.; Leonard, K.J.; Bei, H.; Zinkle, S.J. Microstructural Stability and Mechanical Behavior of FeNiMnCr High Entropy Alloy under Ion Irradiation. Acta Mater. 2016, 113, 230–244. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Hu, X.; Yang, T.; Kumar, N.K.; Wirth, B.D.; Zinkle, S.J. Neutron Irradiation Response of a Co-Free High Entropy Alloy. J. Nucl. Mater. 2019, 527, 151838. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, M.C.; Diao, H.; Yang, T.; Liu, J.; Zuo, T.; Zhang, Y.; Lu, Z.; Cheng, Y.; Zhang, Y.; et al. Aluminum Alloying Effects on Lattice Types, Microstructures, and Mechanical Behavior of High-Entropy Alloys Systems. JOM 2013, 65, 1848–1858. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, Y.-L.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Microstructure Characterization of Al x CoCrCuFeNi High-Entropy Alloy System with Multiprincipal Elements. Met. Mat. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, F.J.; Chen, G.L. Phase Transformation Induced by Lattice Distortion in Multiprincipal Component CoCrFeNiCuxAl1−x Solid-Solution Alloys. Appl. Phys. Lett. 2008, 92, 241917. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Shaysultanov, D.G.; Stepanov, N.D.; Salishchev, G.A.; Senkov, O.N. Tensile Properties of an AlCrCuNiFeCo High-Entropy Alloy in as-Cast and Wrought Conditions. Mater. Sci. Eng. A 2012, 533, 107–118. [Google Scholar] [CrossRef]
- Tung, C.-C.; Yeh, J.-W.; Shun, T.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. On the Elemental Effect of AlCoCrCuFeNi High-Entropy Alloy System. Mater. Lett. 2007, 61, 1–5. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in Multi-Component AlCoCrCuFeNi High-Entropy Alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Ivchenko, M.V.; Pushin, V.G.; Uksusnikov, A.N.; Wanderka, N.; Kourov, N.I. Specific Features of Cast High-Entropy AlCrFeCoNiCu Alloys Produced by Ultrarapid Quenching from the Melt. Phys. Met. Metallogr. 2013, 114, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Lavernia, E.J.; Srivatsan, T.S. The Rapid Solidification Processing of Materials: Science, Principles, Technology, Advances, and Applications. J. Mater. Sci. 2010, 45, 287–325. [Google Scholar] [CrossRef]
- Kok, Y.; Tan, X.P.; Wang, P.; Nai, M.L.S.; Loh, N.H.; Liu, E.; Tor, S.B. Anisotropy and Heterogeneity of Microstructure and Mechanical Properties in Metal Additive Manufacturing: A Critical Review. Mater. Des. 2018, 139, 565–586. [Google Scholar] [CrossRef]
- Brif, Y.; Thomas, M.; Todd, I. The Use of High-Entropy Alloys in Additive Manufacturing. Scr. Mater. 2015, 99, 93–96. [Google Scholar] [CrossRef]
- Joseph, J.; Jarvis, T.; Wu, X.; Stanford, N.; Hodgson, P.; Fabijanic, D.M. Comparative Study of the Microstructures and Mechanical Properties of Direct Laser Fabricated and Arc-Melted Al x CoCrFeNi High Entropy Alloys. Mater. Sci. Eng. A 2015, 633, 184–193. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Wang, C.; Descoins, M.; Mangelinck, D.; Tor, S.B.; Jägle, E.A.; Zaefferer, S.; Raabe, D. Reducing Hot Tearing by Grain Boundary Segregation Engineering in Additive Manufacturing: Example of an AlxCoCrFeNi High-Entropy Alloy. Acta Mater. 2021, 204, 116505. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Niu, P.; Zhang, Z.; Yuan, T.; Yuan, J.; Li, K. Microstructures and Properties of Equimolar AlCoCrCuFeNi High-Entropy Alloy Additively Manufactured by Selective Laser Melting. Intermetallics 2020, 120, 106746. [Google Scholar] [CrossRef]
- Jung, H.Y.; Peter, N.J.; Gärtner, E.; Dehm, G.; Uhlenwinkel, V.; Jägle, E.A. Bulk Nanostructured AlCoCrFeMnNi Chemically Complex Alloy Synthesized by Laser-Powder Bed Fusion. Addit. Manuf. 2020, 35, 101337. [Google Scholar] [CrossRef]
- Guan, S.; Solberg, K.; Wan, D.; Berto, F.; Welo, T.; Yue, T.M.; Chan, K.C. Formation of Fully Equiaxed Grain Microstructure in Additively Manufactured AlCoCrFeNiTi0.5 High Entropy Alloy. Mater. Des. 2019, 184, 108202. [Google Scholar] [CrossRef]
- Scipioni Bertoli, U.; Guss, G.; Wu, S.; Matthews, M.J.; Schoenung, J.M. In-Situ Characterization of Laser-Powder Interaction and Cooling Rates through High-Speed Imaging of Powder Bed Fusion Additive Manufacturing. Mater. Des. 2017, 135, 385–396. [Google Scholar] [CrossRef]
- Hooper, P.A. Melt Pool Temperature and Cooling Rates in Laser Powder Bed Fusion. Addit. Manuf. 2018, 22, 548–559. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Chen, S.-K.; Chen, T.-J.; Chu, P.-C.; Yeh, J.-W.; Lin, S.-J. Electrical, Magnetic, and Hall Properties of AlxCoCrFeNi High-Entropy Alloys. J. Alloys Compd. 2011, 509, 1607–1614. [Google Scholar] [CrossRef]
- Borkar, T.; Chaudhary, V.; Gwalani, B.; Choudhuri, D.; Mikler, C.V.; Soni, V.; Alam, T.; Ramanujan, R.V.; Banerjee, R. A Combinatorial Approach for Assessing the Magnetic Properties of High Entropy Alloys: Role of Cr in AlCoxCr1–XFeNi. Adv. Eng. Mater. 2017, 19, 1700048. [Google Scholar] [CrossRef]
- Zuo, T.; Gao, M.C.; Ouyang, L.; Yang, X.; Cheng, Y.; Feng, R.; Chen, S.; Liaw, P.K.; Hawk, J.A.; Zhang, Y. Tailoring Magnetic Behavior of CoFeMnNiX (X = Al, Cr, Ga, and Sn) High Entropy Alloys by Metal Doping. Acta Mater. 2017, 130, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Shi, J.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J. Annealing on the Structure and Properties Evolution of the CoCrFeNiCuAl High-Entropy Alloy. J. Alloys Compd. 2010, 502, 295–299. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Cao, D.; Zhang, J.; Zhang, P.; Tao, Q.; Yang, X. Concurrence of Spinodal Decomposition and Nano-Phase Precipitation in a Multi-Component AlCoCrCuFeNi High-Entropy Alloy. J. Mater. Res. Technol. 2019, 8, 726–736. [Google Scholar] [CrossRef]
- Peter, N.J.; Duarte, M.J.; Liebscher, C.H.; Srivastava, V.C.; Uhlenwinkel, V.; Jägle, E.A.; Dehm, G. Early Stage Phase Separation of AlCoCr0.75Cu0.5FeNi High-Entropy Powder at the Nanoscale. J. Alloys Compd. 2020, 820, 153149. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-471-47741-9. [Google Scholar]
- Singh, S.; Wanderka, N.; Kiefer, K.; Siemensmeyer, K.; Banhart, J. Effect of Decomposition of the Cr–Fe–Co Rich Phase of AlCoCrCuFeNi High Entropy Alloy on Magnetic Properties. Ultramicroscopy 2011, 111, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Dutta, B.; Körmann, F.; Lu, W.; Zhou, X.; Liu, C.; Silva, A.K.; Wiedwald, U.; Spasova, M.; Farle, M.; et al. Beyond Solid Solution High-Entropy Alloys: Tailoring Magnetic Properties via Spinodal Decomposition. Adv. Funct. Mater. 2021, 31, 2007668. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mandal, G.K.; Ciftci, N.; Uhlenwinkel, V.; Mädler, L. Processing of High-Entropy AlCoCr0.75Cu0.5FeNi Alloy by Spray Forming. J. Mater. Eng. Perform. 2017, 26, 5906–5920. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Humbeeck, J.V.; Kruth, J.-P. A Study of the Microstructural Evolution during Selective Laser Melting of Ti–6Al–4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- McMahon, C.; Soe, B.; Loeb, A.; Vemulkar, A.; Ferry, M.; Bassman, L. Boundary Identification in EBSD Data with a Generalization of Fast Multiscale Clustering. Ultramicroscopy 2013, 133, 16–25. [Google Scholar] [CrossRef]
- Lehto, P.; Remes, H.; Saukkonen, T.; Hänninen, H.; Romanoff, J. Influence of Grain Size Distribution on the Hall–Petch Relationship of Welded Structural Steel. Mater. Sci. Eng. A 2014, 592, 28–39. [Google Scholar] [CrossRef] [Green Version]
- ISO Standard 6507-1: 2018. In Metallic Materials—Vickers Hardness Test—Part 1: Test Method; International Organization for Standardization: Geneva, Switzerland, 2018.
- Huang, S.; Sing, S.L.; de Looze, G.; Wilson, R.; Yeong, W.Y. Laser Powder Bed Fusion of Titanium-Tantalum Alloys: Compositions and Designs for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2020, 108, 103775. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Gao, P.; Yu, H.; Yang, J.; Wang, Z.; Zeng, X. Selective Laser Melting of an Equiatomic AlCrCuFeNi High-Entropy Alloy: Processability, Non-Equilibrium Microstructure and Mechanical Behavior. J. Alloys Compd. 2019, 771, 387–397. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H.; Nie, X.; Yin, J.; Hu, Z.; Zeng, X. Effect of Zirconium Addition on Crack, Microstructure and Mechanical Behavior of Selective Laser Melted Al-Cu-Mg Alloy. Scr. Mater. 2017, 134, 6–10. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Z.; Xiao, Y.; Horstemeyer, M.F.; Cui, X.; Chen, L. Insight into the Mechanisms of Columnar to Equiaxed Grain Transition during Metallic Additive Manufacturing. Addit. Manuf. 2019, 26, 22–29. [Google Scholar] [CrossRef]
- Unnikrishnan, R.; Northover, S.M.; Jazaeri, H.; Bouchard, P.J. Investigating Plastic Deformation around a Reheat-Crack in a 316H Austenitic Stainless Steel Weldment by Misorientation Mapping. Procedia Struct. Integr. 2016, 2, 3501–3507. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, K.; Saito, W.; Mori, M.; Matsumoto, H.; Sato, S.; Chiba, A. Abnormal Grain Growth in Commercially Pure Titanium during Additive Manufacturing with Electron Beam Melting. Materialia 2019, 6, 100281. [Google Scholar] [CrossRef]
- Yang, X.; Ge, Y.; Lehtonen, J.; Hannula, S.-P. Hierarchical Microstructure of Laser Powder Bed Fusion Produced Face-Centered-Cubic-Structured Equiatomic CrFeNiMn Multicomponent Alloy. Materials 2020, 13, 4498. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ding, Q.; Zhong, Y.; Zou, J.; Wu, J.; Chiu, Y.-L.; Li, J.; Zhang, Z.; Yu, Q.; Shen, Z. Dislocation Network in Additive Manufactured Steel Breaks Strength–Ductility Trade-Off. Mater. Today 2018, 21, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Scipioni Bertoli, U.; Wolfer, A.J.; Matthews, M.J.; Delplanque, J.-P.R.; Schoenung, J.M. On the Limitations of Volumetric Energy Density as a Design Parameter for Selective Laser Melting. Mater. Des. 2017, 113, 331–340. [Google Scholar] [CrossRef] [Green Version]
- King, W.E.; Barth, H.D.; Castillo, V.M.; Gallegos, G.F.; Gibbs, J.W.; Hahn, D.E.; Kamath, C.; Rubenchik, A.M. Observation of Keyhole-Mode Laser Melting in Laser Powder-Bed Fusion Additive Manufacturing. J. Mater. Processing Technol. 2014, 214, 2915–2925. [Google Scholar] [CrossRef]
- Rubenchik, A.M.; King, W.E.; Wu, S.S. Scaling Laws for the Additive Manufacturing. J. Mater. Processing Technol. 2018, 257, 234–243. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamoto, T.; Ozaki, T.; Sugita, K.; Mizuno, M.; Araki, H. Microstructural Formation and Characterization Mechanisms of Selective Laser Melted Al–Si–Mg Alloys with Increasing Magnesium Content. Mater. Sci. Eng. A 2019, 754, 786–798. [Google Scholar] [CrossRef]
- Kruth, J.P.; Froyen, L.; Van Vaerenbergh, J.; Mercelis, P.; Rombouts, M.; Lauwers, B. Selective Laser Melting of Iron-Based Powder. J. Mater. Processing Technol. 2004, 149, 616–622. [Google Scholar] [CrossRef]
- Sonawane, A.; Roux, G.; Blandin, J.-J.; Despres, A.; Martin, G. Cracking Mechanism and Its Sensitivity to Processing Conditions during Laser Powder Bed Fusion of a Structural Aluminum Alloy. Materialia 2021, 15, 100976. [Google Scholar] [CrossRef]
- Rappaz, M.; Drezet, J.-M.; Gremaud, M. A New Hot-Tearing Criterion. Met. Mat. Trans. A 1999, 30, 449–455. [Google Scholar] [CrossRef]
- Kontis, P.; Chauvet, E.; Peng, Z.; He, J.; da Silva, A.K.; Raabe, D.; Tassin, C.; Blandin, J.-J.; Abed, S.; Dendievel, R.; et al. Atomic-Scale Grain Boundary Engineering to Overcome Hot-Cracking in Additively-Manufactured Superalloys. Acta Mater. 2019, 177, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Kou, S. Solidification and Liquation Cracking Issues in Welding. JOM 2003, 55, 37–42. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Nguyen, Q.B.; Ng, F.L.; An, X.H.; Liao, X.Z.; Liaw, P.K.; Nai, S.M.L.; Wei, J. Hierarchical Microstructure and Strengthening Mechanisms of a CoCrFeNiMn High Entropy Alloy Additively Manufactured by Selective Laser Melting. Scr. Mater. 2018, 154, 20–24. [Google Scholar] [CrossRef]
- Belli, Y.; Okada, M.; Thomas, G.; Homma, M.; Kaneko, H. Microstructure and Magnetic Properties of Fe-Cr-Co-V Alloys. J. Appl. Phys. 1978, 49, 2049–2051. [Google Scholar] [CrossRef] [Green Version]



| Alloy | Method | Cooing Rate (K/s) | Phase Structure | Ref. |
|---|---|---|---|---|
| AlCoCrCuFeNi | Splat quenching | 106–107 | BCC | [13] |
| AlCoCr0.75Cu0.5FeNi | Gas atomization | 103–104 | BCC A2 + BCC B2 + Cu precipitates | [30] |
| AlCoCrCuFeNi | Spray casting | 102–103 | Dendrites: Al-Ni-rich and Fe-Cr-rich structures, with Cu-rich precipitates. Interdendrites: Cu-rich precipitates. | [29] |
| AlCoCrCuFeNi | Casting | 10–20 | Dendrites: Al-Ni-rich, Cr-Fe-rich, Al-Ni-Fe-rich, and Cu-rich precipitates. Interdendrites: Cu-rich. | [13] |
| Sample # | Power (W) | Scanning Speed (mm/s) | VED (J/mm3) |
|---|---|---|---|
| 1 | 200 | 1200 | 92.59 |
| 2 | 200 | 1500 | 74.07 |
| 3 | 200 | 2000 | 55.56 |
| 4 | 180 | 1800 | 55.56 |
| 5 | 160 | 1600 | 55.56 |
| Sample | Element (at%) | |||||
|---|---|---|---|---|---|---|
| Al | Cu | Fe | Ni | Co | Cr | |
| Powder [30] | 19.5 | 9.57 | 18.8 | 19.5 | 19.6 | 12.9 |
| VED = 92.59 J/mm3 (P = 200 W, v = 1200 mm/s) | 17.8 ± 0.12 | 9.3 ± 0.01 | 19.2 ± 0.09 | 20.1 ± 0.05 | 20.5 ± 0.04 | 13.1 ± 0.03 |
| VED = 74.07 J/mm3 (P = 200 W, v = 1500 mm/s) | 17.8 ± 0.35 | 9.3 ± 0.02 | 19.2 ± 0.13 | 20.0 ± 0.08 | 20.5 ± 0.10 | 13.2 ± 0.08 |
| VED = 55.56 J/mm3 (P = 200 W, v = 2000 mm/s) | 18.1 ± 0.10 | 9.4 ± 0.02 | 19.1 ± 0.03 | 19.8 ± 0.07 | 20.3 ± 0.02 | 13.3 ± 0.03 |
| VED = 55.56 J/mm3 (P = 180 W, v = 1800 mm/s) | 17.7 ± 0.30 | 9.5 ± 0.05 | 19.1 ± 0.08 | 20.0 ± 0.07 | 20.4 ± 0.07 | 13.3 ± 0.08 |
| VED = 55.56 J/mm3 (P = 160 W, v = 1600 mm/s) | 17.6 ± 0.26 | 9.5 ± 0.02 | 19.2 ± 0.04 | 20.0 ± 0.10 | 20.4 ± 0.13 | 13.3 ± 0.06 |
| PECS sample | 19.3 ± 0.15 | 9.6 ± 0.09 | 18.7 ± 0.07 | 19.6 ± 0.06 | 19.9 ± 0.09 | 12.9 ± 0.08 |
| Sample | Ms (Am2/kg) | Ms (300 K)/Ms (10 K) | |
|---|---|---|---|
| 10 K | 300 K | ||
| Powder (20–63 μm) | 71.3 | 55.3 | 0.78 |
| Powder (< 200 μm) | 68.4 | 53.8 | 0.79 |
| VED = 92.59 J/mm3 (P = 200 W, v = 1200 mm/s) | 80.5 | 65.3 | 0.81 |
| VED = 74.07 J/mm3 (P = 200 W, v = 1500 mm/s) | 80.1 | 64.4 | 0.80 |
| VED = 55.56 J/mm3 (P = 200 W, v = 2000 mm/s) | 78.7 | 63.0 | 0.80 |
| VED = 55.56 J/mm3 (P = 180 W, v = 1800 mm/s) | 78.9 | 63.0 | 0.80 |
| VED = 55.56 J/mm3 (P = 160 W, v = 1600 mm/s) | 78.9 | 62.6 | 0.79 |
| PECS sample | 33.9 | 17.6 | 0.52 |
| Alloy | Method | Ms (Am2/kg) | Annealing Temperature and Time | Ms after Annealing (Am2/kg) | Ref. |
|---|---|---|---|---|---|
| AlCoCr0.75Cu0.5FeNi (powder, 20–63 μm) | Gas atomization | 55.3 (at 9 T and 300 K) | - | - | This work |
| AlCoCr0.75Cu0.5FeNi (VED = 92.59 J/mm3 (P = 200 W, v = 1200 mm/s)) | LPBF | 65.3 (at 9 T and 300 K) | - | - | This work |
| AlCoCr0.75Cu0.5FeNi (VED = 55.56 J/mm3 (P = 180 W, v = 1800 mm/s)) | LPBF | 60.1 (at 2 T and 305 K) | 800 K, 10 min | 75.9 (at 2 T and 305 K) | This work |
| AlCoCrCuFeNi | Splat quenching | 46.0 (at 14 T and 300 K) | - | - | [32] |
| AlCoCrCuFeNi | Casting | 44.0 (at 14 T and 300 K) | - | - | [32] |
| AlCoCrCuFeNi | Casting | 38.2 (at 1.5 T and room temperature) | 1273.15 K, 2 h | 16.1 (at 1.5 T and room temperature) | [28] |
| Fe15Co15Ni20Mn20Cu30 | Homogenization | 12.0 (at 2 T and 300 K) | 873.15 K, 240 h | 21.0 (at 2 T and 300 K) | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Heczko, O.; Lehtonen, J.; Björkstrand, R.; Salmi, M.; Uhlenwinkel, V.; Ge, Y.; Hannula, S.-P. Microstructure and Properties of Additively Manufactured AlCoCr0.75Cu0.5FeNi Multicomponent Alloy: Controlling Magnetic Properties by Laser Powder Bed Fusion via Spinodal Decomposition. Materials 2022, 15, 1801. https://doi.org/10.3390/ma15051801
Yang X, Heczko O, Lehtonen J, Björkstrand R, Salmi M, Uhlenwinkel V, Ge Y, Hannula S-P. Microstructure and Properties of Additively Manufactured AlCoCr0.75Cu0.5FeNi Multicomponent Alloy: Controlling Magnetic Properties by Laser Powder Bed Fusion via Spinodal Decomposition. Materials. 2022; 15(5):1801. https://doi.org/10.3390/ma15051801
Chicago/Turabian StyleYang, Xuan, Oleg Heczko, Joonas Lehtonen, Roy Björkstrand, Mika Salmi, Volker Uhlenwinkel, Yanling Ge, and Simo-Pekka Hannula. 2022. "Microstructure and Properties of Additively Manufactured AlCoCr0.75Cu0.5FeNi Multicomponent Alloy: Controlling Magnetic Properties by Laser Powder Bed Fusion via Spinodal Decomposition" Materials 15, no. 5: 1801. https://doi.org/10.3390/ma15051801
APA StyleYang, X., Heczko, O., Lehtonen, J., Björkstrand, R., Salmi, M., Uhlenwinkel, V., Ge, Y., & Hannula, S.-P. (2022). Microstructure and Properties of Additively Manufactured AlCoCr0.75Cu0.5FeNi Multicomponent Alloy: Controlling Magnetic Properties by Laser Powder Bed Fusion via Spinodal Decomposition. Materials, 15(5), 1801. https://doi.org/10.3390/ma15051801








