Abstract
Molybdenum disulfide (MoS2) is the most well-known transition metal chalcogenide for lithium storage applications because of its simple preparation process, superior optical, physical, and electrical properties, and high stability. However, recent research has shown that bare MoS2 nanosheet (NS) can be reformed to the bulk structure, and sulfur atoms can be dissolved in electrolytes or form polymeric structures, thereby preventing lithium insertion/desertion and reducing cycling performance. To enhance the electrochemical performance of the MoS2 NSs, B2O3 nanoparticles were decorated on the surface of MoS2 NSs via a sintering technique. The structure of B2O3 decorated MoS2 changed slightly with the formation of a lattice spacing of ~7.37 Å. The characterization of materials confirmed the formation of B2O3 crystals at 30% weight percentage of H3BO3 starting materials. In particular, the MoS2_B3 sample showed a stable capacity of ~500 mAh·g−1 after the first cycle. The cycling test delivered a high reversible specific capacity of ~82% of the second cycle after 100 cycles. Furthermore, the rate performance also showed a remarkable recovery capacity of ~98%. These results suggest that the use of B2O3 decorations could be a viable method for improving the stability of anode materials in lithium storage applications.
1. Introduction
Low-dimensional layered structures of transition metal chalcogenides (TMCs) have attracted increased attention because of their superior properties, such as high conductivity, high stability, easy processing, and easy computing, in two-dimensional (2D) structures [1,2,3,4,5,6]. Therefore, various research has been undertaken to utilize TMCs in applications that traditionally used graphene materials [7,8,9,10]. Among them, MoS2 is the most well-known TMC material. MoS2 nanosheets (NSs) can be easily obtained through either top-down approaches, such as scotch tape, sonication, and chemical exfoliation, or bottom-up approaches, such as hydrothermal, chemical vapor deposition, and microwave-assisted methods. 2D MoS2 NSs possess high conductivity, flexibility, and a large surface area, thereby making them potential candidates for anode materials in lithium storage applications. The MoS2 NSs have a theoretical capacity of ~670 mAh·g−1, which is twice that of graphite (~372 mAh∙g−1) [11]. However, previous reports have shown that the MoS2 NS anodes undergo fast degradation due to the dissolution of sulfur atoms and dislocation of MoS2 nanosheets during the cycling process [12,13]. Moreover, the conversion reaction of MoS2 to form Li2S, the solid electrolyte interface (SEI) layer, and the degradation of the electrolyte resulted in the formation of a gel-like polymeric layer, which led to fast capacity fading [14]. Many attempts have been made to enhance the stability of MoS2 NSs based on the use of graphene/carbon nanotube (CNT)/carbon cloth as skeletons, carbon coating layers, or the addition of foreign materials (such as TiO2, MnO, Ag, and Sn) to prevent the restacking of MoS2 and co-contribute to the electrochemical conversion reaction with lithium [11,14,15,16,17,18,19,20]. For example, Kong et al. demonstrated that MoS2 nanoplates, with coverage of rolled-up graphene layers, form a core–shell MoS2@graphitic nanotube, which showed a high rate performance and high capacity without using a binder [21]. Yoo et al. used CNTs as skeletons to grow MoS2 via microwave irradiation [22]. The cylindrical-structured MoS2 on CNTs exhibited advantageous electrochemical properties, such as high rate and high stability, as anode materials in lithium-ion batteries (LIBs). Ren et al. combined both graphene and CNTs as a frame structure for the decoration of MoS2 nanoparticles (NPs), which delivered a high reversible capacity of ~600 mAh∙g−1 for 200 cycles [23]. Qu et al. decorated Fe2O3 NPs on MoS2 NSs via a hydrothermal method and sintering process, in which the anodes exhibited high-rate performances and a high reversible capacity of ~900 mAh∙g−1 [24]. Zhao et al. prepared the composition MoO3/MoS2, which has core–sheath structure, via a sulfurization technique [25]. The MoO3/MoS2 core–sheath anodes exhibited a negative fading phenomenon and achieved a capacity of ~1500 mAh∙g−1 after 150 cycles. Even though many attempts on improving the electrochemical performance of MoS2 NS have been made, the mechanisms are still not clearly revealed and further improvement in stability is still needed to meet the requirements of practical applications.
Recently, lithium nickel cobalt manganese oxide (NMC) cathode materials have been effectively enhanced their stability performance by using boron compounds, such as cobalt boride (CoxB) and B2O3, for surface modifications [26,27,28]. Yoon et al. revealed that CoxB metallic glass in Ni-rich NMC can effectively enhance the stability of cathode materials via reactive wetting [26]. Li et al. utilized B2O3 as a surface-modification material to enhance the performance of the NMC111 cathode [27]. The use of B2O3 also resulted in graphene combined with a MoS2 hierarchical structure, which improved the photo/electro properties of the graphene/MoS2 composition for bio applications [29]. Riyanto et al. reported that a boron-doped graphene quantum structure with MoS2 could deliver a high capacity of ~1000 mAh∙g−1 [30]. However, the effect of B2O3 in lithium-ion batteries has not been investigated. B2O3 is a low-cost material with low environmental pollution and easy processing, and it plays an important role in many applications such as thermochemical energy storage, the addition of glass fibers, and the synthesis of boron compound materials such as BN [31,32]. B2O3 is believed to enhance the electrochemical properties of MoS2 as it is conducted on 2D graphene materials.
In this study, we report the use of boron-oxide-nanoparticle-decorated MoS2 NSs as anode materials in LIBs. The MoS2 NSs were prepared using a chemical exfoliation method, and the decoration of B2O3 was carried out using a facile sintering technique. The results showed enhanced cycling stability in the MoS2 anode when B2O3 formed a crystal structure, delivering a reversible capacity of ~500 mAh∙g−1. These results suggest that the use of B2O3 can be a viable strategy for stabilizing anode materials for lithium storage applications.
2. Materials and Methods
2.1. Chemical Materials
Molybdenum (VI) sulfide (MoS2, powder, 98%), boric acid (H3BO3, powder > 99.5%), solution of n-butyllithium in hexane (2.5 M), 1-methyl-2-pyrrolidone (NMP, anhydrous, 99.5%), and polyvinylidene fluoride (PVDF, MW 534,000) were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Super-P amorphous carbon black (C, ~40 nm, 99.99%) was purchased from Alpha Aesar Inc. (Tewksbury, MA, USA).
2.2. Exfoliation of MoS2 NSs
The exfoliation of the MoS2 NSs was performed according to the method outlined in previous reports [16,33,34]. In brief, 1.0 g of MoS2 powder and 3 mL of butyllithium/hexane were mixed in a 10-mL vessel (placed in a glove box) to prevent the self-heating of butyllithium. The 1.6 M butyllithium/hexane was prepared by diluting the delivered 2.5 M butyllithium/hexane solution into hexane solvent. The mixture was maintained for 2 days to form LixMoS2. LixMoS2 was then collected via centrifugation to remove the hexane and residual butyllithium. The obtained LixMoS2 was added to 200 mL of deionized (DI) water and placed in a sonication bath for 2 h to exfoliate MoS2. Finally, 1T-MoS2 was washed with DI water four times to remove lithium ions and then freeze-dried using a Labconco freeze dryer (Labconco Corp., Kansas, MO, USA).
2.3. Preparation of Boron Oxide Decorated MoS2 NS
For boron oxide decoration, the MoS2 NSs were collected after washing four times with DI water. The amount of MoS2 was determined by weighing the same amount of MoS2 NS in the solution after freeze-drying. The boric acid to MoS2 NS weight ratios were approximately 10, 20, and 30%. The mixtures were dispersed in DI water by sonication for 1 h, then freeze-dried, and sintered at 400 °C for 2 h in a tube furnace under Ar gas. The collected powder was denoted as MoS2_B1, -B2, and -B3 with increasing amounts of boric acid (10, 20, and 30 wt%, respectively).
2.4. Material Characterization
The structure of the materials was measured by X-ray diffraction (XRD) (D/MAX-2200 Rigaku Tokyo, Japan) over the 2θ range of 10–70°. The morphologies, sizes, and detailed structures of B2O3 decorated MoS2 NS were analyzed using scanning electron microscopy (SEM) (Hitachi S4700, Tokyo, Japan) and transmission electron microscopy (TEM, TECNAI G2F30, FEI Corp., Hillsboro, OR, USA).
2.5. Electrochemical Measurements
To evaluate the electrochemical performance of the materials and their lithium storage capability, the materials were assembled as working electrodes in half-cell LIBs using a coin-type cell (CR 2032, Rotech Inc., Gwangju, Korea) with a lithium reference electrode. The active material was mixed with PVDF and carbon super P at a weight ratio of 70:15:15 in a NMP solution to form a slurry. The working electrode was prepared by casting the slurry on a copper electrode, using the doctor blading method, followed by drying in a vacuum oven at 70 °C for 24 h. The battery structures were assembled under Ar gas in a glovebox with positive pressure. The separator and electrolyte were polyethylene and 1 M LiPF6 in ethylene carbonate/diethylene carbonate (EC: DEC = 1:1 by volume). The galvanostatic electrochemical charge–discharge performances of the cells were measured using a battery cycle tester (WBCS3000, WonAtech, Seocho-gu, Seoul, Korea) across the voltage range of 0.01–3.0 V versus Li/Li+. Cyclic voltammetry (CV) tests, across a voltage range of 0.01–3.0 V, and electrochemical impedance spectroscopy (EIS), over a frequency range of 100 kHz to 0.1 Hz, were performed using ZIVE MP1 (WonAtech, Seocho-gu, Seoul, Korea). All the specific capacities were calculated based on the weights of the active materials.
3. Results and Discussion
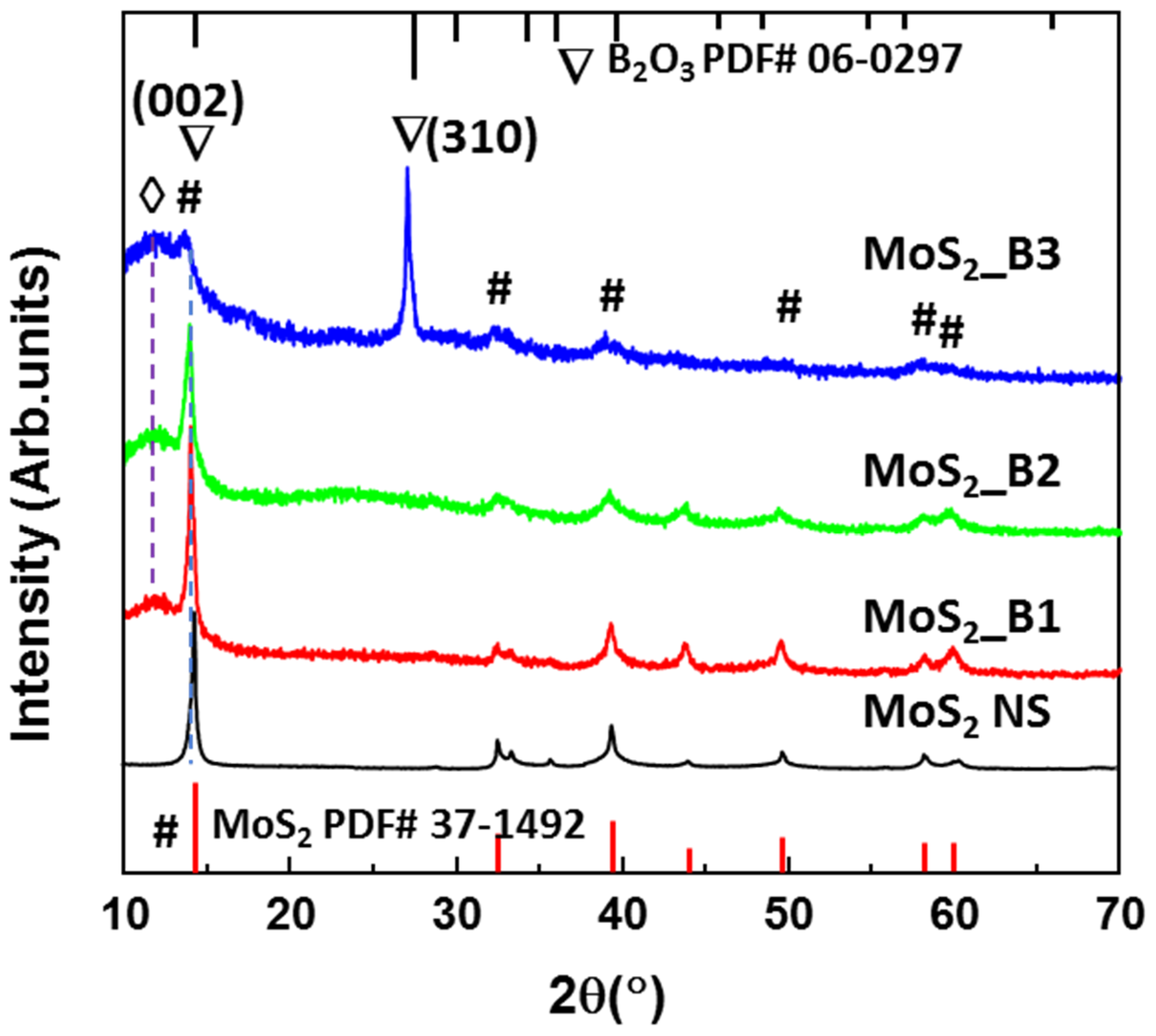
Figure 1 shows the XRD patterns of the MoS2 NSs and MoS2_B1, -B2, and -B3 samples synthesized with 10, 20, and 30 wt% boric acid. The MoS2 NS exhibited a main peak at ~14.2°, indicating the main orientation of the (002) plane in the 2D structure, as per JCPDS #37-1492. The other weak peaks of MoS2 indicated the presence of multiple layers of these materials. These results are consistent with MoS2 NSs synthesized by various methods, such as hydrothermal or sonication methods [19,20,35]. The B2O3 at lower concentrations of 10% and 20% did not exhibit the peak of boric oxide, which can be due to the amorphous structures on the MoS2 NS surface. When increasing the boric acid to 30 wt%, the crystallinity of B2O3 was observed. The structure of B2O3 matched the cubic structure of B2O3 in JCPDS card #06-0297 with a high lattice constant (a = 10.05 Å). This lattice constant was sufficiently high compared to the 0.76 Å of lithium ion. Therefore, B2O3 coverage on MoS2 may not affect lithiation/delithiation. In addition, the XRD patterns of the MoS2_B1, -B2 and B3 samples show a broad peak at ~12°. According to Bragg’s law, the lattice spacing can be calculated from the equation , where is the X-ray wavelength and is the diffraction angle. Therefore, the lattice spacing of this peak is ~7.37 Å, and this can be attributed to the expansion of the MoS2 layers or the stacking of MoS2 NSs with B2O3 NPs. This stacking layer had a large lithium-ion radius, thereby generating a facile path for the insertion/desertion of these ions.
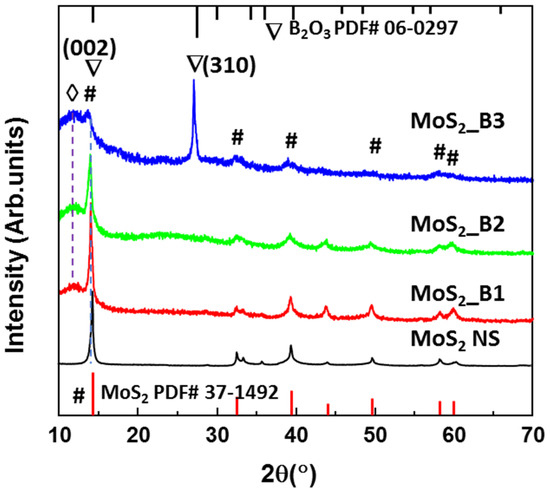
Figure 1.
X-ray diffraction patterns of MoS2 NS and B2O3 decorated MoS2_B1, -B2, and -B3 with starting H3BO3 weight percentages of 10, 20, and 30%, respectively. The symbol #, ∇, and ◊ indicate the peaks of MoS2 and B2O3 lattices, and stacking layer, respectively.
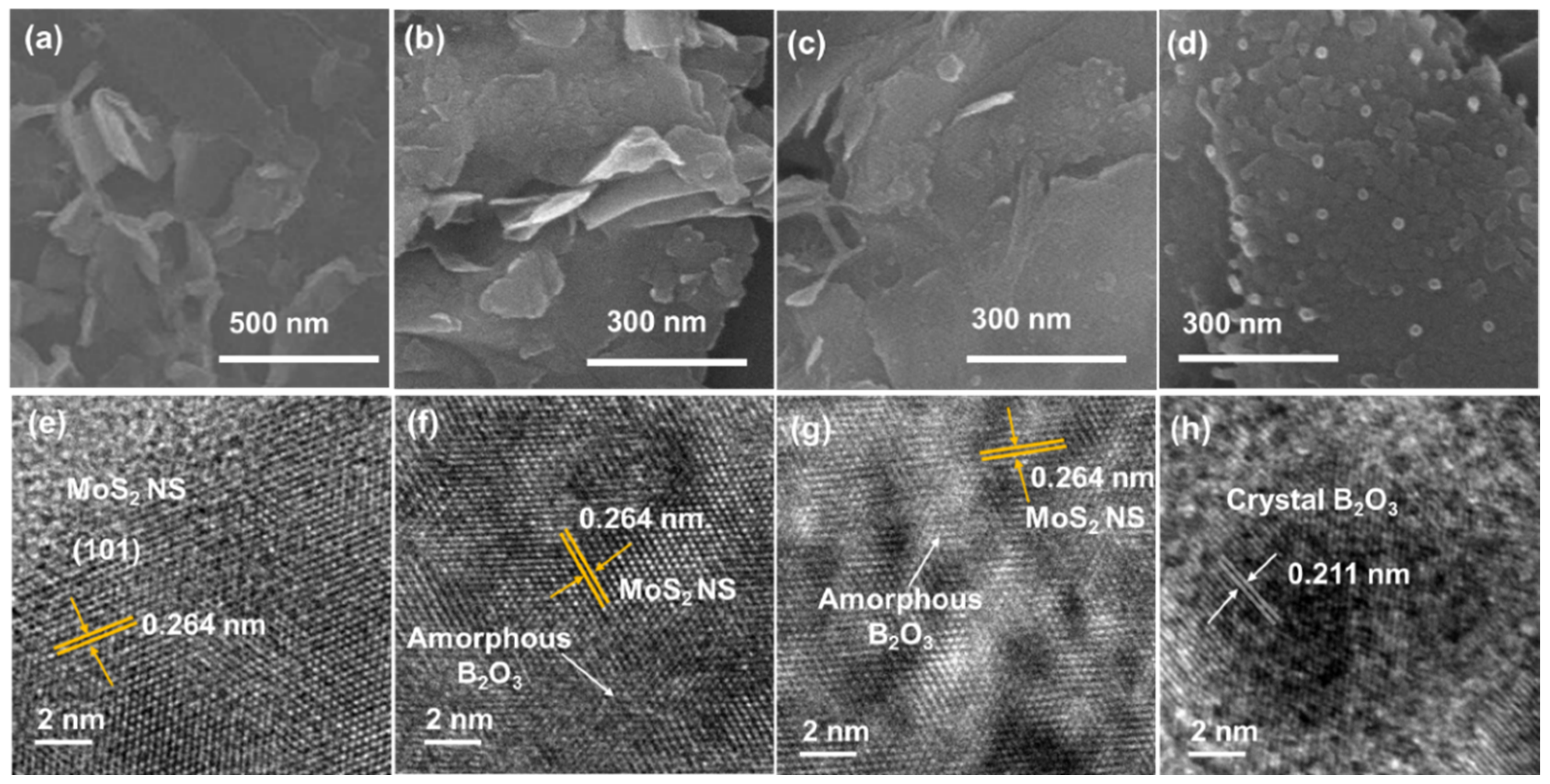
To confirm the morphologies of the MoS2 NSs and their B2O3 decorations, the materials were subjected to SEM and TEM measurements, as shown in Figure 2. As seen in Figure 2a, the MoS2 NSs were exfoliated from the bulk material to nanosheets with a wide size ranging from 200 nm to a few micrometers. The size diversity is due to the strong reaction of intercalated lithium between MoS2 layers and DI water, which broke the NSs into smaller structures and the random shape of the bulk materials. This result is consistent with previous reports of MoS2 NSs prepared using the liquid exfoliation method [16,34,36]. Moreover, the MoS2 NSs with low amounts of B2O3 (10 and 20 wt% of boric acid) show a surface with tiny spots or blurred surface on the MoS2 NS, which are the amorphous structure B2O3 NP decorations, as illustrated in Figure 2b,c. The MoS2_B2 sample had larger B2O3 particles on its surface. When the B2O3 increased to 30 wt%, the SEM image in Figure 2d reveals B2O3 NPs with sizes in the range of ~10–20 nm. The crystallinity of B2O3 depends on the amount of boric acid, which could be due to the large surface area of MoS2 NS. At low concentration, the sintering of low amount of boric acid on MoS2 created imperfect lattices, leading to the low crystalline structure or amorphous structure of B2O3. On the other hand, when the concentration of boric acid was high enough (>30 wt%), the complete lattices of B2O3 NPs formed, indicating the high crystalline structure of B2O3 NPs. Therefore, it is suggested that a low amount of B2O3 only forms an amorphous structure and a high amount of B2O3 (>30 wt%) is sufficient to form a crystalline structure on the surface of MoS2.
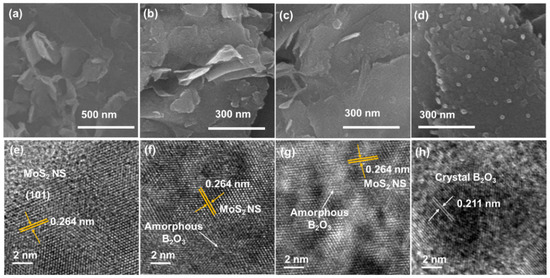
Figure 2.
(a–d) Scanning electron microscopy and (e–h) transmission electron microscopy images of MoS2 NS and MoS2_B1, -B2, and -B3, respectively.
TEM measurements were conducted to further reveal the structure of the MoS2 NSs and B2O3 NPs on the MoS2. Figure 2e shows a high-resolution TEM (HRTEM) image of the MoS2 NSs. The surface image clearly shows a lattice plane spacing of approximately 0.264 nm, which corresponds to the (101) plane of MoS2. Thus, MoS2 NSs with high crystallinity were obtained. However, in the MoS2_B1 samples, the MoS2 NSs were hindered by a blurred surface, which indicated the amorphous structure of B2O3, as illustrated in Figure 2f. The blurred surface area increased in MoS2_B2 owing to the increasing amount of B2O3 amorphous structure, as shown in Figure 2g. In addition, crystalline B2O3 was observed in the MoS2_B3 samples (Figure 2h). The lattice spacing was measured as 0.211 nm, which corresponds to the d-spacing of the B2O3 crystal. These results strongly indicated the presence of well-decorated B2O3 NPs on the MoS2 NS surface.
The electrochemical properties of B2O3-decorated MoS2 were recorded by CV tests at a low scanning rate of 0.1 mV∙s−1, in the range of 0.0–3.0 V (vs. Li/Li+) (Figure 3). The reaction at the anode can be expressed by the following equation:
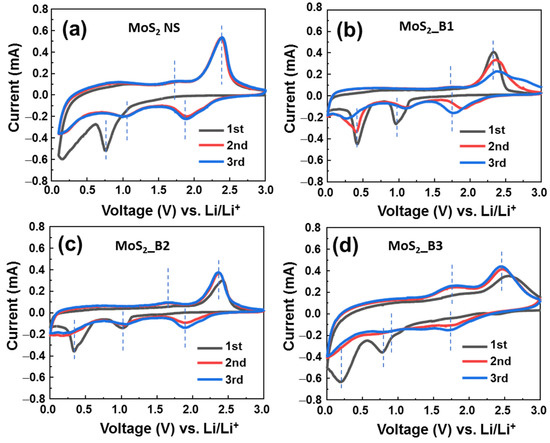
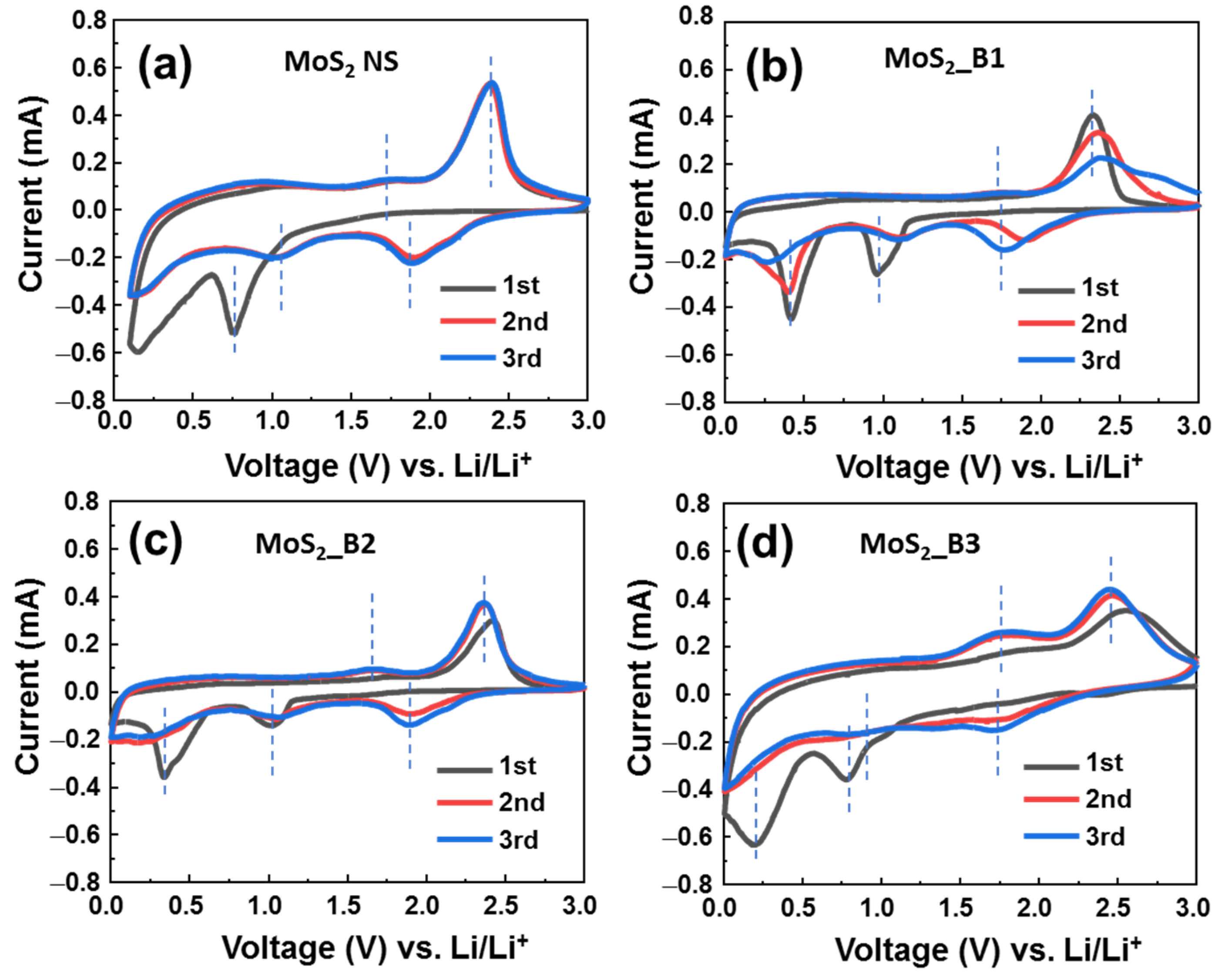
Figure 3.
Cyclic voltammograms of (a) MoS2 NS and (b–d) of MoS2/B1, -B2, and -B3 electrodes, respectively.
For lithiation:
For delithiation:
Finally, the solid electrolyte reaction at first cycles:
As shown in Figure 3a, the bare MoS2 materials show a cathodic peak in the first cycle at ~0.76 V, which is the lithiation process to form LixMoS2 and the deep lithiation to form Mo and Li2S, as shown in Equations (1) and (2). The peak between 0.1–0.5 V could be due to the formation of the SEI layer (5). These results are consistent with previous reports on 1T MoS2 in the first CV cycle [15,16]. From the second cycle, redox couple peaks were recorded at 1.05/1.72 V and 1.87/2.38 V, which are the reactions in Equations (2) and (3); and Equations (1) and (4), respectively. The CV curves of anodes MoS2_B1 and B2 were similar. In these two anodes, the first cycle shows cathodic peaks at ~0.96 and 0.41 V, which correspond to the reactions (1) and (2), respectively. The anodic peaks were located at ~1.7 and 2.3 V, which correspond to the reactions (3) and (4), respectively. SEI layer formation was recorded together with the peak of reaction (2) at ~0.41 V. It is noted that the bare MoS2 NSs and MoS2_B1 and -B2 electrodes show the strong redox couple peaks at 1.87/2.38 V vs. Li+/Li (corresponding to (1) and (4) reactions) and the weak redox couple peaks at ~1.05/1.72 V vs. Li+/Li (corresponding to (2) and (3) reactions). This emphasizes the hard oxidation of Mo to Mo4+, thus leading to the degradation of the cycling stability. In contrast, for MoS2_B3 electrode, the formation of B2O3 crystals was significantly effective in improving the electrochemical properties of the MoS2 NSs. In the first cycle, the cathodic scan showed two peaks at 0.78 and 0.20 V vs. Li+/Li, which corresponds to the lithium insertion into MoS2 (Equation (1)) and the deep insertion of Li into MoS2/formation of SEI layer (Equations (2) and (5)). The peaks of the MoS2_B3 anode were positioned at lower potential compared to those of MoS2_B1 and -B2 electrodes, which were at ~0.9 and 0.4 V vs. Li+/Li. This peak shift could be due to the formation of B2O3 crystalline introducing a different interface to the electrolyte in comparison to the amorphous B2O3, which leads to the harder diffusion of Li in the first cycle. From the second cycle, the redox couple peaks were recorded at 0.82/1.73 V and 1.77/2.42 V, which correspond to the reactions (2) and (3); and Equations (1) and (4), respectively. The third cycle showed a similar curve to the second cycle, indicating the stable electrochemical reaction after the first cycle. Furthermore, the relative intensity of Mo’s oxidation peak located at ~1.73 V for the MoS2_B3 anode (Equation (3)) was significantly enhanced in comparison to those of MoS2_B1 and -B2 and bare MoS2 NSs anodes. It is noted that the insertion of Li in MoS2 at high potential is relative to the formation of a gel-like polymeric SEI layer due to the S dissolution in electrolyte [37]. The MoS2_B1, -B2, and bare MoS2 NSs anodes show a high cathodic peak at ~1.87 V after three cycles, which is higher than that located at 1.77 V of MoS2_B3 anode, indicating the higher amount of S was dissolved in electrolyte. Therefore, the MoS2_B3 anode has high amount of recovered MoS2 NS, resulting in the high oxidation peak intensity of Mo to Mo4+. This could be due to the stability of crystalline B2O3 allowing the insert/desertion of Li ions [38]. Moreover, the sulfur atoms have high electron affinity, thus, they could not pass through the B2O3 lattice [39]. It indicates that the crystalline B2O3 effectively protected the MoS2 layer, preventing the loss of S atoms and the formation of gel-like polymeric SEI layer.
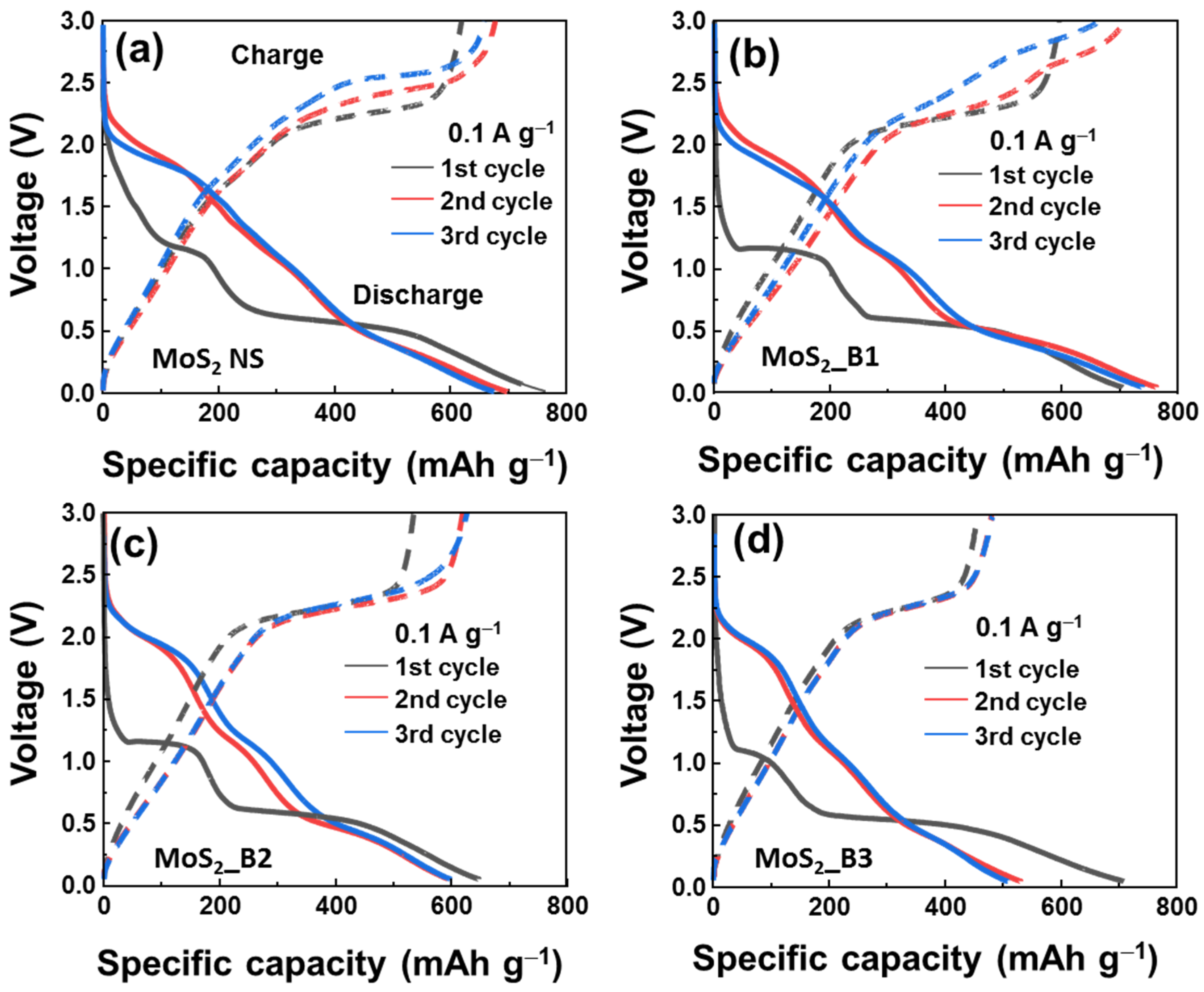
To further observe the effect of B2O3 on the MoS2 materials, the initial voltage profiles of B2O3 decorated samples are shown in Figure 4. The first three cycles of MoS2_B1, -B2, and MoS2 NSs seem to be unstable, showing a clear change from the first to the second and third cycles. The voltage plateau of the first discharge curve was slightly reduced from the MoS2 NSs to the MoS2_B1, -B2, and -B3 samples, where two plateaus at 1.1/0.51 to 1.1/0.51, 1.1/0.50, and 1.1/0.48, respectively, are shown. This indicates that the B2O3 crystals in the MoS2_B3 samples changed the lithium insertion potential. In the second and third cycles, the voltage plateaus were similar for the MoS2_B3 electrode, thereby indicating stable electrochemical properties from the second cycle. In addition, the initial discharge capacity of these anodes was high, but it reduced after each cycle owing to the formation of the SEI layer and degradation behavior in lithium ion batteries, such as cracks, sulfur dispersion in the electrolyte, and dendrite growth [40]. The initial discharge capacities for the bare MoS2 NSs and MoS2_B1, -B2, and -B3 were 747.1, 717.7, 638.1, and 717.2 mAh∙g−1, respectively. The difference in the initial discharge capacities also depended on the formation of the SEI layer and the binding of B2O3 to MoS2. The B2O3 was reported as a low lithium ion storage capability [41]. Therefore, the increased amount of B2O3 in MoS2 led to the decreased charge/discharge capacities of MoS2 anode materials. In particular, the charge/discharge capacities of the MoS2_B3 anode in the third cycle were 505.2/475.0 mAh∙g−1.
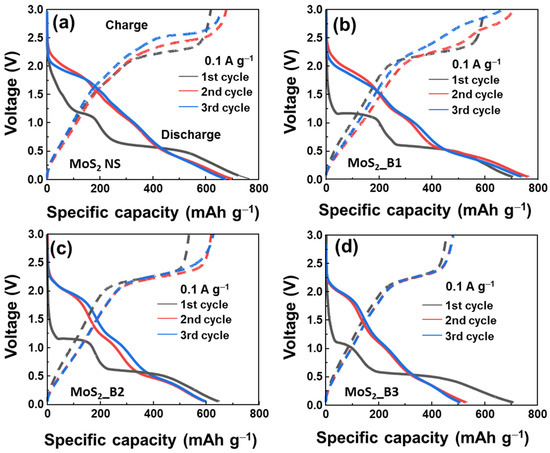
Figure 4.
Initial voltage profiles of (a) MoS2 NS and (b–d) MoS2_B1, -B2, and -B3 electrodes, respectively.
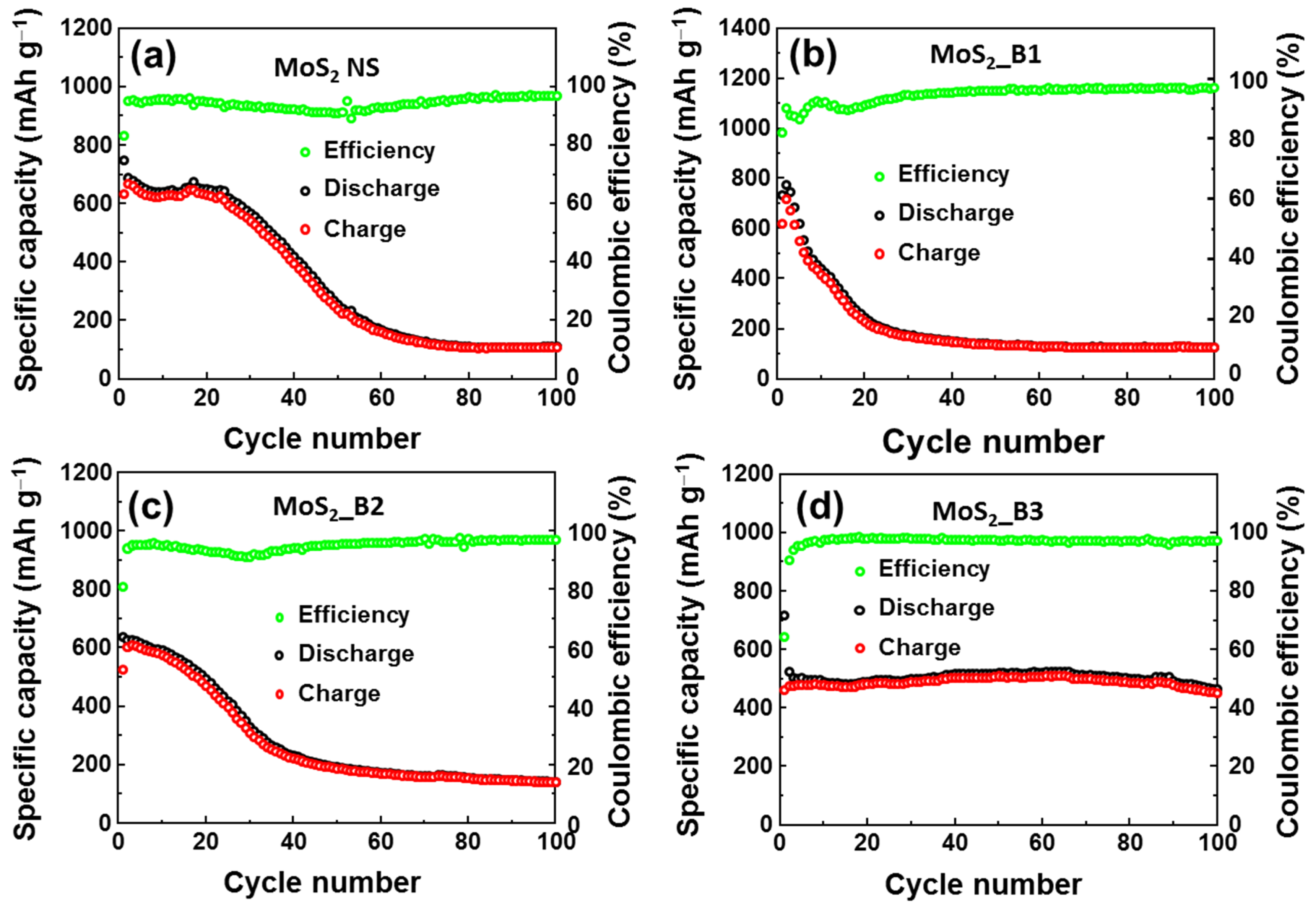
To evaluate the stability of the anode materials, cycling tests were performed at a current rate of 0.1 A∙g−1 for 100 cycles, as illustrated in Figure 5a–d. Detailed comparison of specific capacities of as-prepared anode materials are also shown in Table 1. The MoS2 NSs showed stability for ~20 cycles, and its capacity was subsequently dramatically reduced and maintained at only ~109 mAh∙g−1 at the 100th cycle (Figure 5a). The addition of B2O3 also resulted in very fast degradation, and the remaining capacity was ~125 mAh∙g−1 and ~140 mAh∙g−1 at the 100th cycle in the MoS2_B1 and B2 anodes, respectively (Figure 5b,c). The MoS2_B1 and B2 showed the enhancement of lattice spacing of MoS2, facilitating the insertion/desertion of Li ions. However, the amorphous B2O3 could not prevent the loss of S atoms. Therefore, MoS2_B1 and B2 anodes exhibited inferior stability to the pure MoS2 NS. In contrast, the crystalline B2O3 in the MoS2_B3 electrode showed a high capacity in the first cycle, and it demonstrated prolonged cycling stability for 100 cycles. As shown in Figure 5d, the capacity of MoS2_B3 at the 100th cycle was ~451 mAh∙g−1, which was 86.2% of the second cycle (~510 mAh∙g−1) and ~62.9% of the first cycle. Therefore, it can be concluded that the enhancement of the redox reaction with the B2O3 crystals was effective in improving the stability of the MoS2 NSs.
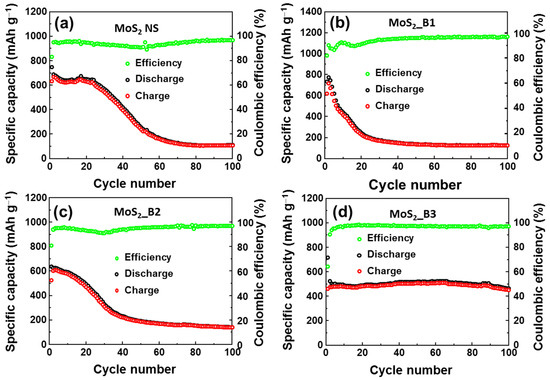
Figure 5.
Cyclic performance of (a) MoS2 NS and (b–d) MoS2_B1, -B2, and -B3 electrodes, respectively.

Table 1.
Comparison of specific capacities of bare MoS2 NS and B2O3 decorated MoS2 anodes.
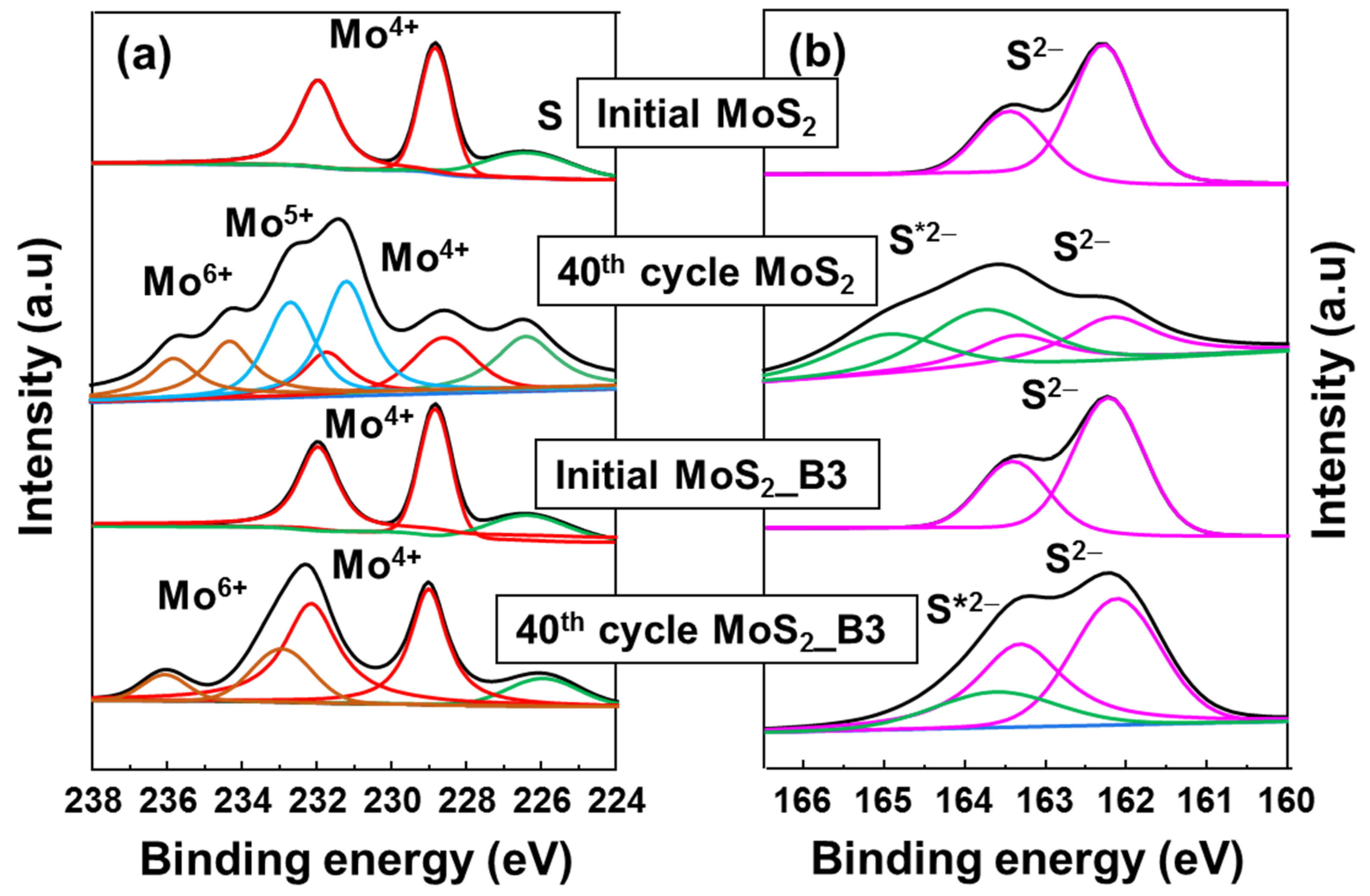
The protective role of B2O3 was further confirmed via ex-situ XPS spectra, as shown in Figure 6. Both bare MoS2 NS and MoS2_B3 anodes were compared at the initial state and at 3.0 V 40 cycles. The initial state of MoS2 and B2O3 decorated samples presented the same conditions of Mo4+ and S2−. However, after 40 cycles, bare MoS2 NSs showed significant change in Mo 4f peak. Mo4+ peak intensity reduced, and the Mo5+ and Mo6+ peaks appeared. Moreover, in S 2p spectrum, the S 2p peak split to S2− peak at ~162 eV and a S*2− peak at ~163.6 eV, which might be related to the formation of polymeric SEI layer due to the S dissolution or the unrecoverable Li2S [42,43], indicating unstable MoS2 NS anode. On the other hand, the MoS2_B3 anode show a better stability, where the main peak of Mo 4f assigned to Mo4+ was maintained with partial Mo6+ peaks. It is noted that the Mo6+ peak might appear due to the sample preparation method as pointed out in previous reports [44,45,46]. The S 2p peak of MoS2_B3 showed a small change with S*2− peak, which might be due to a partial loss of S to polymeric layer or unrecoverable Li2S. These results indicate that B2O3 layer efficiently protected MoS2 layer, preventing the loss of S to electrolyte. The small amount of S loss can be further improved after optimizing the B2O3 layers.
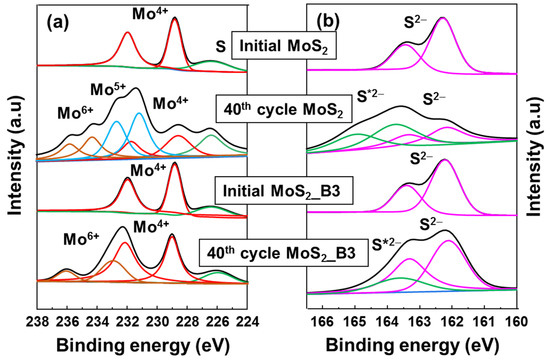
Figure 6.
Ex-situ high resolution XPS spectra of (a) Mo 4f and (b) S 2p for MoS2 NS and MoS2_B3 anodes at initial state and after cycling for 40 cycles. The symbol * indicates the shifted S 2p peaks.
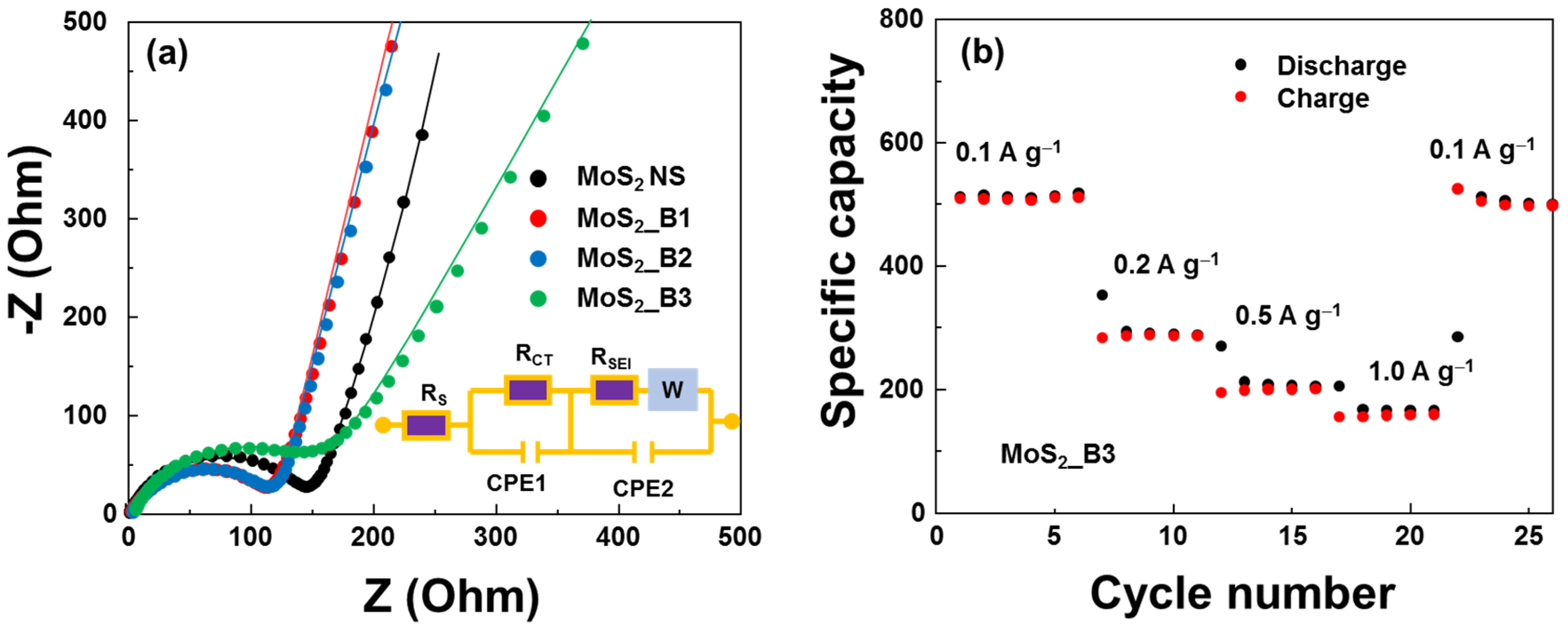
The electrical properties of the anode materials were evaluated using EIS measurements, as shown in Figure 7a. The equivalent circuit (modified Randle’s model) contained series resistance (RS), charge-transfer resistance (RCT), SEI layer resistance (RSEI), a diffusion Warburg impedance element, and constant phase elements (CPE1 and CPE2). The extracted RCTs of the MoS2 NS and MoS2_B1, -B2, and -B3 samples were 150.3, 118.4, 118.2, and 148.6 Ω, respectively. The addition of boron oxide did not significantly affect the resistance of the anode material. MoS2_B1 and -B2 showed reduced resistance. Then, the resistance increased in MoS2_B3 when B2O3 formed crystallinity owing to the low conductivity of B2O3. However, this resistance was still lower than that of the bare MoS2. As a 2D layered structure material, the conductivity of MoS2 decreases when the number of layers is reduced. In addition, the presence of B2O3 NPs may prevent the restacking of MoS2 NSs and the NS material from forming a bulk structure, thereby enhancing the conductivity of the anode material. The rate performance of MoS2_B3 is shown in Figure 7b. An increase in the current rate led to a decrease in capacity. At 1.0 A∙g−1, the capacity was maintained at ~155 mAh∙g−1. Nevertheless, the MoS2_B3 electrode can be recovered to almost 98% when decreasing the current rate to 0.1 A∙g−1, thus illustrating a highly reversible rate performance.
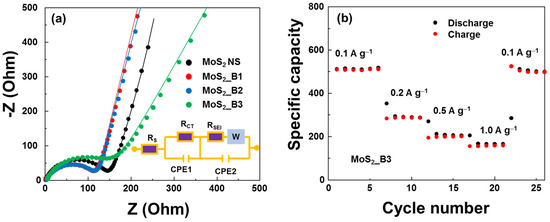
Figure 7.
(a) Nyquist plots of MoS2 and MoS2_B1, -B2, and -B3 anodes; and (b) rate performance of MoS2_B3 anodes.
The recent works on the modification of MoS2 are shown in Table 2. The initial discharge capacities were high above 800 or even 1200 or 1400 mAh∙g−1. This might be due to the contribution to the capacity of the modified materials. From our method, B2O3 does not mainly contribute to the capacity, but effectively protects the MoS2 layer, which maintains the highly stable capacity. Moreover, the sintering method and the utilization of the boric acid are cost-effective ways. Therefore, it can be readily scale-up to the industrial size. We also believe that the use of carbon-based and co-active materials can further improve the electrochemical performance of the materials presented in this study for lithium ion storage application.

Table 2.
Comparison of the electrochemical performance of the modified MoS2 anodes in lithium-ion batteries.
4. Conclusions
In summary, B2O3 NP-decorated MoS2 NSs were successfully fabricated via a facile chemical exfoliation and sintering process. The XRD, SEM, and TEM measurements confirmed that the crystalline B2O3 could be formed at high boric acid content of over 30 wt%. The presence of B2O3 created the lattice spacing of ~7.37 Å in MoS2 NS. Crystal B2O3 formed with a lattice spacing of ~2.58 Å, improving the redox reaction in the conversion of MoS2 during the cycling process. The high intensity of Mo oxidation peak and the lower potential of lithium insertion into MoS2 indicated B2O3 layer played a role as a protective layer, preventing the dissolution of S atoms into electrolyte. The bare MoS2 material and amorphous B2O3 in MoS2_B1 and -B2 anodes showed fast degradation after 20–40 cycles due to the loss of sulfur into the electrolyte. Meanwhile, the MoS2_B3 electrode with protectable crystalline B2O3 layer demonstrated a stable capacity of ~500 mAh∙g−1 and a high-capacity retention of ~86.2% after 100 cycles. These results suggest that B2O3 NP decorations on anode materials could be a potential approach for high-stability anodes in lithium storage applications.
Author Contributions
T.P.N.: Conceptualization, methodology, validation, visualization, writing, review, and editing. I.T.K.: project administration, funding acquisition, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Capacity Enhancement Project through a Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (2019R1A6C1010016). This research was supported by Korea Basic Institute (National Research facilities and Equipment Center) grant funded by the Ministry of Education (2020R1A6C103A050).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, J.; Shin, H.S. Recent advances in layered transition metal dichalcogenides for hydrogen evolution reaction. J. Mater. Chem. A 2014, 2, 5979–5985. [Google Scholar] [CrossRef]
- Kwon, K.C.; Kim, C.; Le, Q.V.; Gim, S.; Jeon, J.-M.; Ham, J.Y.; Lee, J.-L.; Jang, H.W.; Kim, S.Y. Synthesis of Atomically Thin Transition Metal Disulfides for Charge Transport Layers in Optoelectronic Devices. ACS Nano 2015, 9, 4146–4155. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Song, T.T.; Callsen, M.; Zhou, J.; Chai, J.W.; Feng, Y.P.; Wang, S.J.; Yang, M. Atomically Thin 2D Transition Metal Oxides: Structural Reconstruction, Interaction with Substrates, and Potential Applications. Adv. Mater. Interfaces 2019, 6, 1801160. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A.; Poot, M.; Steele, G.A.; Van der Zant, H.S.; Agraït, N.; Rubio-Bollinger, G. Mechanical properties of freely suspended semiconducting graphene-like layers based on MoS2. Nanoscale. Res. Lett. 2012, 7, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-Like Two-Dimensional Materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef]
- Kim, C.; Nguyen, T.P.; Le, Q.V.; Jeon, J.M.; Jang, H.W.; Kim, S.Y. Performances of Liquid-Exfoliated Transition Metal Dichalcogenides as Hole Injection Layers in Organic Light-Emitting Diodes. Adv. Funct. Mater. 2015, 25, 4512–4519. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen, T.P.; Le, T.H.; Vo, D.V.N.; Nguyen, D.L.T.; Trinh, Q.T.; Kim, I.T.; Le, Q.V. Recent advances in two-dimensional transition metal dichalcogenides as photoelectrocatalyst for hydrogen evolution reaction. J. Chem. Technol. Biotechnol. 2020, 95, 2597–2607. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Shu, H.; Li, F.; Hu, C.; Liang, P.; Cao, D.; Chen, X. The capacity fading mechanism and improvement of cycling stability in MoS2-based anode materials for lithium-ion batteries. Nanoscale 2016, 8, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; von dem Bussche, A.; Qiu, Y.; Valentin, T.M.; Gion, K.; Kane, A.B.; Hurt, R.H. Chemical Dissolution Pathways of MoS2 Nanosheets in Biological and Environmental Media. Environ. Sci. Technol. 2016, 50, 7208–7217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Wang, X.J.; Yang, X.Q.; Xun, S.D.; Liu, G.; Koech, P.K.; Liu, J.; Lemmon, J.P. Electrochemically Induced High Capacity Displacement Reaction of PEO/MoS2/Graphene Nanocomposites with Lithium. Adv. Funct. Mater. 2011, 21, 2840–2846. [Google Scholar] [CrossRef]
- Xiang, T.; Fang, Q.; Xie, H.; Wu, C.; Wang, C.; Zhou, Y.; Liu, D.; Chen, S.; Khalil, A.; Tao, S.; et al. Vertical 1T-MoS2 nanosheets with expanded interlayer spacing edged on a graphene frame for high rate lithium-ion batteries. Nanoscale 2017, 9, 6975–6983. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. Ag Nanoparticle-Decorated MoS2 Nanosheets for Enhancing Electrochemical Performance in Lithium Storage. Nanomaterials 2021, 11, 626. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, C.; Xiao, F.; Wang, J.; Su, X. Synthesis of nano-TiO2-decorated MoS2 nanosheets for lithium ion batteries. J. Chem. 2015, 39, 683–688. [Google Scholar] [CrossRef]
- Lu, L.; Min, F.; Luo, Z.; Wang, S.; Teng, F.; Li, G.; Feng, C. Synthesis and electrochemical properties of tin-doped MoS2 (Sn/MoS2) composites for lithium ion battery applications. J. Nanopart. Res. 2016, 18, 357. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, J.; Wen, S.; Lu, L.; Xue, J. Synthesis of SnO2/MoS2 composites with different component ratios and their applications as lithium ion battery anodes. J. Mater. Chem. A 2014, 2, 17857–17866. [Google Scholar] [CrossRef]
- Li, Z.; Sun, P.; Zhan, X.; Zheng, Q.; Feng, T.; Braun, P.V.; Qi, S. Metallic 1T phase MoS2/MnO composites with improved cyclability for lithium-ion battery anodes. J. Alloys Compd. 2019, 796, 25–32. [Google Scholar] [CrossRef]
- Kong, D.; He, H.; Song, Q.; Wang, B.; Lv, W.; Yang, Q.-H.; Zhi, L. Rational design of MoS2@graphene nanocables: Towards high performance electrode materials for lithium ion batteries. Energy Environ. Sci. 2014, 7, 3320–3325. [Google Scholar] [CrossRef]
- Yoo, H.; Tiwari, A.P.; Lee, J.; Kim, D.; Park, J.H.; Lee, H. Cylindrical nanostructured MoS2 directly grown on CNT composites for lithium-ion batteries. Nanoscale 2015, 7, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ren, R.-P.; Lv, Y.-K. A flexible 3D graphene@CNT@MoS2 hybrid foam anode for high-performance lithium-ion battery. Chem. Eng. J. 2018, 353, 419–424. [Google Scholar] [CrossRef]
- Qu, B.; Sun, Y.; Liu, L.; Li, C.; Yu, C.; Zhang, X.; Chen, Y. Ultrasmall Fe2O3 nanoparticles/MoS2 nanosheets composite as high-performance anode material for lithium ion batteries. Sci. Rep. 2017, 7, 42772. [Google Scholar] [CrossRef]
- Zhao, S.; Zha, Z.; Liu, X.; Tian, H.; Wu, Z.; Li, W.; Sun, L.-B.; Liu, B.; Chen, Z. Core–Sheath Structured MoO3@MoS2 Composite for High-Performance Lithium-Ion Battery Anodes. Energy Fuels 2020, 34, 11498–11507. [Google Scholar] [CrossRef]
- Yoon, M.; Dong, Y.; Hwang, J.; Sung, J.; Cha, H.; Ahn, K.; Huang, Y.; Kang, S.J.; Li, J.; Cho, J. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries. Nat. Energy 2021, 6, 362–371. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Wang, Y.; Wang, R. Investigation of facial B2O3 surface modification effect on the cycling stability and high-rate capacity of LiNi1/3Co1/3Mn1/3O2 cathode. J. Alloys Compd. 2020, 834, 155150. [Google Scholar] [CrossRef]
- Hayashi, A.; Nakai, M.; Tatsumisago, M.; Minami, T.; Katada, M. Structural Studies in Lithium Insertion into SnO-B2O3 Glasses and Their Applications for All-Solid-State Batteries. J. Electrochem. Soc. 2003, 150, A582. [Google Scholar] [CrossRef]
- Vijayakumar, E.; Govinda raj, M.; Neppolian, B.; Kumar Lakhera, S.; John Bosco, A. Hierarchical layered nanostructure of MoS2/boron doped reduced graphene oxide composites under visible light irradiation for effective antibiotic degradation and hexavalent chromium reduction. Mater. Lett. 2021, 296, 129891. [Google Scholar] [CrossRef]
- Riyanto; Sahroni, I.; Bindumadhavan, K.; Chang, P.Y.; Doong, R.A. Boron Doped Graphene Quantum Structure and MoS2 Nanohybrid as Anode Materials for Highly Reversible Lithium Storage. Front. Chem. 2019, 7, 116. [Google Scholar] [CrossRef]
- Balcı, S.; Sezgi, N.A.; Eren, E. Boron Oxide Production Kinetics Using Boric Acid as Raw Material. Ind. Eng. Chem. Res. 2012, 51, 11091–11096. [Google Scholar] [CrossRef]
- Huber, C.; Jahromy, S.S.; Jordan, C.; Schreiner, M.; Harasek, M.; Werner, A.; Winter, F. Boric Acid: A High Potential Candidate for Thermochemical Energy Storage. Energies 2019, 12, 1086. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.P.; Kim, I.T. Self-Assembled Few-Layered MoS2 on SnO2 Anode for Enhancing Lithium-Ion Storage. Nanomaterials 2020, 10, 2558. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, L.; Chen, X.; Du, J.; Xiong, Y. Chemically exfoliated metallic MoS2 nanosheets: A promising supporting co-catalyst for enhancing the photocatalytic performance of TiO2 nanocrystals. Nano Res. 2015, 8, 175–183. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Sohn, W.; Oh, J.H.; Jang, H.W.; Kim, S.Y. Size-Dependent Properties of Two-Dimensional MoS2 and WS2. J. Phys. Chem. C 2016, 120, 10078–10085. [Google Scholar] [CrossRef]
- Voiry, D.; Yamaguchi, H.; Li, J.; Silva, R.; Alves, D.C.B.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 2013, 12, 850–855. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Morris, A.J.; Modarres, M.H.; De Volder, M. Structural Evolution of Electrochemically Lithiated MoS2 Nanosheets and the Role of Carbon Additive in Li-Ion Batteries. Chem. Mater. 2016, 28, 7304–7310. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Li, D.; Tian, D.; Xia, G.; Wang, C.; Xiao, N.; Li, N.; Mack, N.H.; Li, Q.; Wu, G. Promotional role of B2O3 in enhancing hollow SnO2 anode performance for Li-ion batteries. J. Power Sources 2014, 251, 279–286. [Google Scholar] [CrossRef]
- Pritchard, H.O. The Determination of Electron Affinities. Chem. Rev. 1953, 52, 529–563. [Google Scholar] [CrossRef]
- Pender, J.P.; Jha, G.; Youn, D.H.; Ziegler, J.M.; Andoni, I.; Choi, E.J.; Heller, A.; Dunn, B.S.; Weiss, P.S.; Penner, R.M.; et al. Electrode Degradation in Lithium-Ion Batteries. ACS Nano 2020, 14, 1243–1295. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Lee, S.J.; Kim, H.J.; Park, S.B.; Choi, J.W. Li2O–B2O3–GeO2 glass as a high performance anode material for rechargeable lithium-ion batteries. J. Mater. Chem. A 2018, 6, 6860–6866. [Google Scholar] [CrossRef]
- Li, G.; Huang, Q.; He, X.; Gao, Y.; Wang, D.; Kim, S.H.; Wang, D. Self-Formed Hybrid Interphase Layer on Lithium Metal for High-Performance Lithium–Sulfur Batteries. ACS Nano 2018, 12, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, Y.; Liu, J. Sulfur double locked by a macro-structural cathode and a solid polymer electrolyte for lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 10760–10766. [Google Scholar] [CrossRef]
- Huang, L.; Xu, L.; Yang, Y.; Yu, H.; Tao, H.; Li, D.; Dong, X. Superhydrophilic MoS2–Ni3S2 nanoflake heterostructures grown on 3D Ni foam as an efficient electrocatalyst for overall water splitting. J. Mater. Sci. Mater. Electron 2020, 31, 6607–6617. [Google Scholar] [CrossRef]
- Fominski, V.; Demin, M.; Nevolin, V.; Fominski, D.; Romanov, R.; Gritskevich, M.; Smirnov, N. Reactive Pulsed Laser Deposition of Clustered-Type MoSx (x ~ 2, 3, and 4) Films and Their Solid Lubricant Properties at Low Temperature. Nanomaterials 2020, 10, 653. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Singh, J.; Vikraman, D.; Singh, A.K.; Iqbal, M.Z.; Khan, M.F.; Kumar, P.; Choi, D.-C.; Song, W.; An, K.-S.; et al. Large-area, continuous and high electrical performances of bilayer to few layers MoS2 fabricated by RF sputtering via post-deposition annealing method. Sci. Rep. 2016, 6, 30791. [Google Scholar] [CrossRef] [Green Version]
- David, L.; Bhandavat, R.; Barrera, U.; Singh, G. Polymer-Derived Ceramic Functionalized MoS2 Composite Paper as a Stable Lithium-Ion Battery Electrode. Sci. Rep. 2015, 5, 9792. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Chen, W.; Zhou, J.; Yin, F.; Uchaker, E.; Zhang, Q.; Cao, G. Preparation of carbon coated MoS2 flower-like nanostructure with self-assembled nanosheets as high-performance lithium-ion battery anodes. J. Mater. Chem. A 2014, 2, 7862–7872. [Google Scholar] [CrossRef]
- Rana, M.; Boaretto, N.; Mikhalchan, A.; Vila Santos, M.; Marcilla, R.; Vilatela, J.J. Composite Fabrics of Conformal MoS2 Grown on CNT Fibers: Tough Battery Anodes without Metals or Binders. ACS Appl. Energy Mater. 2021, 4, 5668–5676. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).