Nickel-Containing Perovskites, PrNi0.4Fe0.6O3–δ and PrNi0.4Co0.6O3–δ, as Potential Electrodes for Protonic Ceramic Electrochemical Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterization of Materials
2.3. Electrochemical Behavior of PrNi0.4Fe0.6O3–δ and PrNi0.4Co0.6O3–δ Electrodes
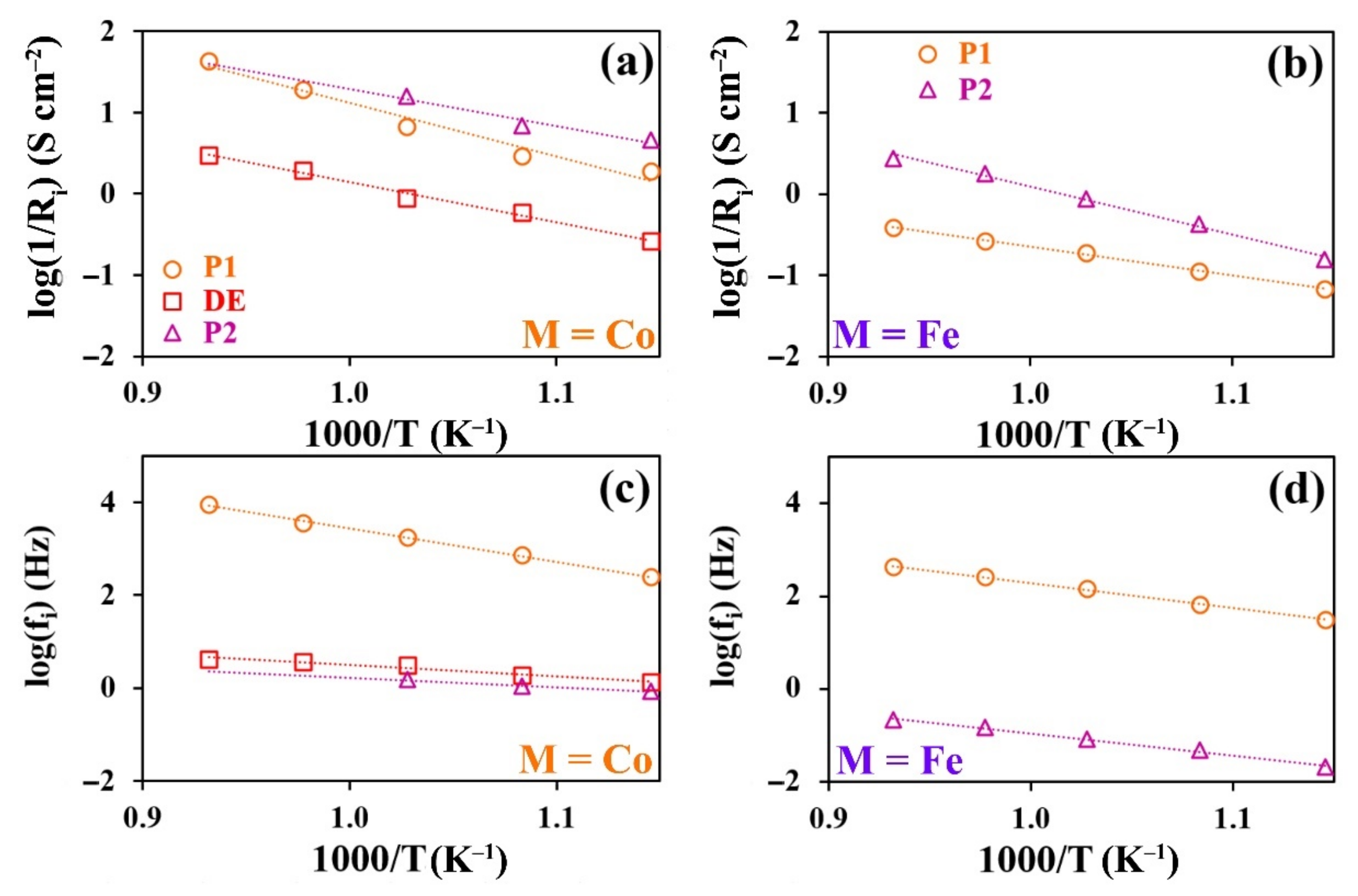
3. Results and Discussion
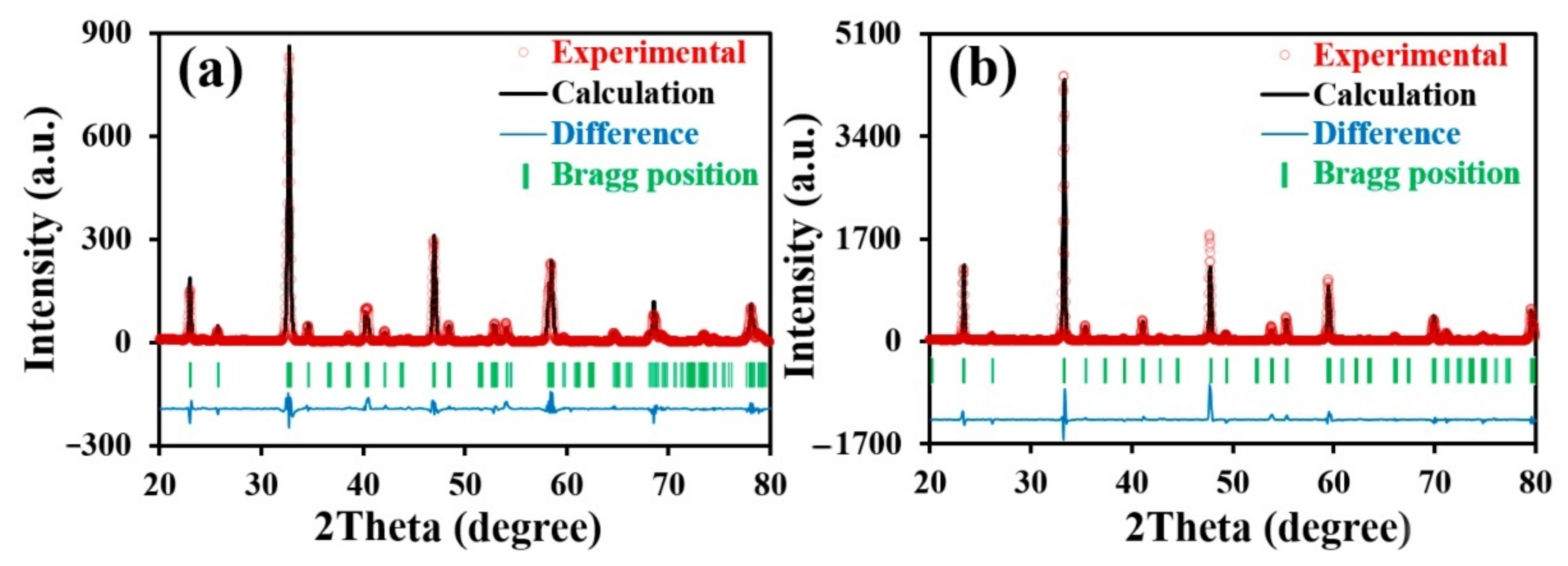
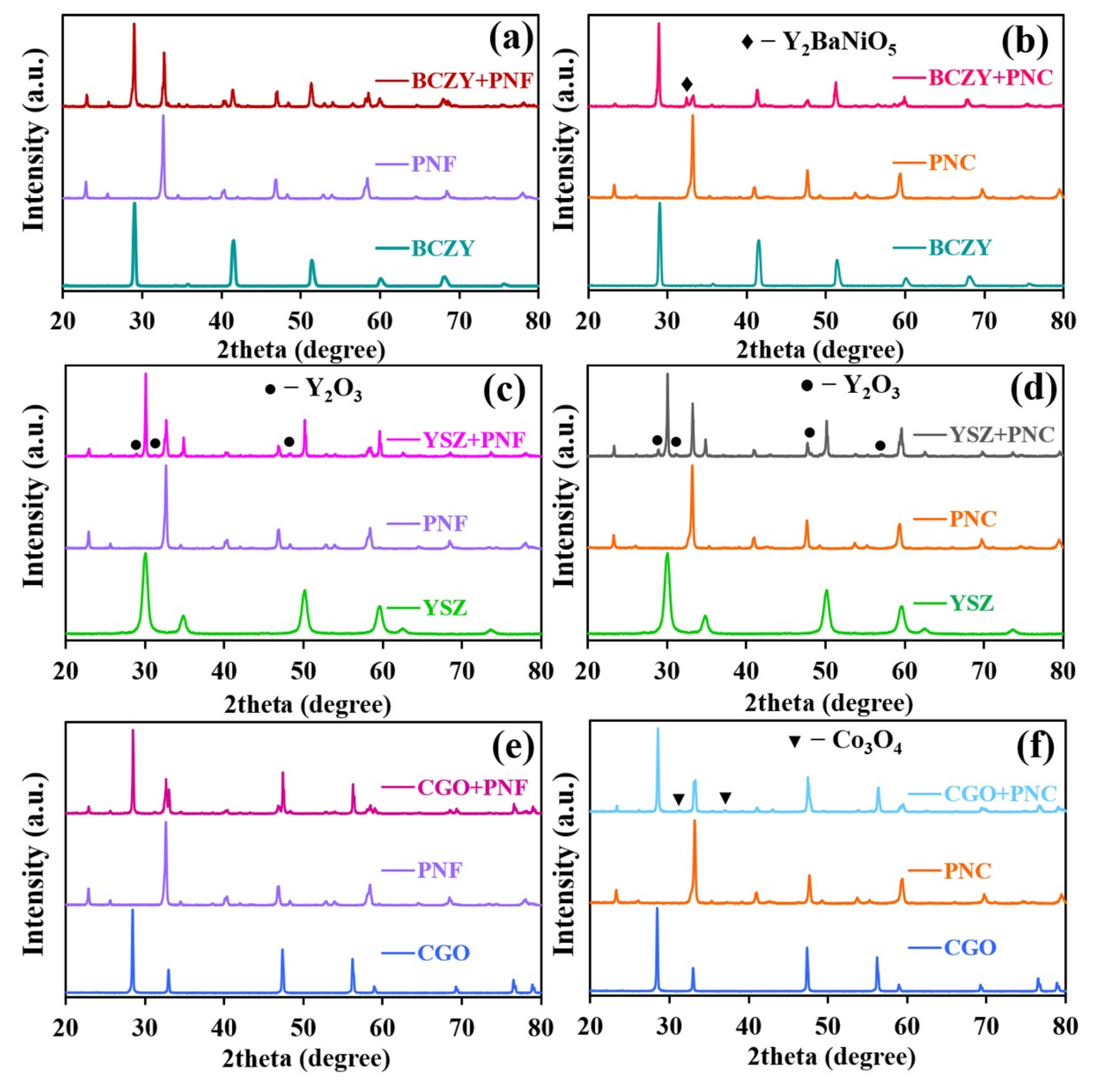
3.1. Phase Structure and Chemical Compatibility
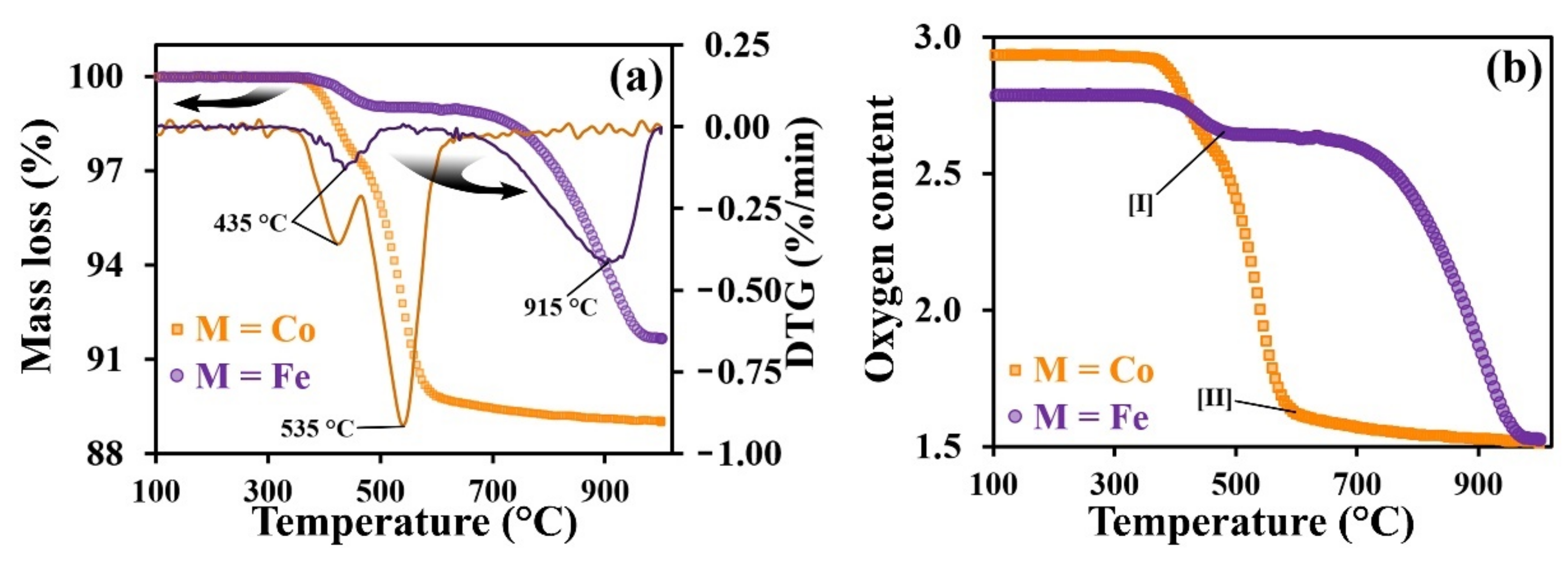
3.2. Thermal Properties
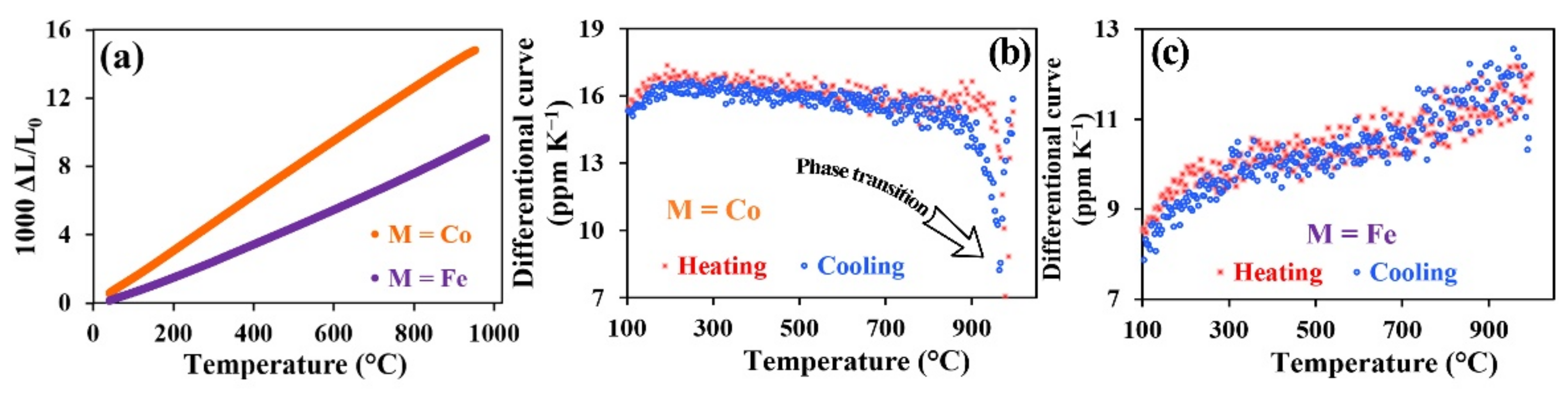
3.3. Thermal Expansion Behavior
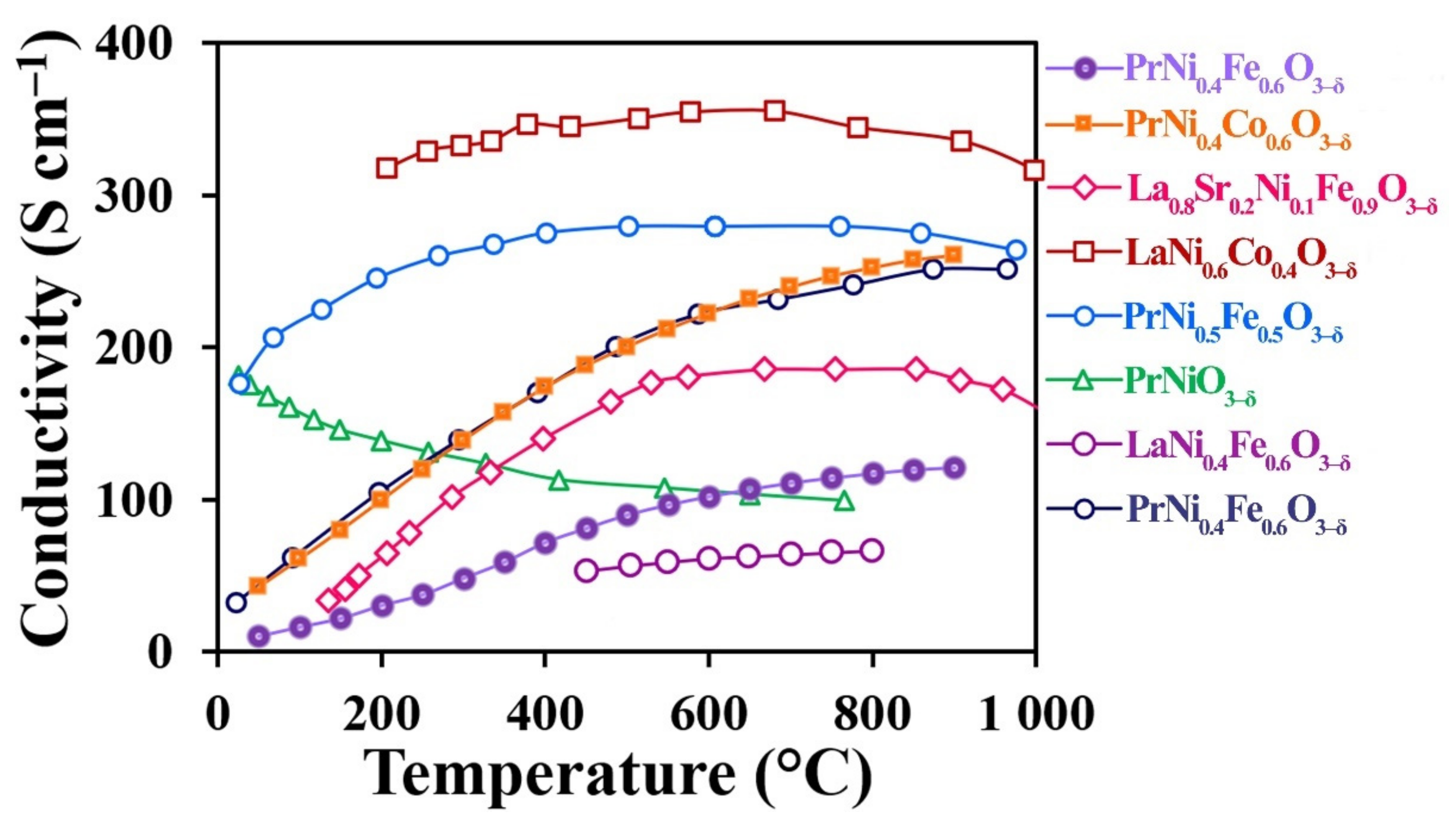
3.4. Total Conductivity
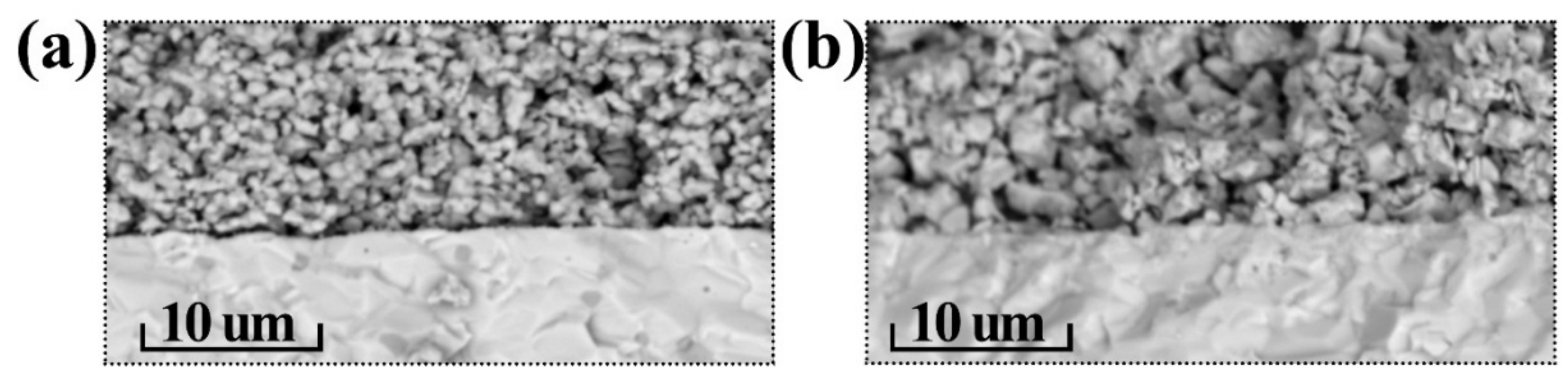
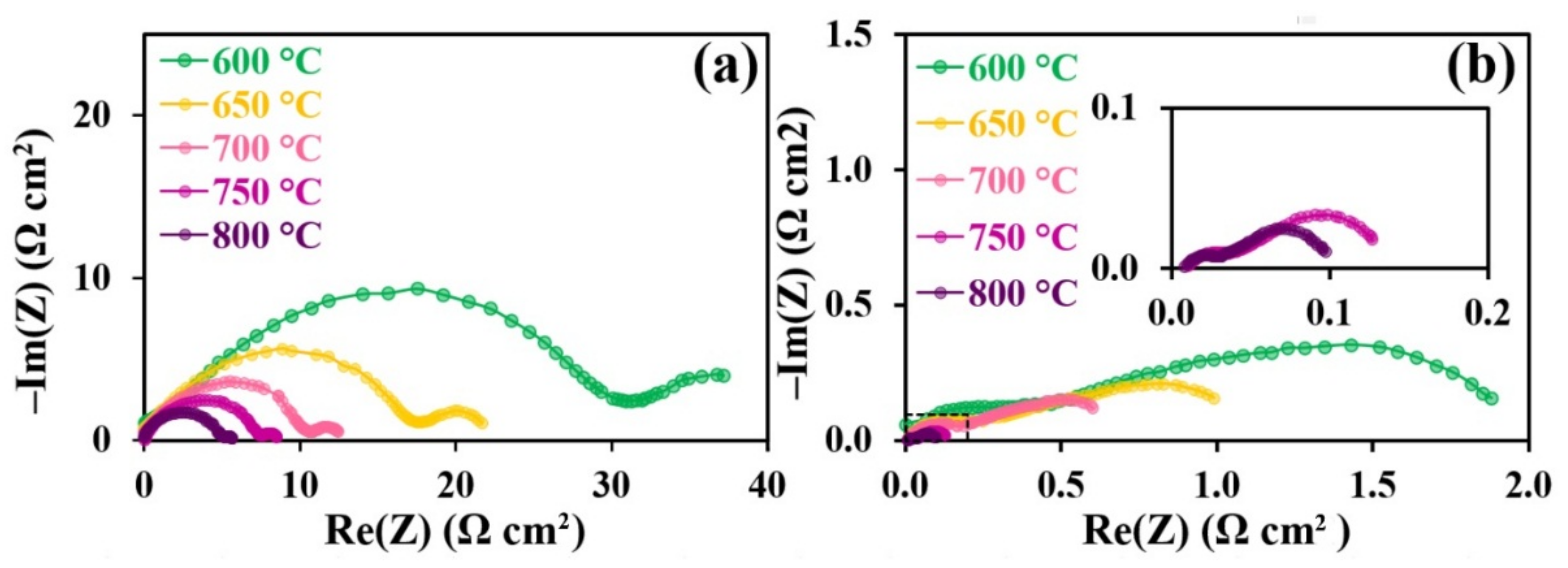
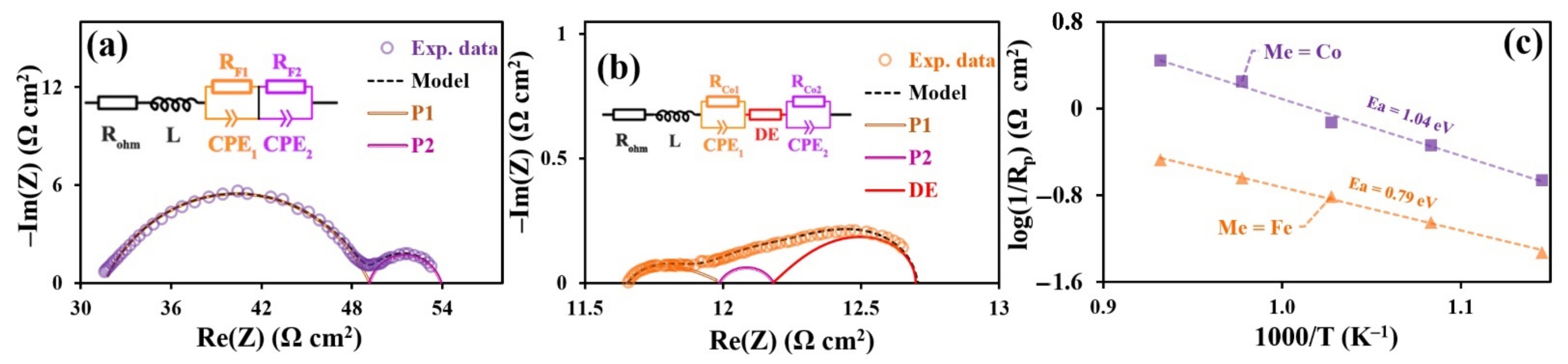
3.5. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The role of green and blue hydrogen in the energy transition—A technological and geopolitical perspective. Sustainability 2020, 13, 298. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Aziz, M. Prospect of hydrogen energy in Asia-Pacific: A perspective review on techno-socio-economy nexus. Int. J. Hydrog. Energy 2021, 46, 35027–35056. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Ali, M.S.; Rahim, N.A. Hydrogen energy vision 2060: Hydrogen as energy carrier in Malaysian primary energy mix—Developing P2G case. Energy Strat. Rev. 2021, 35, 100632. [Google Scholar] [CrossRef]
- Wulf, C.; Zapp, P.; Schreiber, A. Review of power-to-X demonstration projects in Europe. Front. Energy Res. 2020, 8, 191. [Google Scholar] [CrossRef]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Chen, G.; Feldhoff, A.; Weidenkaff, A.; Li, C.; Liu, S.; Zhu, X.; Sunarso, J.; Huang, K.; Wu, X.; Ghoniem, A.F.; et al. Roadmap for sustainable mixed ionic-electronic conducting membranes. Adv. Funct. Mater. 2022, 32, 2105702. [Google Scholar] [CrossRef]
- Irvine, J.; Rupp, J.G.L.M.; Liu, G.; Xu, X.; Haile, S.M.; Qian, X.; Snyder, A.; Freer, R.; Ekren, D.; Skinner, S.; et al. Roadmap on inorganic perovskites for energy applications. J. Phys. Energy 2021, 3, 031502. [Google Scholar] [CrossRef]
- Hanif, M.B.; Rauf, S.; Motola, M.; Babar, Z.U.D.; Li, C.-J.; Li, C.-X. Recent progress of perovskite-based electrolyte materials for solid oxide fuel cells and performance optimizing strategies for energy storage applications. Mater. Res. Bull. 2022, 146, 111612. [Google Scholar] [CrossRef]
- Zhigachev, A.O.; Rodaev, V.V.; Zhigacheva, D.V.; Lyskov, N.V.; Shchukina, M.A. Doping of scandia-stabilized zirconia electrolytes for intermediate-temperature solid oxide fuel cell: A review. Ceram. Int. 2021, 47, 32490–32504. [Google Scholar] [CrossRef]
- Saarinen, V.; Pennanen, J.; Kotisaari, M.; Thomann, O.; Himanen, O.; Di Iorio, S.; Hanoux, P.; Aicart, J.; Couturier, K.; Sun, X.; et al. Design, manufacturing, and operation of movable 2 × 10 kW size rSOC system. Fuel Cells 2021, 21, 477–487. [Google Scholar] [CrossRef]
- Udomsilp, D.; Lenser, C.; Guillon, O.; Menzler, N.H. Performance benchmark of planar solid oxide cells based on material development and designs. Energy Technol. 2021, 9, 2001062. [Google Scholar] [CrossRef]
- Zhou, Z.; Nadimpalli, V.K.; Pedersen, D.B.; Esposito, V. Degradation mechanisms of metal-supported solid oxide cells and countermeasures: A review. Materials 2021, 14, 3139. [Google Scholar] [CrossRef]
- Khan, M.S.; Xu, X.; Knibbe, R.; Zhu, Z. Air electrodes and related degradation mechanisms in solid oxide electrolysis and reversible solid oxide cells. Renew. Sustain. Energy Rev. 2021, 143, 110918. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Ma, L.; Li, W.; Liu, X. Degradation of solid oxide electrolysis cells: Phenomena, mechanisms, and emerging mitigation strategies—A review. J. Mater. Sci. Technol. 2020, 55, 35–55. [Google Scholar] [CrossRef]
- Bello, I.T.; Zhai, S.; Zhao, S.; Li, Z.; Yu, N.; Ni, M. Scientometric review of proton-conducting solid oxide fuel cells. Int. J. Hydrog. Energy 2021, 46, 37406–37428. [Google Scholar] [CrossRef]
- Su, H.; Hu, Y.H. Degradation issues and stabilization strategies of protonic ceramic electrolysis cells for steam electrolysis. Energy Sci. Eng. 2021, 1–20. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Ran, R.; Cao, J.; Shao, Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Progr. Nat. Sci. Mater. Int. 2020, 30, 764–774. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. Materials AIILnInO4 with ruddlesden-popper structure for electrochemical applications: Relationship between ion (oxygen-ion, proton) conductivity, water uptake, and structural changes. Materials 2021, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Fop, S. Solid oxide proton conductors beyond perovskites. J. Mater. Chem. A 2021, 9, 18836–18856. [Google Scholar] [CrossRef]
- Hossain, M.K.; Biswas, M.C.; Chanda, R.K.; Rubel, M.H.K.; Khan, M.I.; Hashizume, K. A review on experimental and theoretical studies of perovskite barium zirconate proton conductors. Emergent Mater. 2021, 4, 999–1027. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, J.; Zhao, Z.; Amoroso, J.; Tong, J.; Brinkman, K.S. Review: Recent progress in low-temperature proton-conducting ceramics. J. Mater. Sci. 2019, 54, 9291–9312. [Google Scholar] [CrossRef] [Green Version]
- Zamudio-García, J.; Caizán-Juanarena, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. A review on recent advances and trends in symmetrical electrodes for solid oxide cells. J. Power Sources 2022, 520, 230852. [Google Scholar] [CrossRef]
- Ni, C.; Zhou, J.; Zhang, Z.; Li, S.; Ni, J.-P.; Wu, K.; Irvine, J.T.S. Iron-based electrode materials for solid oxide fuel cells and electrolysers. Energy Environ. Sci. 2021, 14, 6287–6319. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Chizhova, E.A.; Kharytonau, D.S.; Medvedev, D.A. Layered oxygen-deficient double perovskites as promising cathode materials for solid oxide fuel cells. Materials 2021, 15, 141. [Google Scholar] [CrossRef]
- Skutina, L.; Filonova, E.; Medvedev, D.; Maignan, A. Undoped Sr2MMoO6 double perovskite molybdates (M = Ni, Mg, Fe) as promising anode materials for solid oxide fuel cells. Materials 2021, 14, 1715. [Google Scholar] [CrossRef]
- Medvedev, D.A. Current drawbacks of proton-conducting ceramic materials: How to overcome them for real electrochemical purposes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100549. [Google Scholar] [CrossRef]
- Basu, R.N.; Tietz, F.; Wessel, E.; Buchkremer, H.P.; Stöver, D. Microstructure and electrical conductivity of LaNi0.6Fe0.4O3 prepared by combustion synthesis routes. Mater. Res. Bull. 2004, 39, 1335–1345. [Google Scholar] [CrossRef]
- Pikalova, E.; Bogdanovich, N.; Kolchugin, A.; Shubin, K.; Ermakova, L.; Eremeev, N.; Farlenkov, A.; Khrustov, A.; Filonova, E.; Sadykov, V. Development of composite LaNi0.6Fe0.4O3-δ-based air electrodes for solid oxide fuel cells with a thin-film bilayer electrolyte. Int. J. Hydrog. Energy 2021, 46, 16947–16964. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Medvedev, D.; Pikalova, E.; Plaksin, S.; Brouzgou, A.; Demin, A.; Tsiakaras, P. A detailed analysis of thermal and chemicalcompatibility of cathode materials suitable for BaCe0.8Y0.2O3−δ and BaZr0.8Y0.2O3−δ proton electrolytes for solid oxide fuel cell application. Int. J. Hydrog. Energy 2017, 42, 1715–1723. [Google Scholar] [CrossRef]
- Adachi, Y.; Hatada, N.; Kato, M.; Hirota, K.; Uda, T. Experimental validation of high electrical conductivity in Ni-rich LaNi1−xFexO3 solid solutions (x ≤ 0.4) in high-temperature oxidizing atmospheres. Mater. Adv. 2021, 2, 3257–3263. [Google Scholar] [CrossRef]
- Harrison, C.M.; Slater, P.R.; Steinberger-Wilckens, R. Lanthanum nickelates and their application in solid oxide cells—Thse LaNi1-xFexO3 system and other ABO3-type nickelates. Solid State Ion. 2021, 373, 115799. [Google Scholar] [CrossRef]
- Hou, J.; Zhu, Z.; Qian, J.; Liu, W. A new cobalt-free proton-blocking composite cathode La2NiO4+δ–LaNi0.6Fe0.4O3−δ for BaZr0.1Ce0.7Y0.2O3−δ-based solid oxide fuel cells. J. Power Sources 2014, 264, 67–75. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodriquez-Carvajal, J. WinPLOTR: A windows tool for powder diffraction pattern analysis. Mater. Sci. Forum 2001, 378–381, 118–123. [Google Scholar] [CrossRef] [Green Version]
- ZView® for Windows. Available online: http://www.scribner.com/software/68-general-electrochemistr376-zview-for-windows/ (accessed on 15 February 2022).
- An, H.; Shin, D.; Ji, H.-I. Effect of nickel addition on sintering behavior and electrical conductivity of BaCe0.35Zr0.5Y0.15O3−δ. J. Korean Ceram. Soc. 2019, 56, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, L.; Liu, M.; Tang, Z.; Liu, M. Enhanced sinterability of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ by addition of nickel oxide. J. Power Sources 2011, 196, 9980–9984. [Google Scholar] [CrossRef]
- Gorbova, E.; Maragou, V.; Medvedev, D.; Demin, A.; Tsiakaras, P. Influence of sintering additives of transition metals on the properties of gadolinium-doped barium cerate. Solid State Ion. 2008, 179, 887–890. [Google Scholar] [CrossRef]
- Basbus, J.F.; Caneiro, A.; Suescun, L.; Lamas, D.G.; Mogni, L.V. Anomalous X-ray diffraction study of Pr-substituted BaCeO3−δ. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 455–462. [Google Scholar] [CrossRef]
- Wang, B.; Bi, L.; Zhao, X.S. Exploring the role of NiO as a sintering aid in BaZr0.1Ce0.7Y0.2O3–δ electrolyte for proton-conducting solid oxide fuel cells. J. Power Sources 2018, 399, 207–214. [Google Scholar] [CrossRef]
- Xie, H.-H.; Gao, Q.; Li, L.; Lei, G.; Hu, X.-R.; Deng, J.-B. First-principles study of structural, electronic, magnetic and thermodynamic properties of the double perovskite Ba2CeCoO6. Solid State Commun. 2016, 239, 49–54. [Google Scholar] [CrossRef]
- Moriga, T.; Hayashi, M.; Sakamoto, T.; Orihara, M.; Nakabayashi, I. Reduction processes of rare-earth nickelate perovskites LnNiO3 (Ln=La, Pr, Nd). Solid State Ion. 2002, 154–155, 251–255. [Google Scholar] [CrossRef]
- Kostogloudis, G.C.; Ftikos, C. Structural, thermal and electrical properties of Pr0.5Sr0.5Co1−yNiyO3−δ perovskite-type oxides. Solid State Ion. 1998, 109, 43–53. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sec. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- S’wierczek, K.; Marzec, J.; Pa’ubiak, D.; Zaja, W.; Molenda, J. LFN and LSCFN perovskites—Structure and transport properties. Solid State Ion. 2006, 177, 1811–1817. [Google Scholar] [CrossRef]
- Vibhu, V.; Flura, A.; Nicollet, C.; Fourcade, S.; Penin, N.; Bassat, J.-M.; Grenier, J.-C.; Rougier, A.; Pouchard, M. Characterization of PrNiO3−δ as oxygen electrode for SOFCs. Solid State Sci. 2018, 81, 26–31. [Google Scholar] [CrossRef]
- Vashook, V.; Rebello, J.; Chen, J.Y.; Vasylechko, L.; Trots, D.; Zosel, J.; Guth, U. Thermal expansion, oxygen non-stoichiometry and diffusion mobility in some ferrites-nickelites. Solid State Phenom. 2013, 200, 86–92. [Google Scholar] [CrossRef]
- Rebello, J.; Vashook, V.; Trots, D.; Guth, U. Thermal stability, oxygen non-stoichiometry, electrical conductivity and diffusion characteristics of PrNi0.4Fe0.6O3−δ, a potential cathode material for IT-SOFCs. J. Power Sources 2011, 196, 3705–3712. [Google Scholar] [CrossRef]
- Hashimoto, S.; Kammer, K.; Larsen, P.H.; Poulsen, F.W.; Mogensen, M. A study of Pr0.7Sr0.3Fe1−xNixO3−δ as a cathode material for SOFCs with intermediate operating temperature. Solid State Ion. 2005, 176, 1013–1020. [Google Scholar] [CrossRef]
- Tsipis, E.; Kiselev, E.; Kolotygin, V.; Waerenborgh, J.; Cherepanov, V.; Kharton, V. Mixed conductivity, Mössbauer spectra and thermal expansion of (La,Sr)(Fe,Ni)O3−δ perovskites. Solid State Ion. 2008, 179, 2170–2180. [Google Scholar] [CrossRef]
- Sakaki, Y. Ln1−xSrxMnO3 (Ln=Pr, Nd, Sm and Gd) as the cathode material for solid oxide fuel cells. Solid State Ion. 1999, 118, 187–194. [Google Scholar] [CrossRef]
- Lyagaeva, Y.G.; Medvedev, D.A.; Demin, A.V.K.; Tsiakaras, P.; Reznitskikh, O.G. Thermal expansion of materials in the barium cerate-zirconate system. Phys. Solid State 2015, 57, 285–289. [Google Scholar] [CrossRef]
- Flura, A.; Dru, S.; Nicollet, C.; Vibhu, V.; Fourcade, S.; Lebraud, E.; Rougier, A.; Bassat, J.-M.; Grenier, J.-C. Chemical and structural changes in Ln2NiO4+δ (Ln=La, Pr or Nd) lanthanide nickelates as a function of oxygen partial pressure at high temperature. J. Solid State Chem. 2015, 228, 189–198. [Google Scholar] [CrossRef]
- Chiba, R.; Yoshimura, F.; Sakurai, Y. An investigation of LaNi1−xFexO3 as a cathode material for solid oxide fuel cells. Solid State Ion. 1999, 124, 281–288. [Google Scholar] [CrossRef]
- Gateshki, M.; Suescun, L.; Kolesnik, S.; Mais, J.; Świerczek, K.; Short, S.; Dabrowski, B. Structural, magnetic and electronic properties of LaNi0.5Fe0.5O3 in the temperature range 5–1000K. J. Solid State Chem. 2008, 181, 1833–1839. [Google Scholar] [CrossRef]
- Niwa, E.; Uematsu, C.; Miyashita, E.; Ohzeki, T.; Hashimoto, T. Conductivity and sintering property of LaNi1−xFexO3 ceramics prepared by Pechini method. Solid State Ion. 2011, 201, 87–93. [Google Scholar] [CrossRef]
- Hashimoto, S.; Kammer, K.; Poulsen, F.W.; Mogensen, M. Conductivity and electrochemical characterization of PrFe1−xNixO3−δ at high temperature. J. Alloy. Compd. 2007, 428, 256–261. [Google Scholar] [CrossRef]
- Wu, M.; Cai, H.; Jin, F.; Sun, N.; Xu, J.; Zhang, L.; Han, X.; Wang, S.; Su, X.; Long, W.; et al. Assessment of cobalt–free ferrite–based perovskite Ln0.5Sr0.5Fe0.9Mo0.1O3–δ (Ln = lanthanide) as cathodes for IT-SOFCs. J. Eur. Ceram. Soc. 2021, 41, 2681–2690. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Vdovin, G.K.V.; Medvedev, D.A.; Yaremchenko, A.A. Fluorine-containing oxygen electrodes of the nickelate family for proton-conducting electrochemical cells. Electrochim. Acta 2020, 337, 135808. [Google Scholar] [CrossRef]
- Nasani, N.; Ramasamy, D.; Antunes, I.; Singh, B.; Fagg, D.P. Structural and electrical properties of strontium substituted Y2BaNiO5. J. Alloy. Compd. 2015, 620, 91–96. [Google Scholar] [CrossRef]
- Tarutin, A.P.; Lyagaeva, Y.G.; Vylkov, A.I.; Gorshkov, M.Y.; Vdovin, G.K.V.; Medvedev, D.A. Performance of Pr2(Ni,Cu)O4+δ electrodes in protonic ceramic electrochemical cells with unseparated and separated gas spaces. J. Mater. Sci. Technol. 2021, 93, 157–168. [Google Scholar] [CrossRef]
- Huang, S.; Lu, Q.; Feng, S.; Li, G.; Wang, C. PrNi0.6Co0.4O3–Ce0.8Sm0.2O1.9 composite cathodes for intermediate temperature solid oxide fuel cells. J. Power Sources 2012, 199, 150–154. [Google Scholar] [CrossRef]
- Li, Y.; Cai, J.W.; Alonso, J.A.; Lian, H.Q.; Cui, X.G.; Goodenough, J.B. Evaluation of LaNi0.6M0.4O3 (M = Fe, Co) cathodes in LSGM-electrolyte-supported solid-oxide fuel cells. Int. J. Hydrog. Energy 2017, 42, 27334–27342. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Montini, T.; Tavagnacco, C.; Fonda, E.; Fornasiero, P.; Graziani, M. Preparation, characterization, and electrochemical properties of pure and composite LaNi0.6Fe0.4O3-based cathodes for IT-SOFC. Chem. Mater. 2007, 19, 5926–5936. [Google Scholar] [CrossRef]
- Chrzan, A.; Karczewski, J.; Gazda, M.; Szymczewska, D.; Jasinski, P. Investigation of thin perovskite layers between cathode and doped ceria used as buffer layer in solid oxide fuel cells. J. Solid State Electrochem. 2015, 19, 1807–1815. [Google Scholar] [CrossRef] [Green Version]
- Khoshkalam, M.; Faghihi-Sani, M.A.; Tong, X.; Chen, M.; Hendriksen, P.V. Enhanced Activity of Pr6O11 and CuO Infiltrated Ce0.9Gd0.1O2 Based Composite Oxygen Electrodes. J. Electrochem. Soc. 2020, 167, 024505. [Google Scholar] [CrossRef]
- Chrzan, A.; Ovtar, S.; Chen, M. LaNi1–xCoxO3–δ (x = 0.4 to 0.7) cathodes for solid oxide fuel cells by infiltration. IOP Conf. Ser. Mater. Sci. Eng. 2016, 104, 012019. [Google Scholar] [CrossRef] [Green Version]
- Hjalmarsson, P.; Mogensen, M. La0.99Co0.4Ni0.6O3−δ–Ce0.8Gd0.2O1.95 as composite cathode for solid oxide fuel cells. J. Power Sources 2011, 196, 7237–7244. [Google Scholar] [CrossRef]
- Huang, S.; Feng, S.; Wang, H.; Li, Y.; Wang, C. LaNi0.6Fe0.4O3–Ce0.8Sm0.2O1.9–Ag composite cathode for intermediate temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2011, 36, 10968–10974. [Google Scholar] [CrossRef]
- Natalia Popova’s Web-Sites. Scientific Translation Laboratory. Available online: https://nauka-perevod.ru (accessed on 15 February 2022).
| Composition | Heating | Cooling | ||||||
|---|---|---|---|---|---|---|---|---|
| T* | 100–T* | T*–1000 | 100–1000 | T* | 100–T* | T*–1000 | 100–1000 | |
| M = Co | 580 | 16.5 | 15.5 | 16.0 | 550 | 16.1 | 15.2 | 15.7 |
| M = Fe | 620 | 9.9 | 10.5 | 10.2 | 630 | 9.6 | 11.1 | 10.4 |
| Composition | Temperature Interval, °C | TEC·106, K−1 | References |
|---|---|---|---|
| PrNi0.4Fe0.6O3–δ | 100–1000 | 10.4 | This work |
| PrNi0.4Co0.6O3–δ | 15.7 | ||
| PrNiO3–δ | 25–1000 | 12.7 | [49] |
| PrFe0.7Ni0.3O3–δ | 25–900 | 8.4–11.0 | [50] |
| PrNi0.4Fe0.6O3–δ | 400–800 | 10.5–11.4 | [51] |
| Pr0.7Sr0.3Fe0.6Ni0.4O3–δ | 25–1000 | 14.1 | [52] |
| Pr0.7Sr0.3Fe0.5Ni0.5O3–δ | 25–1000 | 13.5 | [52] |
| Pr0.5Sr0.5Co0.6Ni0.4O3–δ | 100–255 | 15.7 | [46] |
| 400–800 | 21.6 | ||
| 800–1000 | 27.5 | ||
| La0.9Sr0.1Fe0.6Ni0.4O3–δ | 420–700 | 12.2 | [53] |
| 700–1150 | 13.1 |
| Electrode | Electrolyte | Rp, Ω cm2 | References | |
|---|---|---|---|---|
| 600 °C | 700 °C | |||
| PrNi0.4Fe0.6O3–δ | BCZY | 21.1 | 6.46 | This work |
| PrNi0.4Co0.6O3–δ | 4.6 | 1.3 | ||
| PrNiO3–δ | Ce0.9Gd0.1O2–δ | 0.91 | 0.13 | [49] |
| Pr0.7Sr0.3Fe0.6Ni0.4O3–δ | 40.9 | 4.7 | [52] | |
| PrFe0.6Ni0.4O3–δ | 20.1 | 2.5 | [60] | |
| PrFe0.5Ni0.5O3–δ | 17.8 | 1.4 | ||
| PrFe0.4Ni0.6O3−δ | 24.6 | 2.8 | ||
| LaFe0.4Ni0.6O3−δ | 3.5 | 0.4 | ||
| PrNi0.6Co0.4O3–δ | Ce0.8Sm0.2O2–δ | 0.7 | 0.1 | [65] |
| LaNi0.6Co0.4O3–δ | La0.8Sr0.2Ga0.83Mg0.17O3–δ | 34.0 | 2.9 | [66] |
| LaNi0.6Fe0.4O3–δ | La0.8Sr0.2Ga0.83Mg0.17O3–δ | 27.3 | 3.2 | |
| LaNi0.6Fe0.4O3–δ | YSZ | 373.2 | 81.9 | [67] |
| LaNi0.6Fe0.4O3–δ | Ce0.8Gd0.2O2–δ | 3.3 | 0.4 | [68] |
| LaNi0.6Fe0.4O3–δ | Ce0.9Gd0.1O2–δ | 21.4 | 2.7 | [69] |
| LaNi0.4Co0.6O3–δ | YSZ | 0.1 | 0.04 | [70] |
| LaCo0.6Ni0.4O3–δ | Ce0.8Gd0.2O2–δ | 326.0 | 45.0 | [71] |
| LaNi0.6Fe0.4O3–δ | Ce0.8Sm0.2O2–δ | 7.6 | 1.2 | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarutin, A.P.; Kasyanova, A.V.; Vdovin, G.K.; Lyagaeva, J.G.; Medvedev, D.A. Nickel-Containing Perovskites, PrNi0.4Fe0.6O3–δ and PrNi0.4Co0.6O3–δ, as Potential Electrodes for Protonic Ceramic Electrochemical Cells. Materials 2022, 15, 2166. https://doi.org/10.3390/ma15062166
Tarutin AP, Kasyanova AV, Vdovin GK, Lyagaeva JG, Medvedev DA. Nickel-Containing Perovskites, PrNi0.4Fe0.6O3–δ and PrNi0.4Co0.6O3–δ, as Potential Electrodes for Protonic Ceramic Electrochemical Cells. Materials. 2022; 15(6):2166. https://doi.org/10.3390/ma15062166
Chicago/Turabian StyleTarutin, Artem P., Anna V. Kasyanova, Gennady K. Vdovin, Julia G. Lyagaeva, and Dmitry A. Medvedev. 2022. "Nickel-Containing Perovskites, PrNi0.4Fe0.6O3–δ and PrNi0.4Co0.6O3–δ, as Potential Electrodes for Protonic Ceramic Electrochemical Cells" Materials 15, no. 6: 2166. https://doi.org/10.3390/ma15062166







