The Effects of 3-Dimensional Bioprinting Calcium Silicate Cement/Methacrylated Gelatin Scaffold on the Proliferation and Differentiation of Human Dental Pulp Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
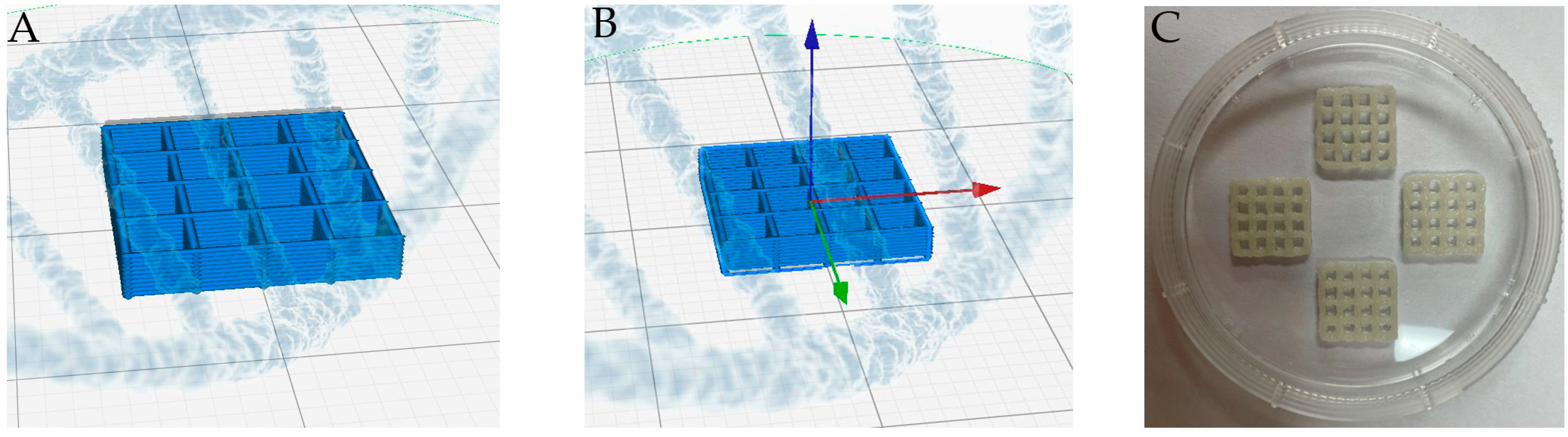
2.2. Fabrication of 3D MTA-GelMa Scaffolds
2.3. Materials
2.4. In Vitro Biocompatibility Evaluation
2.4.1. Isolation and Culture of hDPSCs
2.4.2. Cell Adhesion on the 3D Scaffold
2.4.3. Material Extracts
2.4.4. Cell Viability
2.4.5. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.4.6. Alkaline Phosphatase (ALP) and Alizarin Red S (ARS) Staining
2.5. Statistical Analysis
3. Results
3.1. Morphology of the Scaffolds
3.2. Cell Adhesion
3.3. Cell Viability
3.4. Odontogenic Differentiation and Mineralization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs 2020, 43, 69–86. [Google Scholar] [CrossRef]
- Reddy, L.V.K.; Murugan, D.; Mullick, M.; Begum Moghal, E.T.; Sen, D. Recent approaches for angiogenesis in search of successful tissue engineering and regeneration. Curr. Stem Cell Res. Ther. 2020, 15, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ouyang, L.; Armstrong, J.P.; Stevens, M.M. Advances in the fabrication of biomaterials for gradient tissue engineering. Trends Biotechnol. 2021, 39, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.A.; Bártolo, P.J. Biomimetic boundary-based scaffold design for tissue engineering applications. Methods Mol. Biol. 2021, 2147, 3–18. [Google Scholar] [PubMed]
- Jazayeri, H.E.; Lee, S.-M.; Kuhn, L.; Fahimipour, F.; Tahriri, M.; Tayebi, L. Polymeric scaffolds for dental pulp tissue engineering: A review. Dent. Mater. 2020, 36, e47–e58. [Google Scholar] [CrossRef]
- Ohara, T.; Itaya, T.; Usami, K.; Ando, Y.; Sakurai, H.; Honda, M.J.; Ueda, M.; Kagami, H. Evaluation of scaffold materials for tooth tissue engineering. J. Biomed. Mater. Res. A 2010, 94, 800–805. [Google Scholar] [CrossRef]
- Lv, Y. Application of Physical Stimulation in Stem Cell-based Tissue Engineering. Curr. Stem Cell Res. Ther. 2020, 15, 389–390. [Google Scholar] [CrossRef]
- de Sousa Iwamoto, L.A.; Duailibi, M.T.; Iwamoto, G.Y.; Juliano, Y.; Duailibi, M.S.; Ossamu Tanaka, F.A.; Duailibi, S.E. Tooth tissue engineering: Tooth decellularization for natural scaffold. Future Sci. OA 2016, 2. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Weng, W.; Zhang, Y.; Sun, X.; Yang, H. Applications of gelatin methacryloyl (GelMA) hydrogels in microfluidic technique-assisted tissue engineering. Molecules 2020, 25, 5305. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, X.; Song, W.; Pan, T.; Wang, H.; Ning, T.; Wei, Q.; Xu, H.H.; Wu, B.; Ma, D. Effects of 3-dimensional bioprinting alginate/gelatin hydrogel scaffold extract on proliferation and differentiation of human dental pulp stem cells. J. Endod. 2019, 45, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xu, L.; Wu, J.; Wang, S.; Dong, Y. Gelatin/bioactive glass composite scaffold for promoting the migration and odontogenic differentiation of bone marrow mesenchymal stem cells. Polym. Test. 2021, 93, 106915. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater. Sci. 2021, 9, 2620–2630. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and properties of gelatin methacryloyl (GelMA) hydrogels and their recent applications in load-bearing tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [Green Version]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as biomaterial for tissue engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Van Damme, L.; Van Hoorick, J.; Blondeel, P.; Van Vlierberghe, S. Toward Adipose Tissue Engineering Using Thiol-Norbornene Photo-Crosslinkable Gelatin Hydrogels. Biomacromolecules 2021, 22, 2408–2418. [Google Scholar] [CrossRef]
- Gautam, S.; Sharma, C.; Purohit, S.D.; Singh, H.; Dinda, A.K.; Potdar, P.D.; Chou, C.-F.; Mishra, N.C. Gelatin-polycaprolactone-nanohydroxyapatite electrospun nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111588. [Google Scholar] [CrossRef]
- Chawla, D.; Kaur, T.; Joshi, A.; Singh, N. 3D bioprinted alginate-gelatin based scaffolds for soft tissue engineering. Int. J. Biol. Macromol. 2020, 144, 560–567. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Ho, C.-C.; Huang, T.-H.; Hsu, T.-T.; Shie, M.-Y. The ionic products from mineral trioxide aggregate–induced odontogenic differentiation of dental pulp cells via activation of the Wnt/β-catenin signaling pathway. J. Endod. 2016, 42, 1062–1069. [Google Scholar] [CrossRef]
- Wang, M.; Li, B.; Liu, Y.; Tang, L.; Zhang, Y.; Xie, Q. A Novel Bionic Extracellular Matrix Polymer Scaffold Enhanced by Calcium Silicate for Bone Tissue Engineering. ACS Omega 2021, 6, 35727–35737. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Coelho, C.M.; Sequeira, D.B.; Marques, J.A.; Pereira, J.F.; Sousa, V.; Palma, P.J.; Santos, A.C. Subcutaneous implantation assessment of new calcium-silicate based sealer for warm obturation. Biomedicines 2021, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, A.; Hargreaves, K.M. Microbial modulation of stem cells and future directions in regenerative endodontics. J. Endod. 2017, 43, S95–S101. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.; Cornélio, A.; Mestieri, L.; Fuentes, A.; Salles, L.; Rossa-Junior, C.; Faria, G.; Guerreiro-Tanomaru, J.; Tanomaru-Filho, M. Human dental pulp cells response to mineral trioxide aggregate (MTA) and MTA Plus: Cytotoxicity and gene expression analysis. Int. Endod. J. 2017, 50, 780–789. [Google Scholar] [CrossRef]
- Sequeira, D.B.; Oliveira, A.R.; Seabra, C.M.; Palma, P.J.; Ramos, C.; Figueiredo, M.H.; Santos, A.C.; Cardoso, A.L.; Peça, J.; Santos, J.M. Regeneration of pulp-dentin complex using human stem cells of the apical papilla: In vivo interaction with two bioactive materials. Clin. Oral Investig. 2021, 25, 5317–5329. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Fang, H.-Y.; Hsu, T.-T.; Lin, C.-Y.; Shie, M.-Y. The characteristics of mineral trioxide aggregate/polycaprolactone 3-dimensional scaffold with osteogenesis properties for tissue regeneration. J. Endod. 2017, 43, 923–929. [Google Scholar] [CrossRef]
- Singh, S.; Dutt, D.; Kaur, P.; Singh, H.; Mishra, N.C. Microfibrous paper scaffold for tissue engineering application. J. Biomater. Sci. Polym. Ed. 2020, 31, 1091–1106. [Google Scholar] [CrossRef]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Laird, N.Z.; Acri, T.M.; Chakka, J.L.; Quarterman, J.C.; Malkawi, W.I.; Elangovan, S.; Salem, A.K. Applications of nanotechnology in 3D printed tissue engineering scaffolds. Eur. J. Pharm. Biopharm. 2021, 161, 15–28. [Google Scholar] [CrossRef]
- Wang, F.; Xie, C.; Ren, N.; Bai, S.; Zhao, Y. Human freeze-dried dentin matrix as a biologically active scaffold for tooth tissue engineering. J. Endod. 2019, 45, 1321–1331. [Google Scholar] [CrossRef]
- Farzin, A.; Bahrami, N.; Mohamadnia, A.; Mousavi, S.; Gholami, M.; Ai, J.; Moayeri, R.S. Scaffolds in dental tissue engineering: A review. Arch. Neurosci. 2019, 7, e97014. [Google Scholar] [CrossRef] [Green Version]
- Leu Alexa, R.; Iovu, H.; Ghitman, J.; Serafim, A.; Stavarache, C.; Marin, M.-M.; Ianchis, R. 3D-Printed Gelatin Methacryloyl-Based Scaffolds with Potential Application in Tissue Engineering. Polymers 2021, 13, 727. [Google Scholar] [CrossRef] [PubMed]
- Kuchler-Bopp, S.; Bécavin, T.; Kökten, T.; Weickert, J.; Keller, L.; Lesot, H.; Deveaux, E.; Benkirane-Jessel, N. Three-dimensional micro-culture system for tooth tissue engineering. J. Dent. Res. 2016, 95, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Seo, Y.B.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; Park, C.H. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef]
- Do, A.-V.; Smith, R.; Acri, T.M.; Geary, S.M.; Salem, A.K. 3D printing technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Elsevier: Iowa City, IA, USA, 2018; pp. 203–234. [Google Scholar]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.-R.; Kang, H.-W. Bioprinting of three-dimensional dentin–pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10, 2041731419845849. [Google Scholar] [CrossRef]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D printing of scaffolds and tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Kantaros, A.; Chatzidai, N.; Karalekas, D. 3D printing-assisted design of scaffold structures. Int. J. Adv. Manuf. Technol. 2016, 82, 559–571. [Google Scholar] [CrossRef]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 14023. [Google Scholar] [CrossRef]
- Cao, S.; Han, J.; Sharma, N.; Msallem, B.; Jeong, W.; Son, J.; Kunz, C.; Kang, H.-W.; Thieringer, F.M. In vitro mechanical and biological properties of 3D printed polymer composite and β-tricalcium phosphate scaffold on human dental pulp stem cells. Materials 2020, 13, 3057. [Google Scholar] [CrossRef]
- Vaezi, M.; Zhong, G.; Kalami, H.; Yang, S. Extrusion-based 3D printing technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Elsevier: Leuven, Belgium, 2018; pp. 235–254. [Google Scholar]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef]
- Yu, Y.; Hua, S.; Yang, M.; Fu, Z.; Teng, S.; Niu, K.; Zhao, Q.; Yi, C. Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold. RSC Adv. 2016, 6, 110557–110565. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.-B.; Song, D.; Kim, H.-M.; Kim, S.-Y. Cytotoxicity and Mineralization Potential of Four Calcium Silicate-Based Cements on Human Gingiva-Derived Stem Cells. Coatings 2020, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-Y.; Lee, S.-M.; Lee, J.-H. Initial cytotoxicity of mineral trioxide aggregate (MTA) during setting on human mesenchymal stem cells. Adv. Mater. Sci. Eng. 2019, 2019, 2365104. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Kang, C.-M.; Song, J.S.; Shin, Y.; Kim, S.; Kim, S.-O.; Choi, H.-J. Biological efficacy of two mineral trioxide aggregate (MTA)-based materials in a canine model of pulpotomy. Dent. Mater. J. 2017, 36, 2016–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.J.; Kim, E.; Song, M.; Park, J.-W.; Shin, S.-J. Effects of two fast-setting calcium-silicate cements on cell viability and angiogenic factor release in human pulp-derived cells. Odontology 2016, 104, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.D.; Yadlapati, M.; Dorn, S.O.; Silva, R.; Letra, A. Analysis of radiopacity, pH and cytotoxicity of a new bioceramic material. J. Appl. Oral Sci. 2015, 23, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Kodama, S.; Okiji, T. Evaluation of calcium-releasing and apatite-forming abilities of fast-setting calcium silicate-based endodontic materials. Int. Endod. J. 2015, 48, 124–130. [Google Scholar] [CrossRef]
- Gandolfi, M.; Taddei, P.; Tinti, A.; Prati, C. Apatite-forming ability (bioactivity) of ProRoot MTA. Int. Endod. J. 2010, 43, 917–929. [Google Scholar] [CrossRef]
- Lee, H.; Shin, Y.; Jung, J.; Kim, S.; Lee, J.; Song, J. Biologic response of human deciduous dental pulp cells on newly developed MTA-like materials. J. Korean Acad. Pediatr. Dent. 2015, 42, 291–301. [Google Scholar] [CrossRef]






| Primer Sequences (5′–3′) | |
|---|---|
| dspp | Forward: TTAAATGCCAGTGGAACCAT Reverse: ATTCCCTTCTCCCTTGTGAC |
| dmp-1 | Forward: TGGGGATTATCCTGTGCTCT Reverse: TACTTCTGGGGTCACTGTCG |
| gapdh | Forward: AAGGTGAAGGTCGGACTCAAC Reverse: GGGGTCATTGATGGCAACAATA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.; Qiu, M.; Hwang, Y.-C.; Oh, W.-M.; Koh, J.-T.; Park, C.; Lee, B.-N. The Effects of 3-Dimensional Bioprinting Calcium Silicate Cement/Methacrylated Gelatin Scaffold on the Proliferation and Differentiation of Human Dental Pulp Stem Cells. Materials 2022, 15, 2170. https://doi.org/10.3390/ma15062170
Choi D, Qiu M, Hwang Y-C, Oh W-M, Koh J-T, Park C, Lee B-N. The Effects of 3-Dimensional Bioprinting Calcium Silicate Cement/Methacrylated Gelatin Scaffold on the Proliferation and Differentiation of Human Dental Pulp Stem Cells. Materials. 2022; 15(6):2170. https://doi.org/10.3390/ma15062170
Chicago/Turabian StyleChoi, Dakyung, Manfei Qiu, Yun-Chan Hwang, Won-Mann Oh, Jeong-Tae Koh, Chan Park, and Bin-Na Lee. 2022. "The Effects of 3-Dimensional Bioprinting Calcium Silicate Cement/Methacrylated Gelatin Scaffold on the Proliferation and Differentiation of Human Dental Pulp Stem Cells" Materials 15, no. 6: 2170. https://doi.org/10.3390/ma15062170
APA StyleChoi, D., Qiu, M., Hwang, Y.-C., Oh, W.-M., Koh, J.-T., Park, C., & Lee, B.-N. (2022). The Effects of 3-Dimensional Bioprinting Calcium Silicate Cement/Methacrylated Gelatin Scaffold on the Proliferation and Differentiation of Human Dental Pulp Stem Cells. Materials, 15(6), 2170. https://doi.org/10.3390/ma15062170







