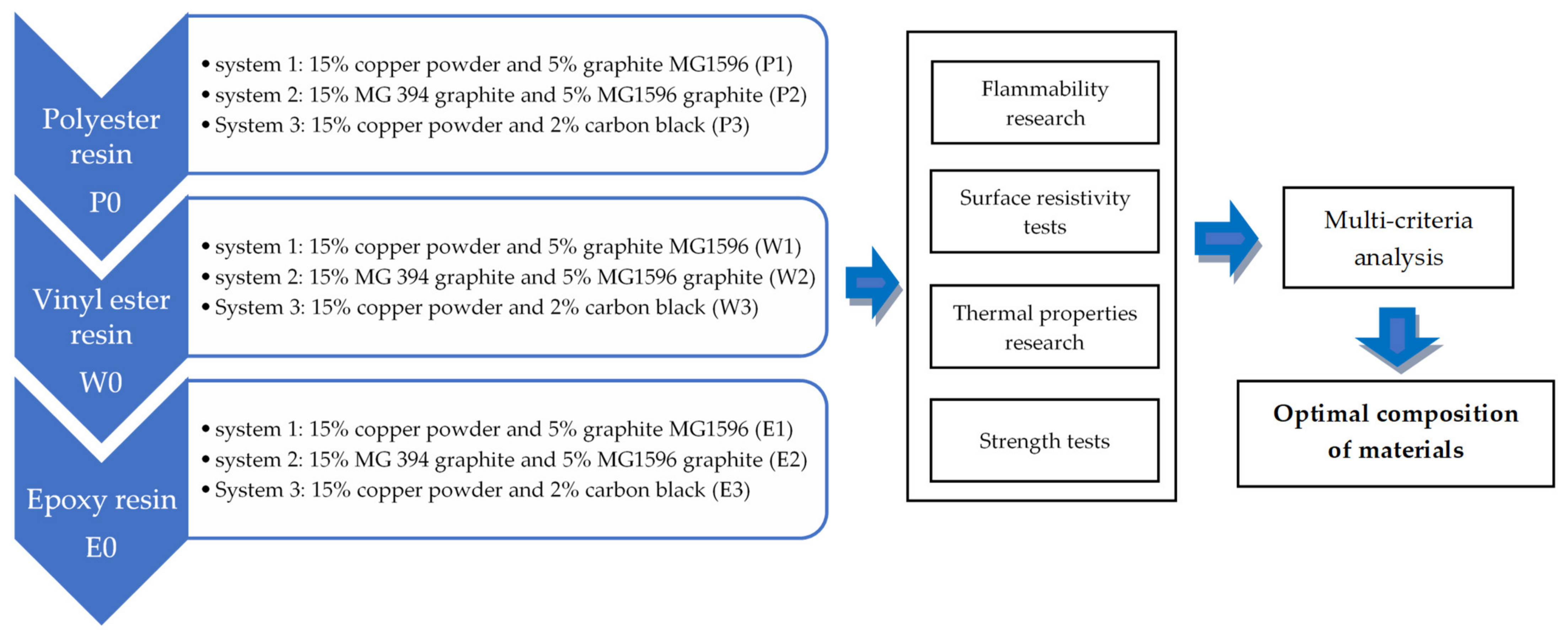
Polyester and Epoxy Resins with Increased Thermal Conductivity and Reduced Surface Resistivity for Applications in Explosion-Proof Enclosures of Electrical Devices
Abstract
:1. Introduction
2. Materials for Research and Methodology
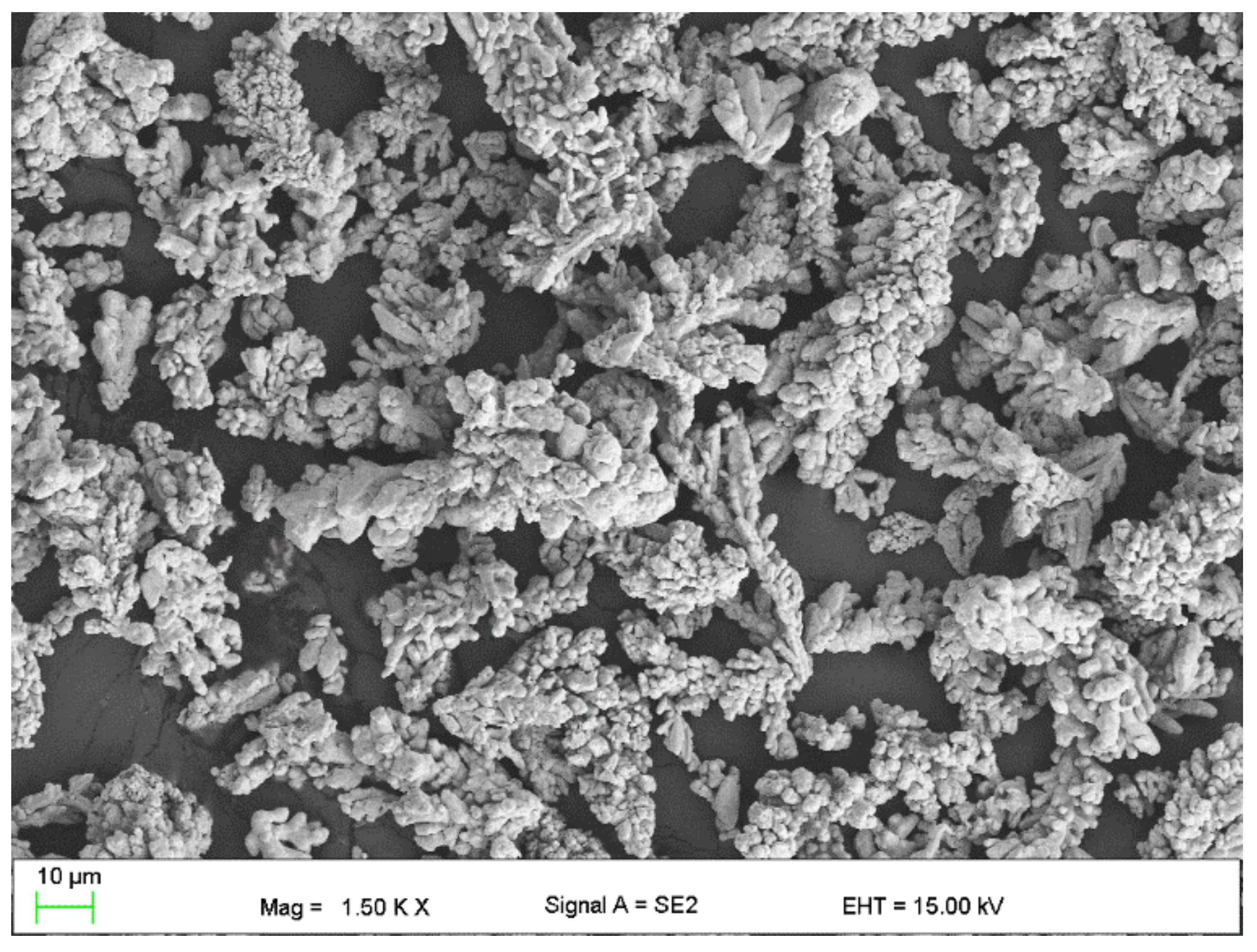
2.1. Material for Research
- Polyester with reduced flammability by the addition of mineral fillers (POLIMAL 1604 TS) cured with a system of 0.4% cobalt accelerator (1% concentration) and 2% LUPAROX K-15, produced by Sarzyna Chemical, Nowa Sarzyna, Poland.
- Vinyl ester (POLIMAL VE-11 MAT) based on brominated bisphenol-A, cured with a system of 1% cobalt accelerator (1% concentration) and 2% low-reactive methyl ethyl ketone peroxide (LUPAROX K-12G), produced by Sarzyna Chemical, Nowa Sarzyna, Poland.
- Epoxy (LG 420 FR from GRM Composites, Olomouc, Czech Republic) with a reduced flammability and an increased resistance to fire, cured with an amine cycloaliphatic hardener with a low viscosity (HG 400) in the ratio 100:25 parts. In addition, the resin was modified in terms of reducing flammability with diethyl-N,N-bis (2-hydroxyethyl)-aminomethyl phosphonate, as well as an agent improving the wettability of the fibers, which facilitates degassing of the resin. Table 1 presents the selected properties of the tested resins.
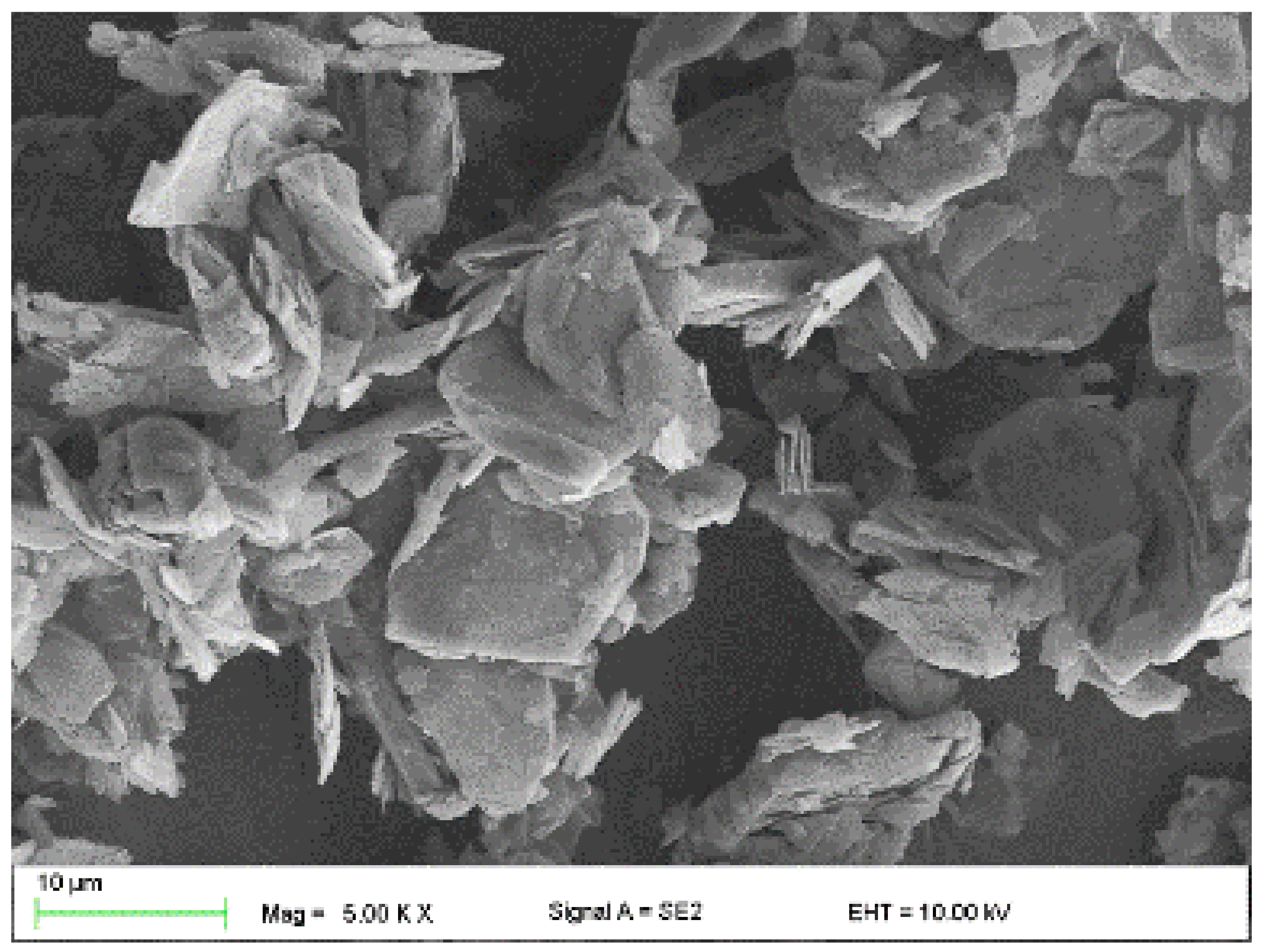
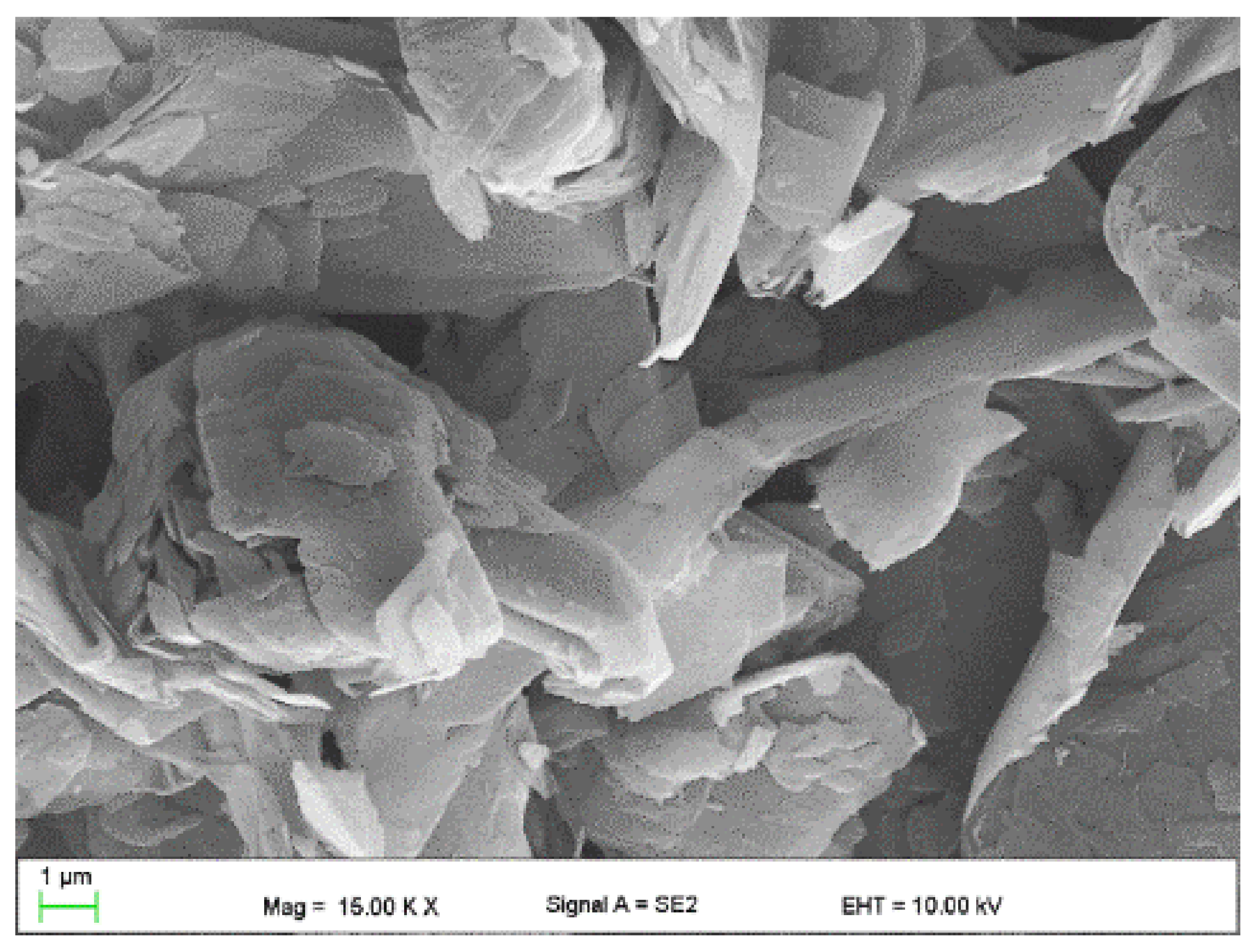
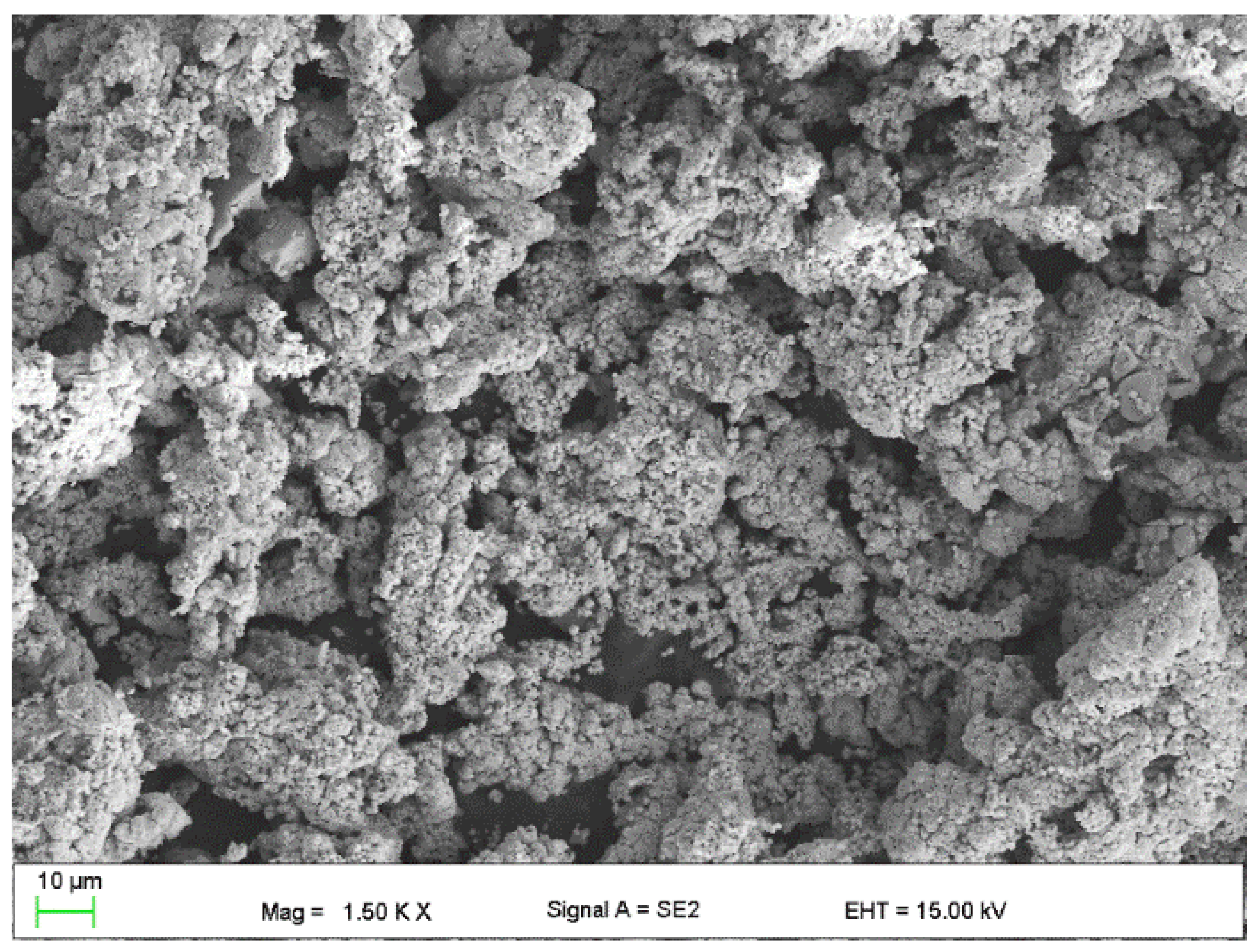
- Copper powder (Lt16 from Stanchem, Niemice, Poland) with a dendritic particle shape and grain size from 150 to 32 µm (Figure 4) with a thermal conductivity of 380 W/mK.
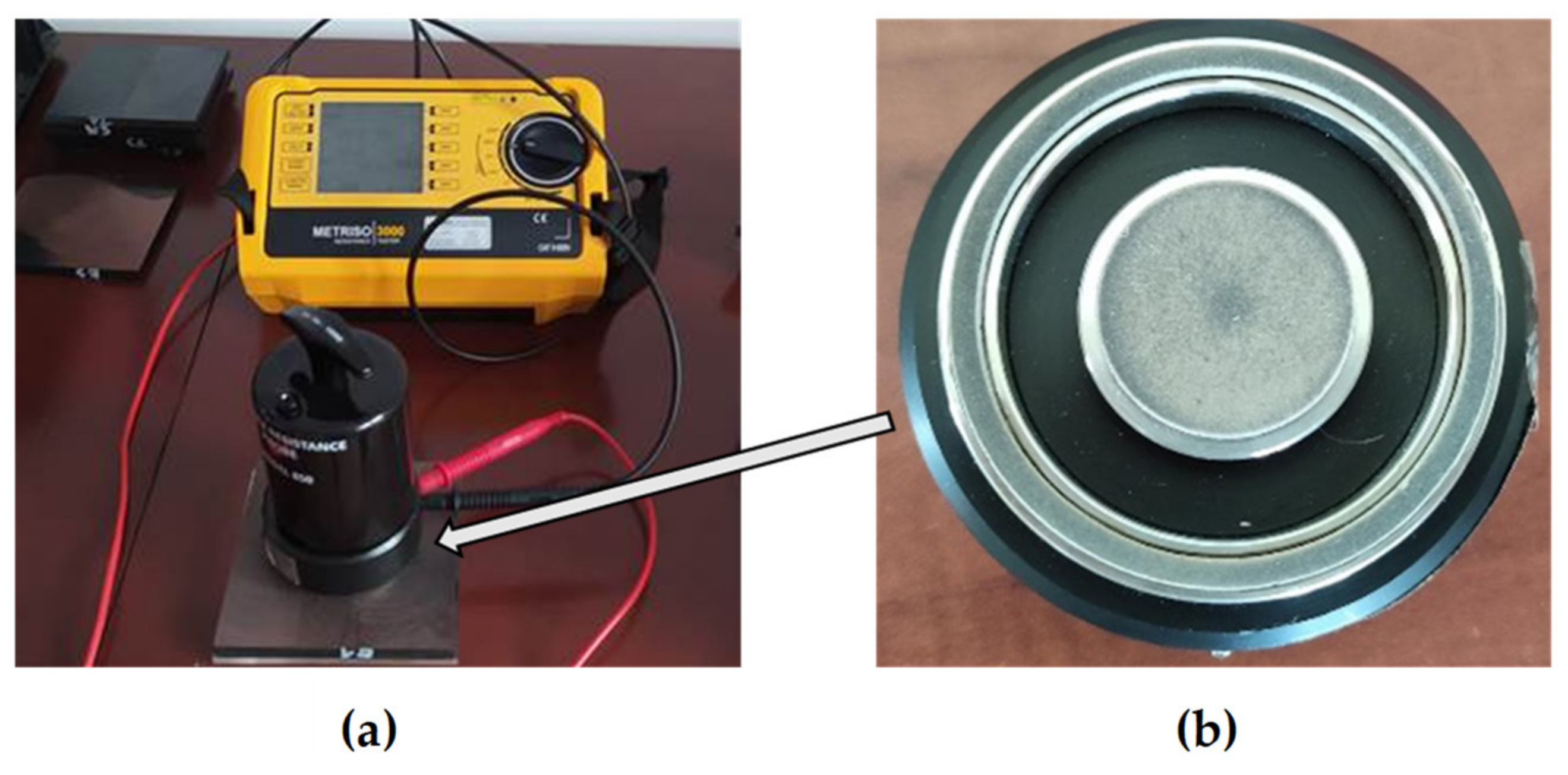
2.2. Thermal Properties Research
2.3. Surface Resistivity
- A 10 V voltage for results below 106 Ω and 100 V for results above 106 Ω;
- A measurement time of 15 s by the recommendations of the IEC 61340-2-3 standard;
- A test temperature of 20 °C and a humidity of 60%.
2.4. Flammability Tests
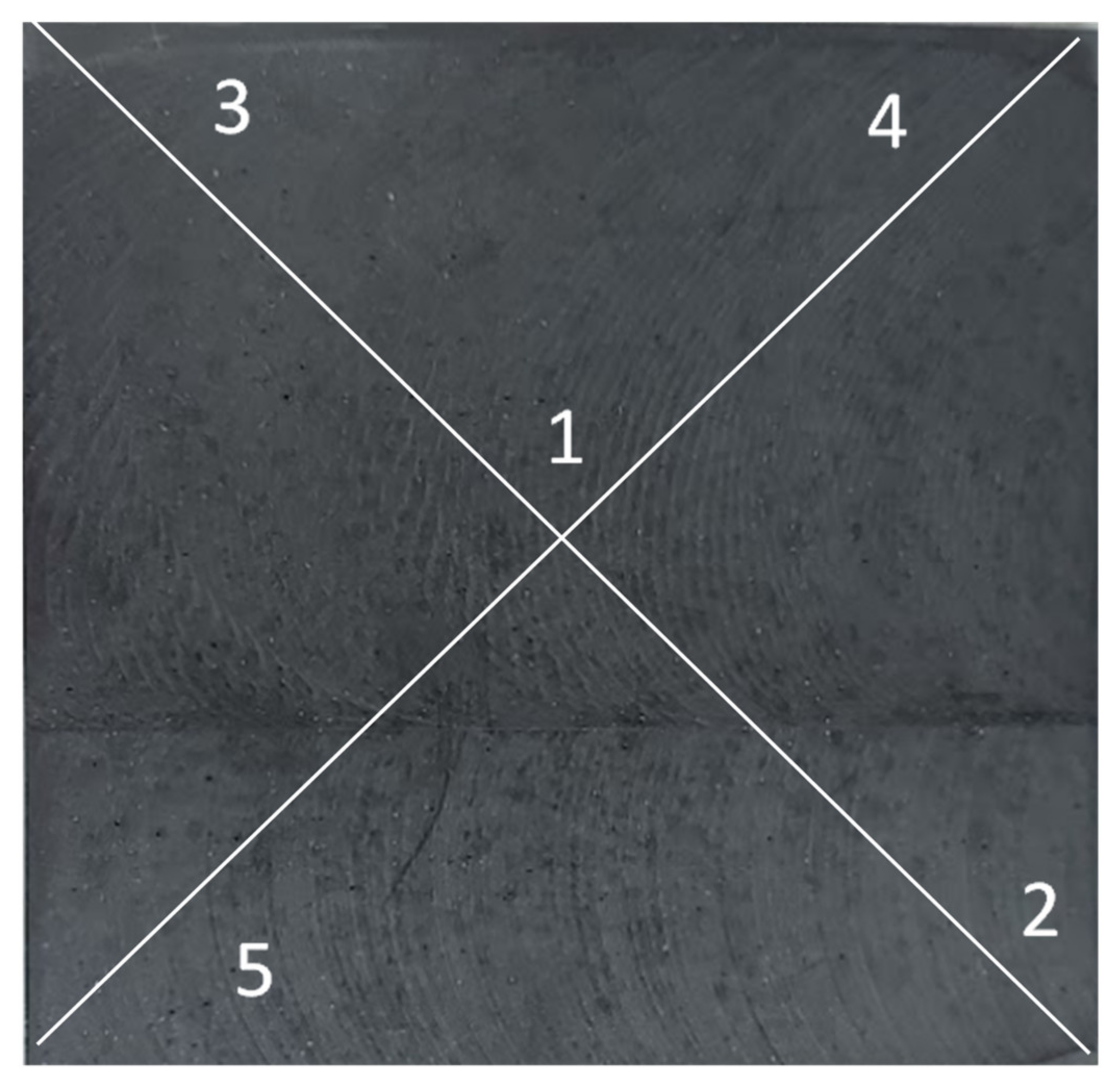
2.5. Flexural Strength Tests
3. Results and Discussion
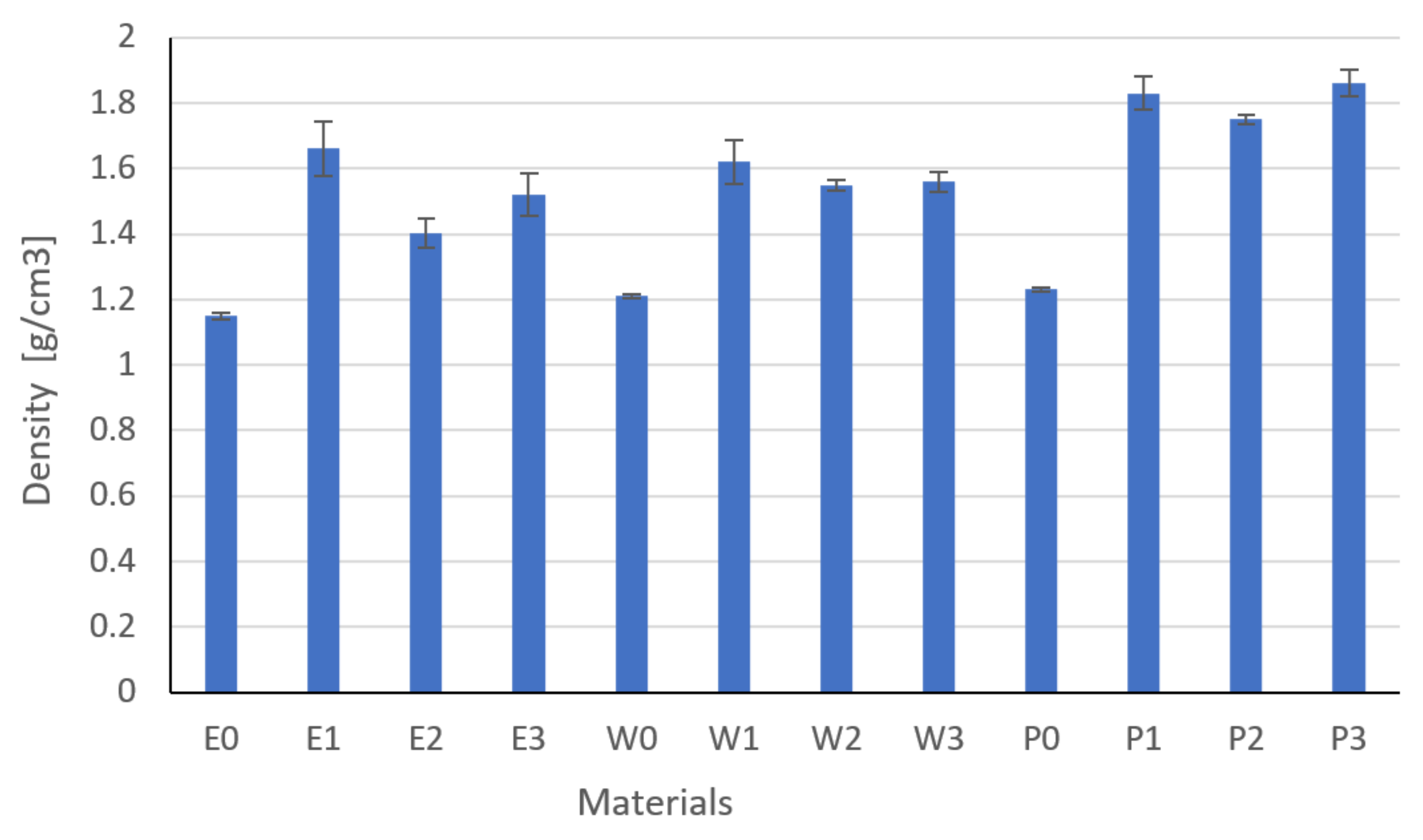
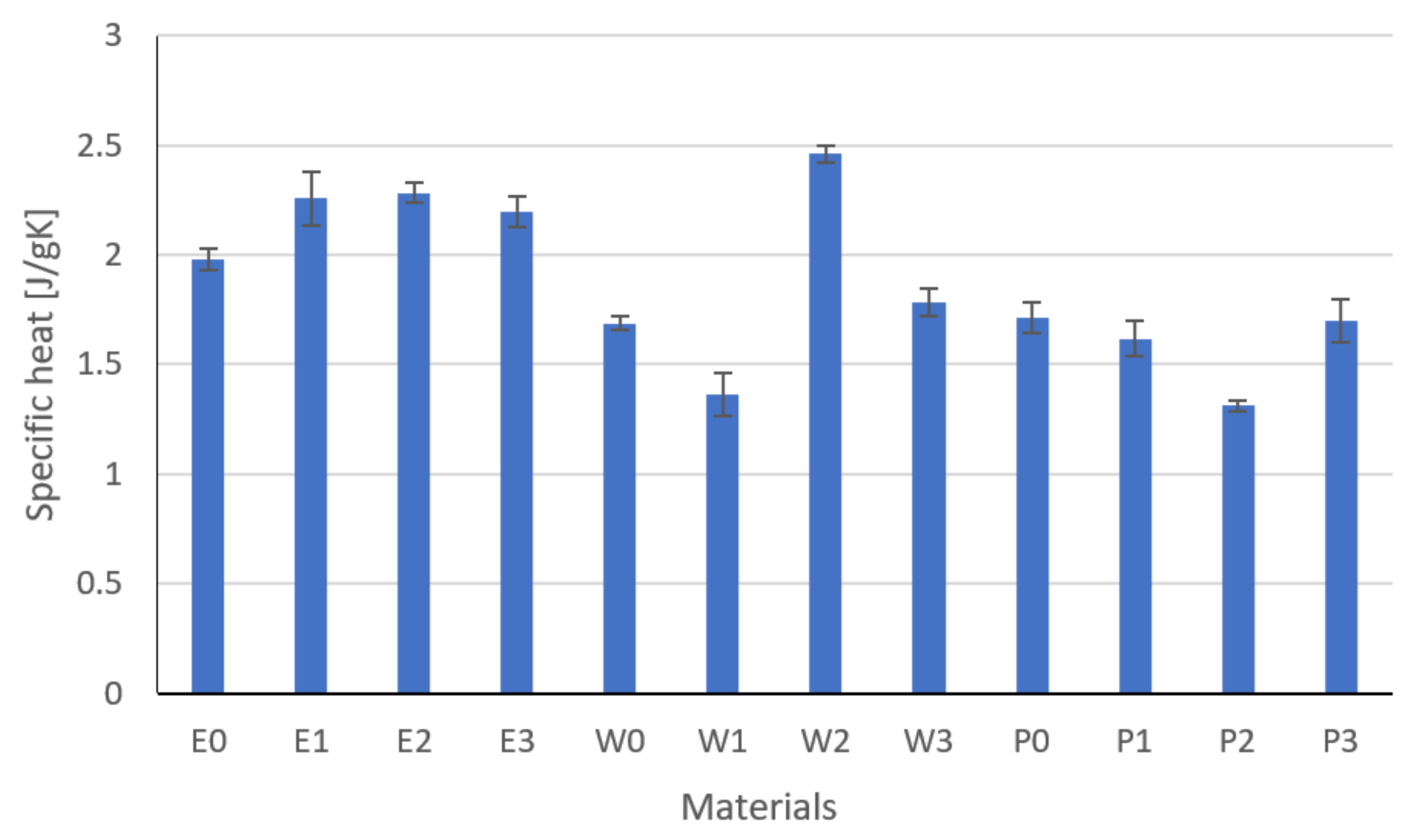
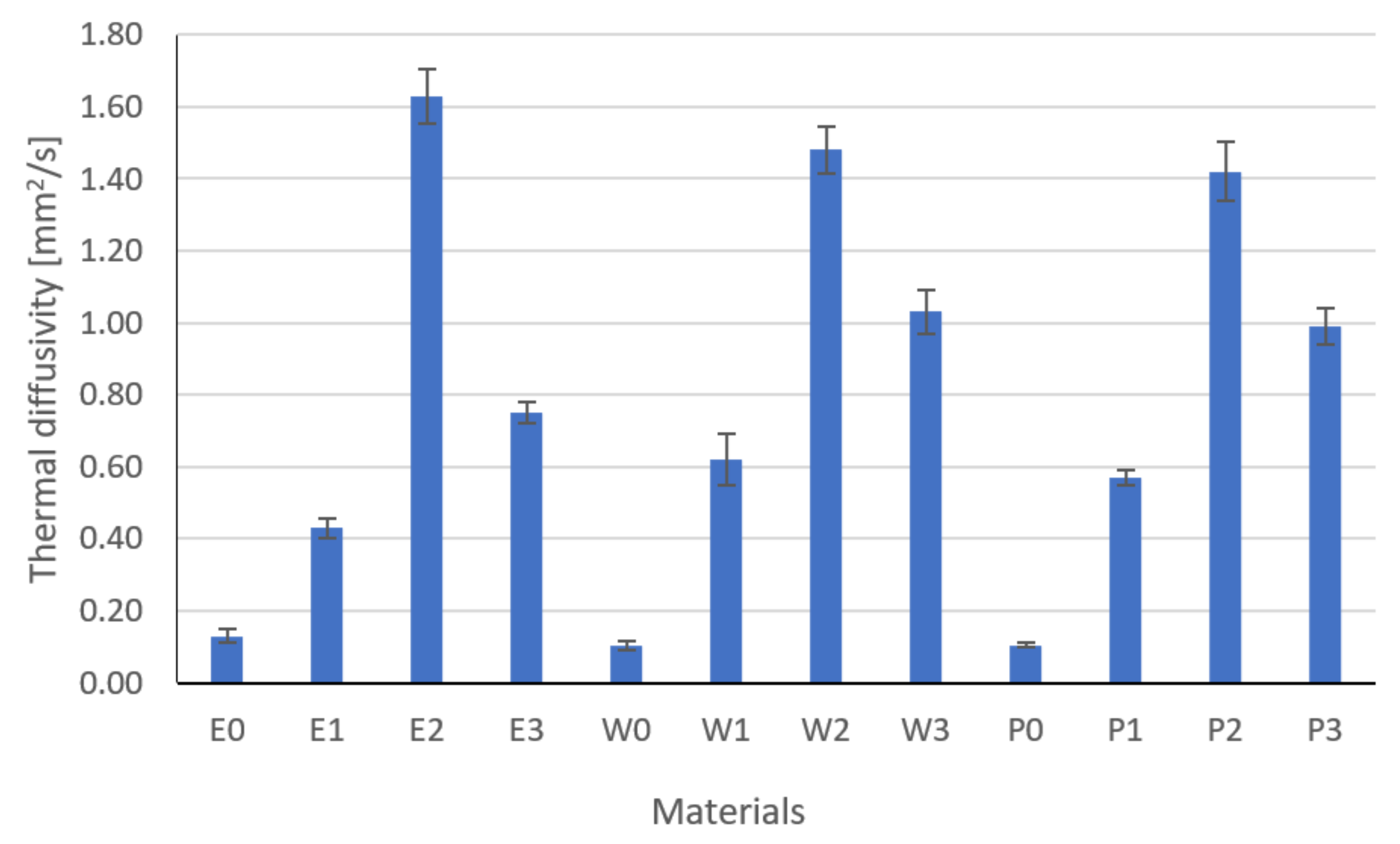
3.1. Thermal Properties Results
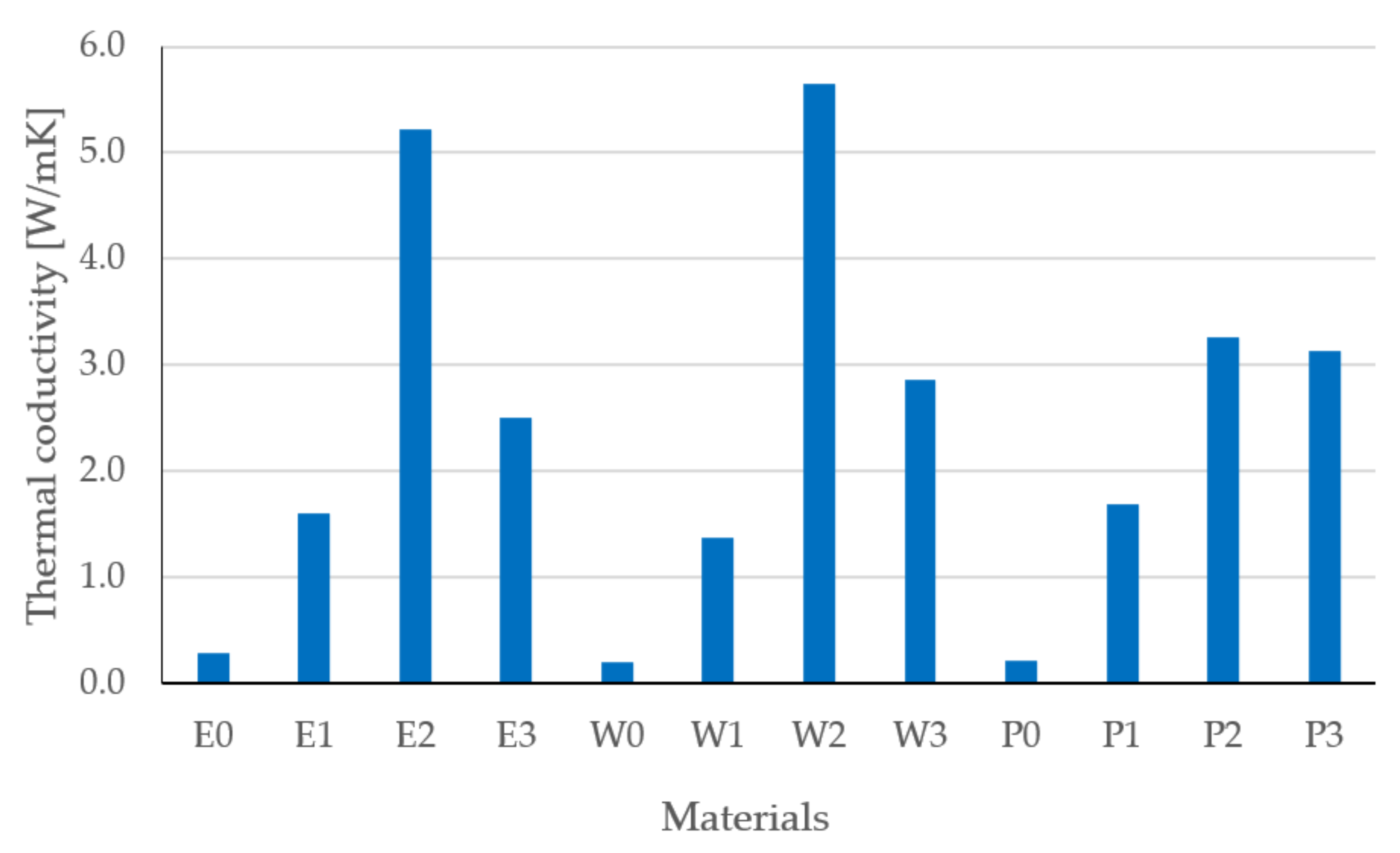
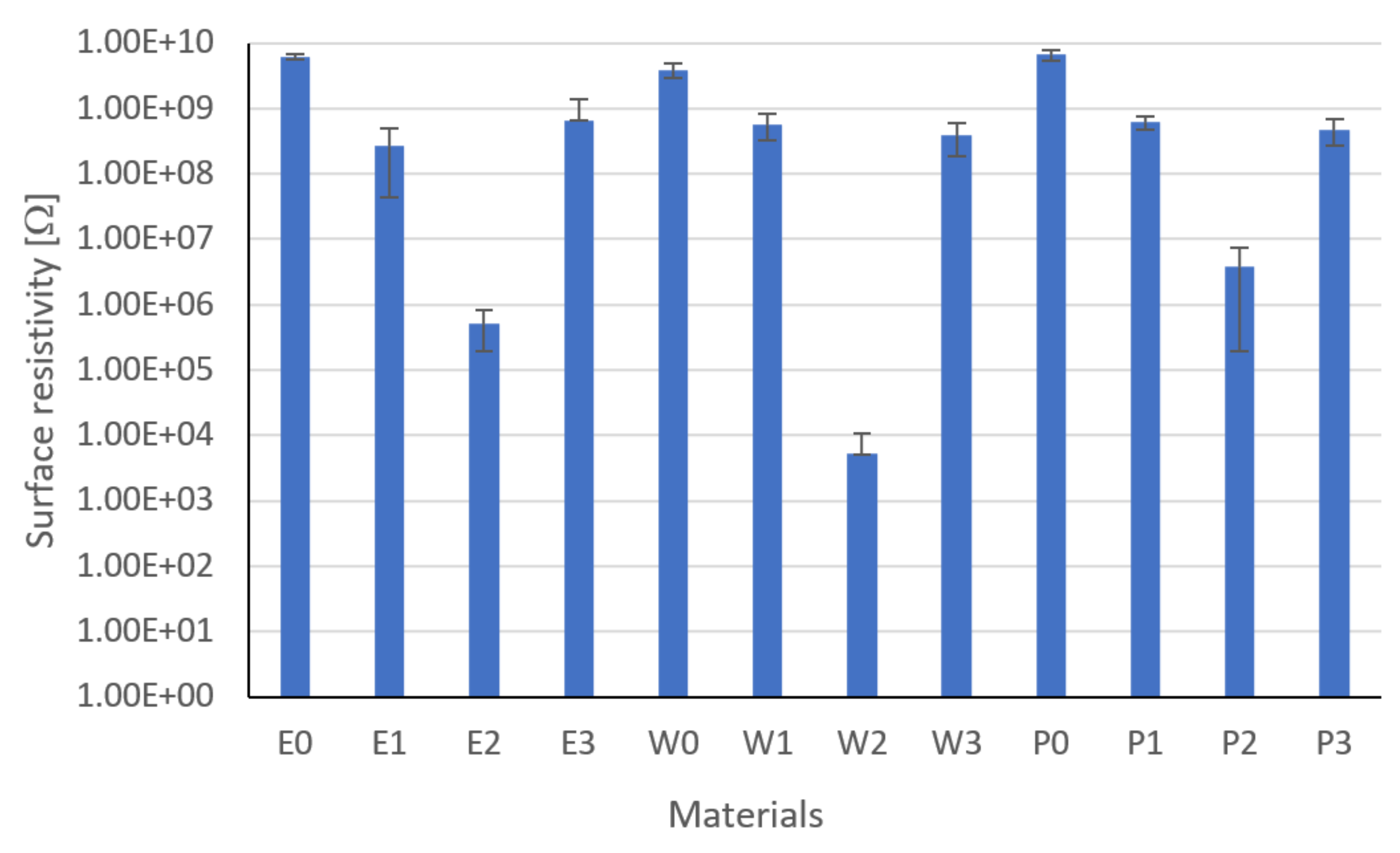
3.2. Surface Resistivity Results
3.3. Flammability Results
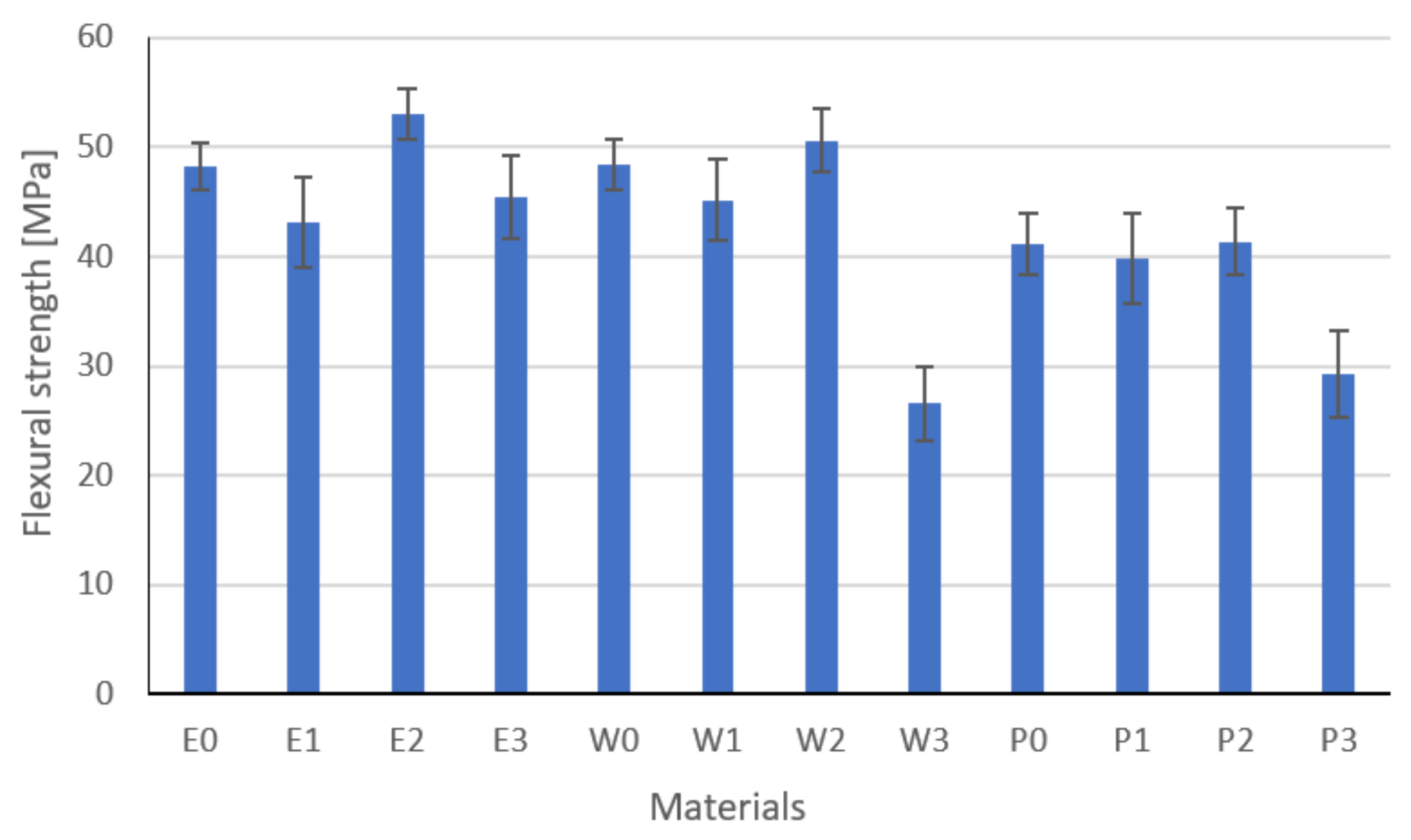
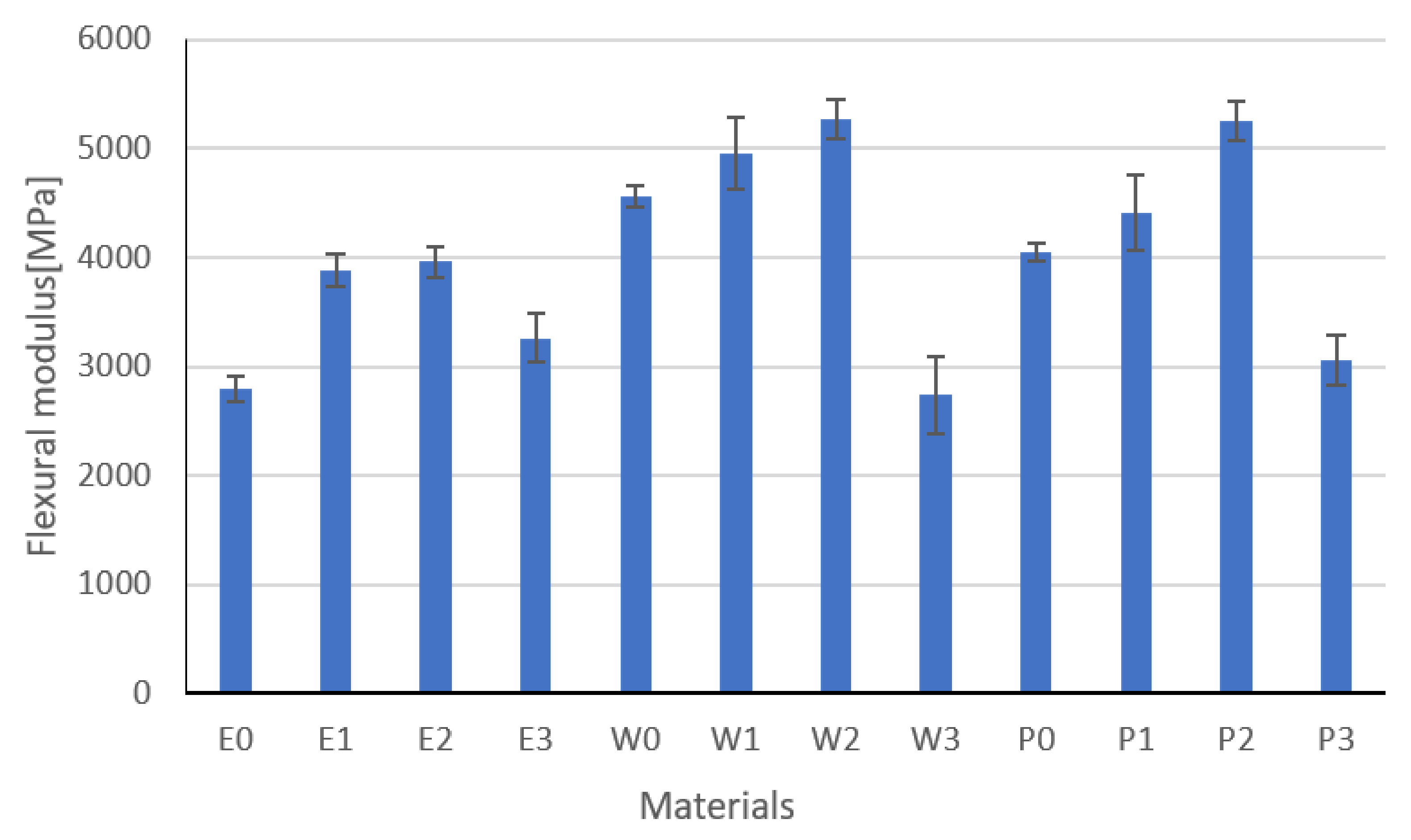
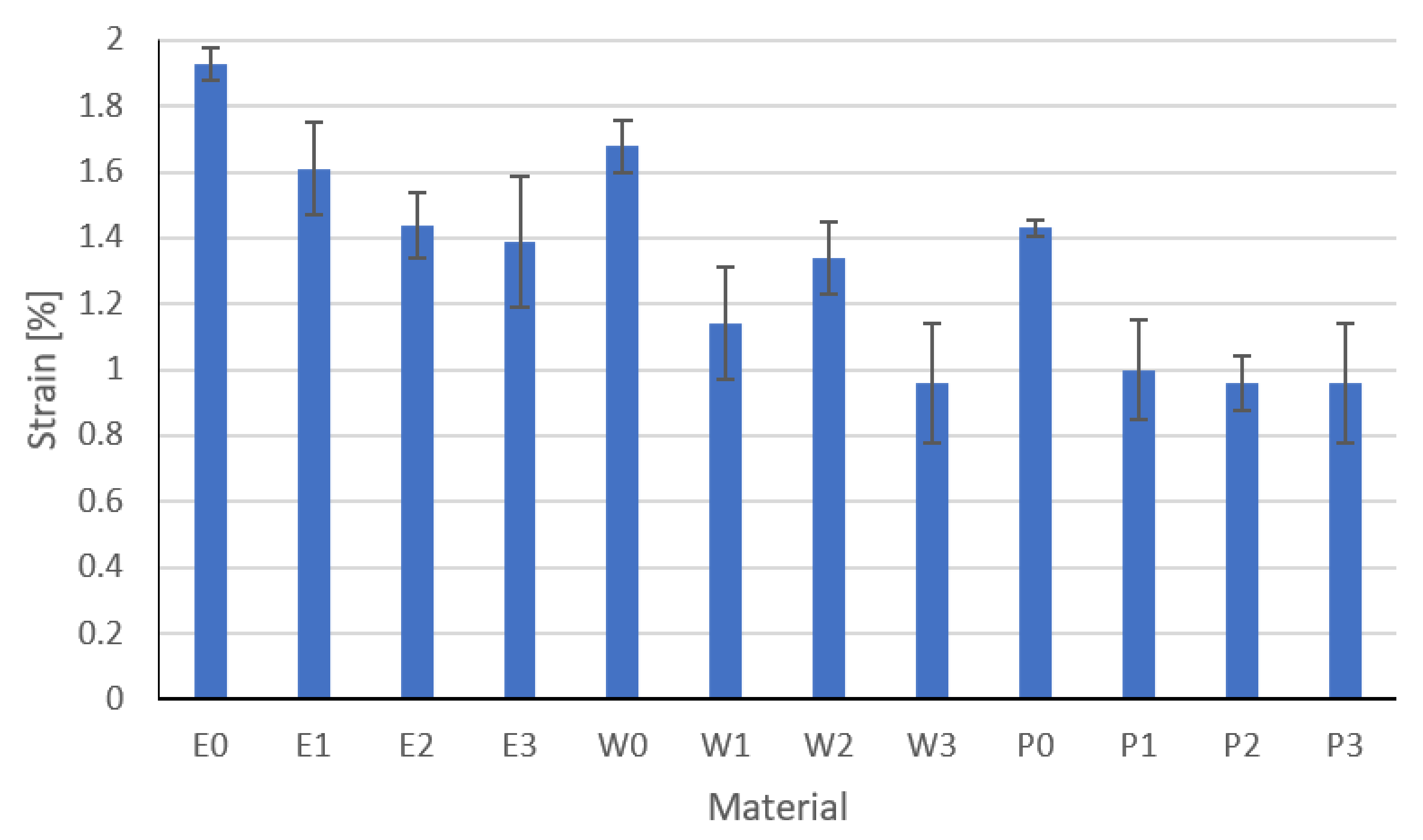
3.4. Flexural Strength Results
3.5. Multi-Criteria Analysis
4. Conclusions
- The type and content of the filler had a significant impact on the thermal conductivity, resistivity, and the tested strength properties, which was confirmed by the one-way analysis of variance.
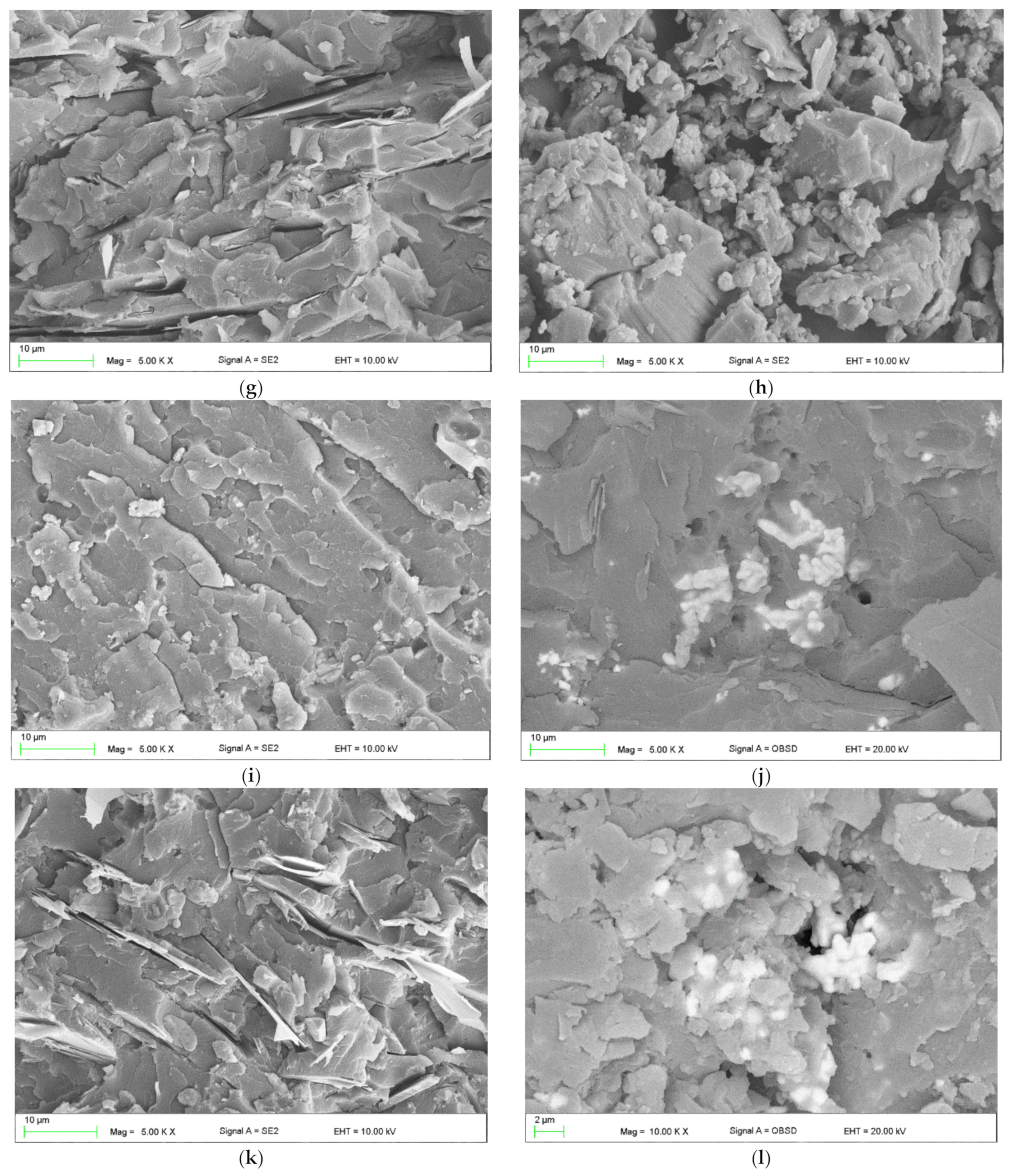
- Modification and the developed technology of introducing fillers into the resin allowed for the production of composites with increased thermal conductivity and lowered resistance. The highest coefficient of thermal conductivity was observed for composites E2, W2, and P2 filled with MG394 and MG1596 flak graphite. The highest resistance was also noted for the materials mentioned. This is the result of the fillers being evenly distributed over the cross-section.
- The lowest properties concerning graphite-modified composites were found in composites filled with copper powder and technical carbon black. This is a result of the uneven distribution of the filler on the cross-section and the phase separation (copper sedimentation).
- The highest values of flexural strength were also shown by graphite-modified composites, which confirms the results of thermal conductivity and resistance.
- The best summary effect of improving the tested properties was presented by composite W2 (176 points in the multi-criteria analysis).
- The developed W2 composite (MG394 and MG1596 graphite and vinyl ester resin) can be used as a matrix material for the production of laminates that are enclosures for electrical devices operating in explosive conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IEC Standard 60079-1; Explosive Atmospheres—Part 1: Equipment Protection by Flameproof Enclosures “d”. International, Electrotechnical Commission (IEC): Geneva, Switzerland, 2014.
- IEC Standard 60079-0; Explosive Atmospheres—Part 0: Equipment—General Requirements. International, Electrotechnical Commission (IEC): Geneva, Switzerland, 2017.
- Dai, K.; Song, L.; Jiang, S.; Yu, B.; Yang, W.; Yuen, R.K.K.; Hu, Y. Unsaturated Polyester Resins Modified with Phosphorus-Containing Groups: Effects on Thermal Properties and Flammability. Polym. Degrad. Stab. 2013, 98, 2033–2040. [Google Scholar] [CrossRef]
- Dai, K.; Song, L.; Yuen, R.K.K.; Jiang, S.; Pan, H.; Hu, Y. Enhanced Properties of the Incorporation of a Novel Reactive Phosphorus- and Sulfur-Containing Flame-Retardant Monomer into Unsaturated Polyester Resin. Ind. Eng. Chem. Res. 2012, 51, 15918–15926. [Google Scholar] [CrossRef]
- Kicko-Walczak, E. New Generation of Fire Retardant Polyester Resins. Macromol. Symp. 2003, 199, 343–350. [Google Scholar] [CrossRef]
- Kaynak, C.; Nakas, G.I.; Isitman, N.A. Mechanical Properties, Flammability and Char Morphology of Epoxy Resin/Montmorillonite Nanocomposites. Appl. Clay Sci. 2009, 46, 319–324. [Google Scholar] [CrossRef]
- Rahatekar, S.S.; Zammarano, M.; Matko, S.; Koziol, K.K.; Windle, A.H.; Nyden, M.; Kashiwagi, T.; Gilman, J.W. Effect of Carbon Nanotubes and Montmorillonite on the Flammability of Epoxy Nanocomposites. Polym. Degrad. Stab. 2010, 95, 870–879. [Google Scholar] [CrossRef]
- Ma, A.; Li, H.; Chen, W.; Hou, Y. Improved Thermal Conductivity of Silicon Carbide/Carbon Fiber/Epoxy Resin Composites. Polym. -Plast. Technol. Eng. 2013, 52, 295–299. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Lv, Z.; Li, N.; Liang, C.; Zhang, Q. Functionalized Graphite Nanoplatelets/Epoxy Resin Nanocomposites with High Thermal Conductivity. Int. J. Heat Mass Transf. 2016, 92, 15–22. [Google Scholar] [CrossRef]
- Ganguli, S.; Roy, A.K.; Anderson, D.P. Improved Thermal Conductivity for Chemically Functionalized Exfoliated Graphite/Epoxy Composites. Carbon 2008, 46, 806–817. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, R.; Wang, J.; Qi, S. Thermal Conductivity Improvement of Epoxy Composite Filled with Expanded Graphite. Ceram. Int. 2015, 41, 13541–13546. [Google Scholar] [CrossRef]
- Agrawal, A.; Satapathy, A. Effects of Aluminium Nitride Inclusions on Thermal and Electrical Properties of Epoxy and Polypropylene: An Experimental Investigation. Compos. Part A Appl. Sci. Manuf. 2014, 63, 51–58. [Google Scholar] [CrossRef]
- Bian, W.; Yao, T.; Chen, M.; Zhang, C.; Shao, T.; Yang, Y. The Synergistic Effects of the Micro-BN and Nano-Al2O3 in Micro-Nano Composites on Enhancing the Thermal Conductivity for Insulating Epoxy Resin. Compos. Sci. Technol. 2018, 168, 420–428. [Google Scholar] [CrossRef]
- Tsekmes, I.A.; Kochetov, R.; Morshuis, P.H.F.; Smit, J.J. Thermal Conductivity of Polymeric Composites: A Review. In Proceedings of the 2013 IEEE International Conference on Solid Dielectrics (ICSD), Bologna, Italy, 30 June–4 July 2013; pp. 678–681. [Google Scholar]
- Guo, L.; Ding, S.; Yuan, S.; Gou, X.; Cai, F.; Wang, D.; Zhao, H. Study on the Thermal Properties and Insulation Resistance of Epoxy Resin Modified by Hexagonal Boron Nitride. Polymers 2021, 21, 681–690. [Google Scholar] [CrossRef]
- Ma, A.-J.; Chen, W.; Hou, Y.; Zhang, G. Dispersion, Mechanical and Thermal Properties of Epoxy Resin Composites Filled with the Nanometer Carbon Black. Polym. -Plast. Technol. Eng. 2010, 49, 916–920. [Google Scholar] [CrossRef]
- Campbell, F.C. Manufacturing Processes for Advanced Composites; Elsevier: Oxford, UK, 2003. [Google Scholar]
- Polimal 1604TS, Datasheet. Available online: https://polymall.com.ua/wp-content/uploads/2020/09/POLIMAL_ENG.pdf (accessed on 10 November 2021).
- Polimal VE 11 MAT, Datasheet. Available online: https://www.polymall.com.ua/uploads/files/public-folder/Polimal_VE-11M.pdf (accessed on 10 November 2021).
- LG 420 FR, Datasheet. Available online: https://www.rc-helper.com.ua/wp-content/uploads/2021/03/Resin-LG-420-FR-1.pdf (accessed on 12 November 2021).
- Walczak, K.; Mamos, J. Fatigue strength of laminate pipes (GRP) used in mining. Kompozyty 2004, 4, 194–199. [Google Scholar]
- Carbon Black FW 200 Datasheet. Available online: https://www.palmerholland.com/Assets/User/Documents/Product/43725/3377/MITM07895.pdf (accessed on 6 February 2022).
- Szymiczek, M. Ultrasonic, and Thermal Testing as a Diagnostic Tool for the Evaluation of Cumulative Discontinuities of the Polyester-Glass Pipes Structure. Eksploatacja Niezawodność 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Parker, W.J.; Jenkins, R.J.; Butler, C.P.; Abbott, G.L. Flash Method of Determining Thermal Diffusivity, Heat Capacity, and Thermal Conductivity. J. Appl. Phys. 1961, 32, 1679–1684. [Google Scholar] [CrossRef]
- ISO 1183-1:2019; Plastics—Methods for Determining the Density of Non-Cellular Plastics—Part 1: Immersion Method, Liquid Pycnometer Method, and Titration Method. ISO: Geneva, Switzerland, 2019.
- ISO 11357-4:2013; Plastics—Differential Scanning Calorimetry (DSC)—Part 4: Determination of Specific Heat Capacity. ISO: Geneva, Switzerland, 2013.
- IEC Standard 61340-2-3; Electrostatics—Part 2–3: Methods of Test for Determining the Resistance and Resistivity of Solid Materials Used to Avoid Electrostatic Charge Accumulation. International, Electrotechnical Commission (IEC): Geneva, Switzerland, 2016.
- IEC Standard 60695-11-10: 2014-02; Fire Hazard Test—Part 11-10: Test Flames—50 W Test Flame Test Methods with the Sample Horizontal and Vertical. International, Electrotechnical Commission (IEC): Geneva, Switzerland, 2014.
- ISO 340:2013-07; Conveyor Belts—Flammability Characteristics on a Laboratory Scale—Requirements and Test Method. ISO: Geneva, Switzerland, 2013.
- ISO/IEC 80079-38:2017-02 p.6.2; Explosive Atmospheres—Part 38: Equipment and Components Used in Explosive Atmospheres Occurring in Underground Workings of the Mine. ISO: Geneva, Switzerland, 2017.
- Morgan, A.B.; Wilkie, C.A. Flame Retardant Polymer Nanocomposites; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- ISO 178-2019; Plastics—Determination of Flexural Properties. ISO: Geneva, Switzerland, 2019.

| Property | Standard | Unit | Polyester Resin | Vinyl Ester Resin | Epoxy Resin |
|---|---|---|---|---|---|
| Viscosity at 25 °C Brookfield | ISO 3219 | mPa s | 550–650 | 300–400 | 900–1200 |
| Gel time at 25 °C | ISO 2535 | min | 34 | 30 | 3 h 17 min |
| Deflection temperature HDT | ISO 75 | °C | 94 | 90 | - |
| Flammability class | EN 60695-11-10:2014-02 | - | V0 | V0 | Undefined |
| Compressive strength | ISO 604 | MPa | 120 | 126 | 132 |
| Flexural strength | ISO 178 | MPa | 90 | 130 | 120 |
| Tensile strength | ISO 527 | MPa | 67 | 80 | 72 |
| Young’s modulus | ISO 527 | MPa | 3700 | 3600 | 3300 |
| Strain at break | ISO 527 | % | 2.3 | 3.5 | 6 |
| Barcol hardness | ASTM D 2583-95 ASTM D 2583 | B/Sh Do | 48 | 40 | 85 o |
| Resin Parameter | Epoxy E0 | Vinyl Ester W0 | Polyester P0 |
|---|---|---|---|
| Test conditions | 23 ± 2 °C/50 ± 5% | 70 ± 2 °C | 23 ± 2 °C/50 ± 5% |
| Burning time after 1st flame application t2 (s) | 34.96 | 39.04 | 3.002 |
| Burning time after 1st flame application t1 (s) | 66.14 | 65.54 | 4.88 |
| Glowing time after the second flame application t3 (s) | 0 | 0 | 0 |
| Total burning time t = S (t1 + t2) (s) | 505.5 | 522.9 | 36.6 |
| Time of burning and glowing after the second flame application (t2 + t3) (s) | 66.14 | 65.54 | 4.88 |
| Inflammation of cotton wool, yes/no | no | no | no |
| Burning, glow to terminals, yes/no | no | no | no |
| Flammability category | Cannot be categorized | V0—10.6 mm | V0—10.3 mm |
| Characteristic | Weights | Materials | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E0 | E1 | E2 | E3 | W0 | W1 | ||||||||
| C | W | C | W | C | W | C | W | C | W | C | W | ||
| Flammability class | 1 | - | 0 | - | 0 | - | 0 | - | 0 | V0 | 11 | V0 | 11 |
| 0 | 0 | 0 | 0 | 11 | 11 | ||||||||
| Thermal conductivity coefficient, W/mK | 5 | 0.29 | 5 | 1.61 | 20 | 5.22 | 55 | 2.51 | 35 | 0.21 | 5 | 1.37 | 25 |
| 1 | 4 | 11 | 7 | 1 | 5 | ||||||||
| Surface resistivity, Ω | 4 | 6.1 × 109 | 8 | 2.7 × 108 | 36 | 5.2 × 105 | 44 | 6.5 × 108 | 24 | 3.9 × 109 | 12 | 5.8 × 108 | 20 |
| 2 | 9 | 11 | 6 | 3 | 5 | ||||||||
| Flexural strength, MPa | 3 | 48.2 | 27 | 43.1 | 18 | 53.01 | 36 | 45.45 | 24 | 48.43 | 30 | 45.19 | 21 |
| 9 | 6 | 12 | 8 | 10 | 7 | ||||||||
| Flexural modulus, GPa | 2 | 2.8 | 4 | 3.89 | 10 | 3.96 | 12 | 3.27 | 8 | 4.56 | 18 | 4.96 | 20 |
| 2 | 5 | 6 | 4 | 9 | 10 | ||||||||
| Σ | 44 | 84 | 147 | 91 | 76 | 97 | |||||||
| Characteristic | Weights | Materials | |||||||||||
| W2 | W3 | P0 | P1 | P2 | P3 | ||||||||
| C | W | C | W | C | W | C | W | C | W | C | W | ||
| Flammability class | 1 | 0 | 11 | V0 | 11 | V0 | 12 | V0 | 12 | V0 | 12 | V0 | 12 |
| 11 | 11 | 12 | 12 | 12 | 12 | ||||||||
| Thermal conductivity coefficient, W/mK | 5 | 5.64 | 60 | 2.86 | 40 | 0.21 | 5 | 1.69 | 30 | 3.25 | 50 | 3.13 | 45 |
| 12 | 8 | 1 | 6 | 10 | 9 | ||||||||
| Surface resistivity, Ω | 4 | 5.2 × 103 | 48 | 3.9 × 108 | 32 | 6.7 × 109 | 4 | 6.2 × 108 | 16 | 3.9 × 106 | 40 | 4.7 × 108 | 28 |
| 12 | 8 | 1 | 4 | 10 | 7 | ||||||||
| Flexural strength, MPa | 3 | 50.61 | 33 | 26.58 | 3 | 41.2 | 12 | 39.84 | 9 | 41.36 | 15 | 29.28 | 2 |
| 11 | 1 | 4 | 3 | 5 | 2 | ||||||||
| Flexural modulus, GPa | 2 | 5.27 | 24 | 2.74 | 2 | 4.05 | 14 | 4.42 | 16 | 5.26 | 22 | 3.06 | 6 |
| 12 | 1 | 7 | 8 | 11 | 3 | ||||||||
| Σ | 176 | 88 | 47 | 83 | 139 | 93 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymiczek, M.; Buła, D. Polyester and Epoxy Resins with Increased Thermal Conductivity and Reduced Surface Resistivity for Applications in Explosion-Proof Enclosures of Electrical Devices. Materials 2022, 15, 2171. https://doi.org/10.3390/ma15062171
Szymiczek M, Buła D. Polyester and Epoxy Resins with Increased Thermal Conductivity and Reduced Surface Resistivity for Applications in Explosion-Proof Enclosures of Electrical Devices. Materials. 2022; 15(6):2171. https://doi.org/10.3390/ma15062171
Chicago/Turabian StyleSzymiczek, Małgorzata, and Dawid Buła. 2022. "Polyester and Epoxy Resins with Increased Thermal Conductivity and Reduced Surface Resistivity for Applications in Explosion-Proof Enclosures of Electrical Devices" Materials 15, no. 6: 2171. https://doi.org/10.3390/ma15062171







