Enhancement of Anticorrosive Performance of Cardanol Based Polyurethane Coatings by Incorporating Magnetic Hydroxyapatite Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
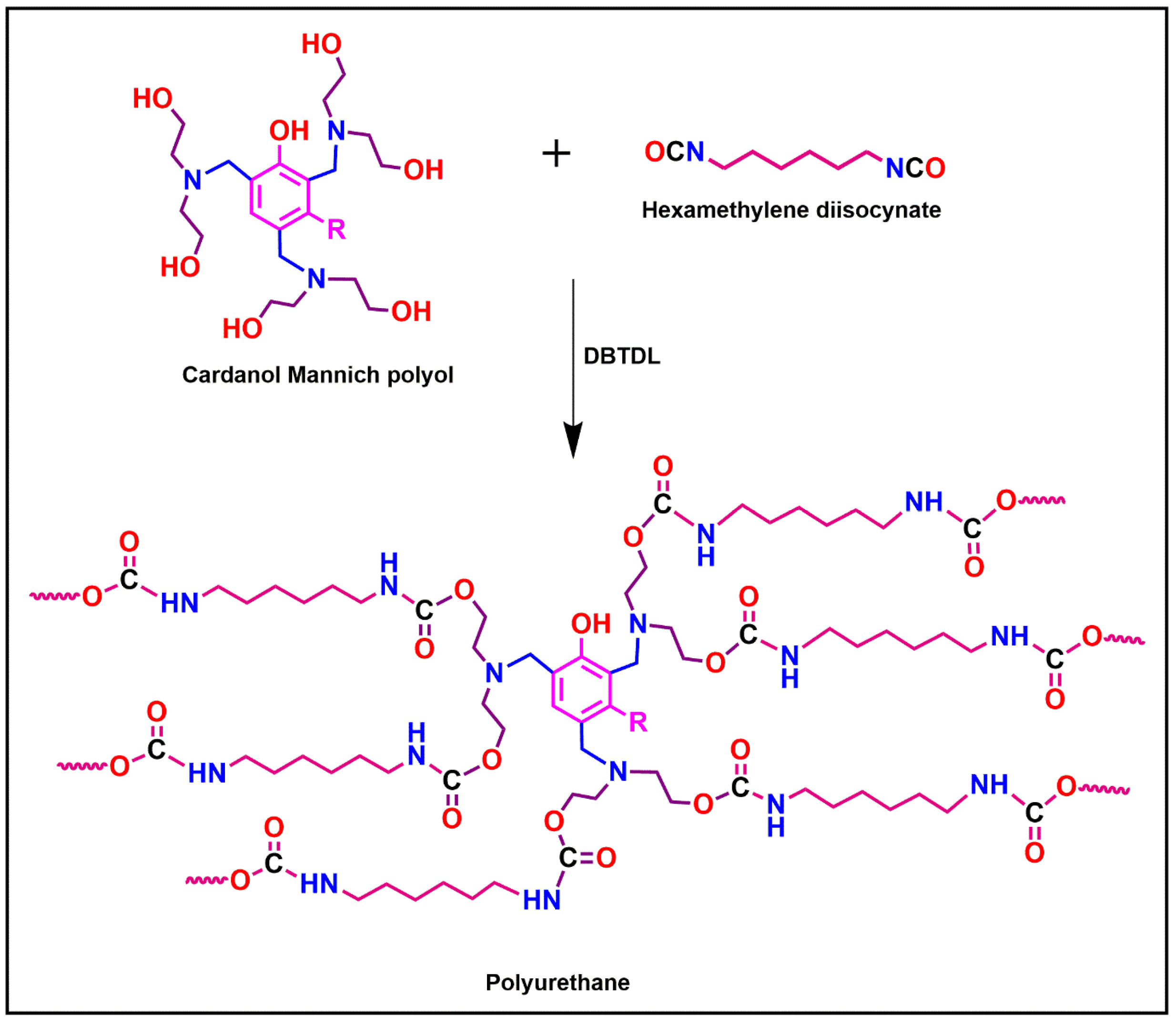
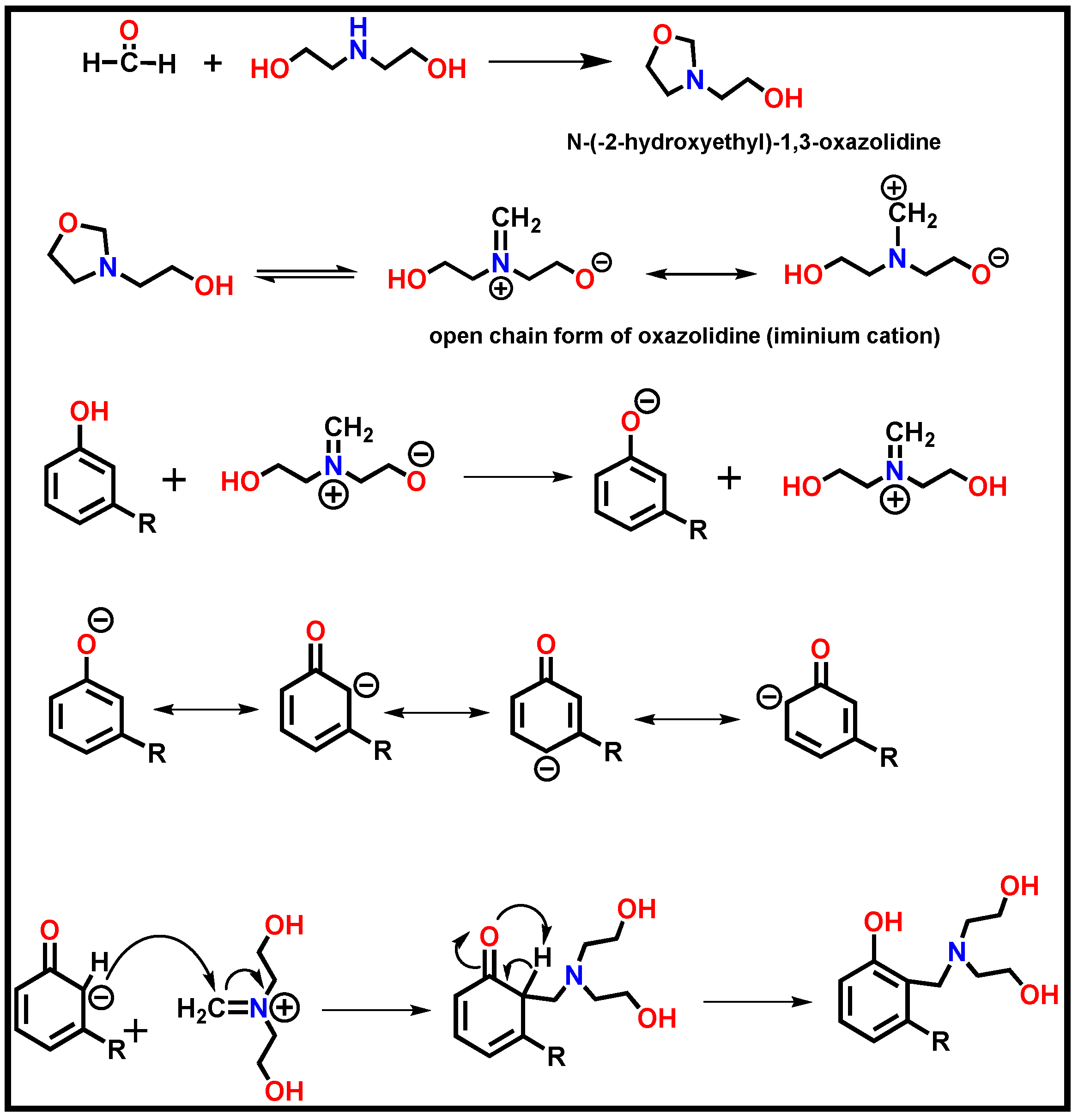
2.1. Synthesis of Cardanol Based Mannich polyol
2.2. Synthesis of Magnetic Hydroxyapatite Nanoparticles
2.3. Formulation of Polyurethane Nanocomposite Coatings
3. Characterization of Materials
3.1. End Group Analysis
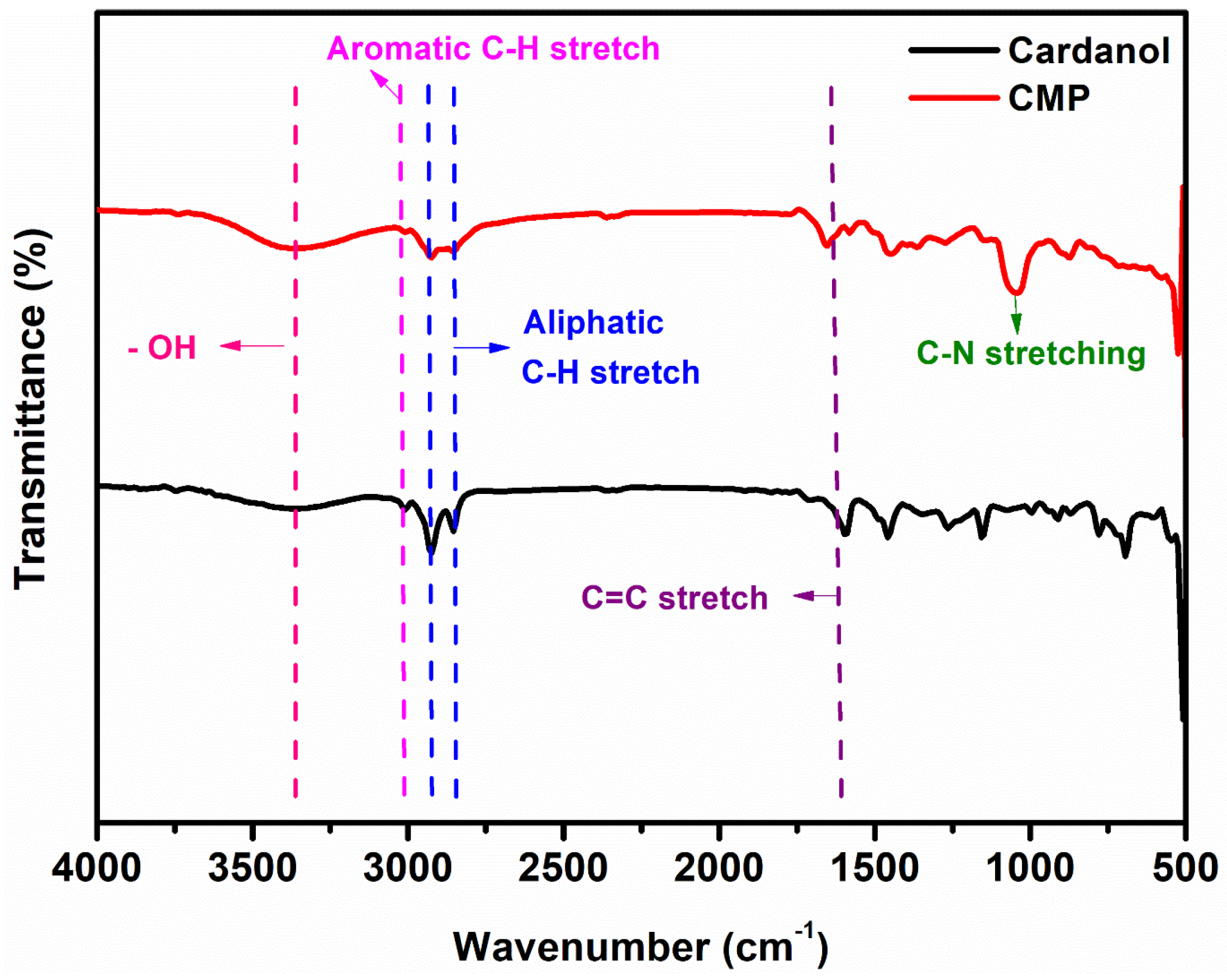
3.2. Structural Analysis
3.3. Testing Properties of Coatings
3.3.1. Gloss Test
3.3.2. Adhesion Test
3.3.3. Pencil Hardness
3.3.4. Flexibility
3.3.5. Contact Angle
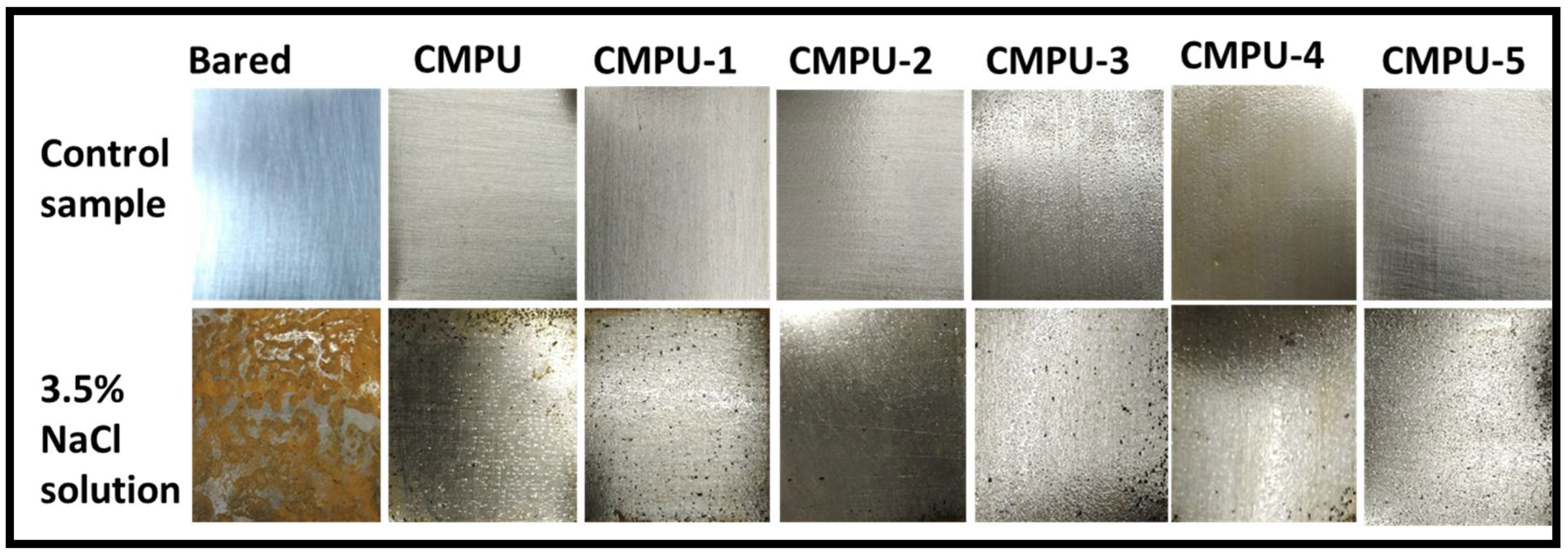
3.3.6. Corrosion Performance
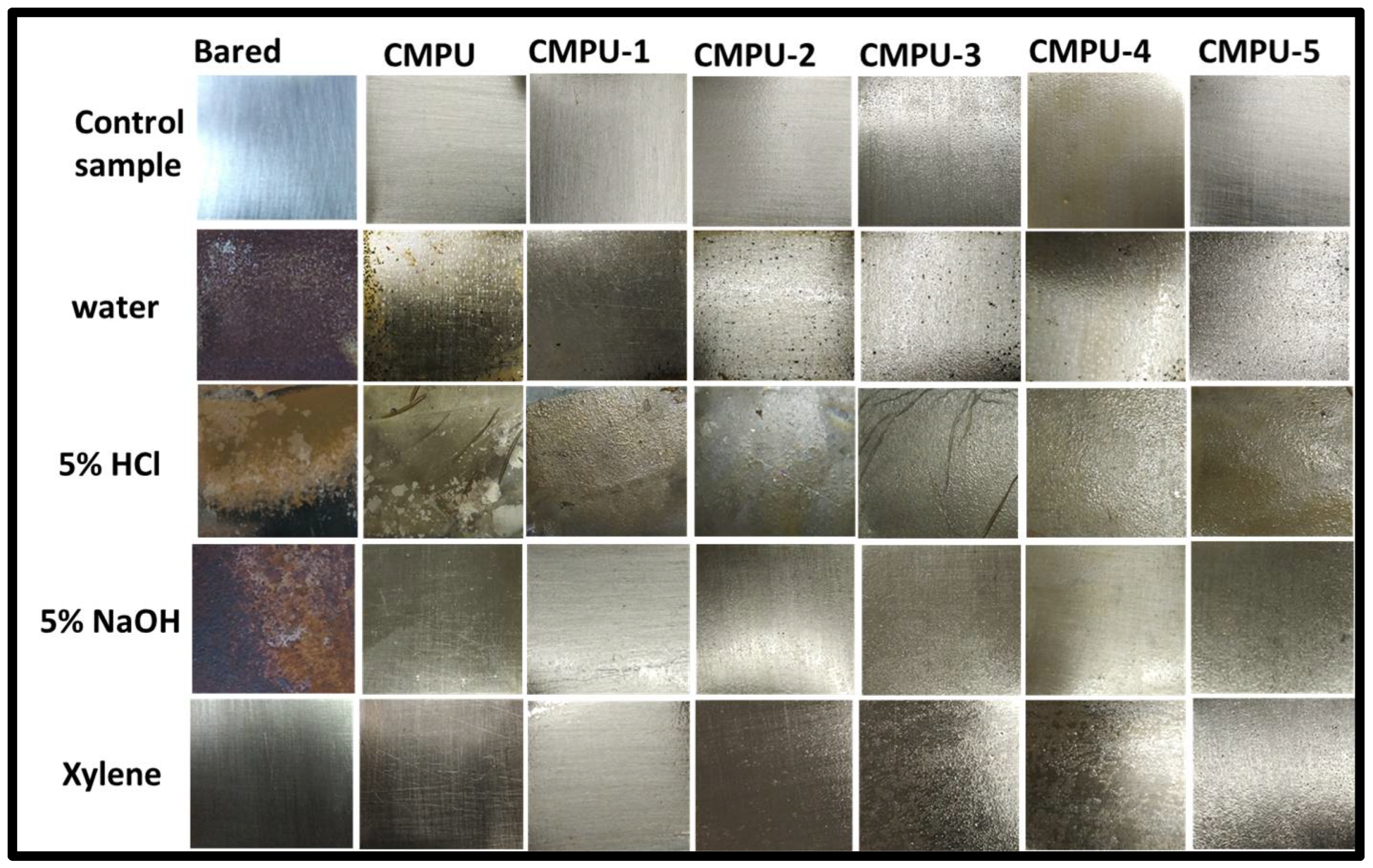
3.3.7. Chemical Resistance
3.3.8. Thermal Property
3.3.9. Surface Morphology
4. Results and Discussion
4.1. Formation of Cardanol Based polyol
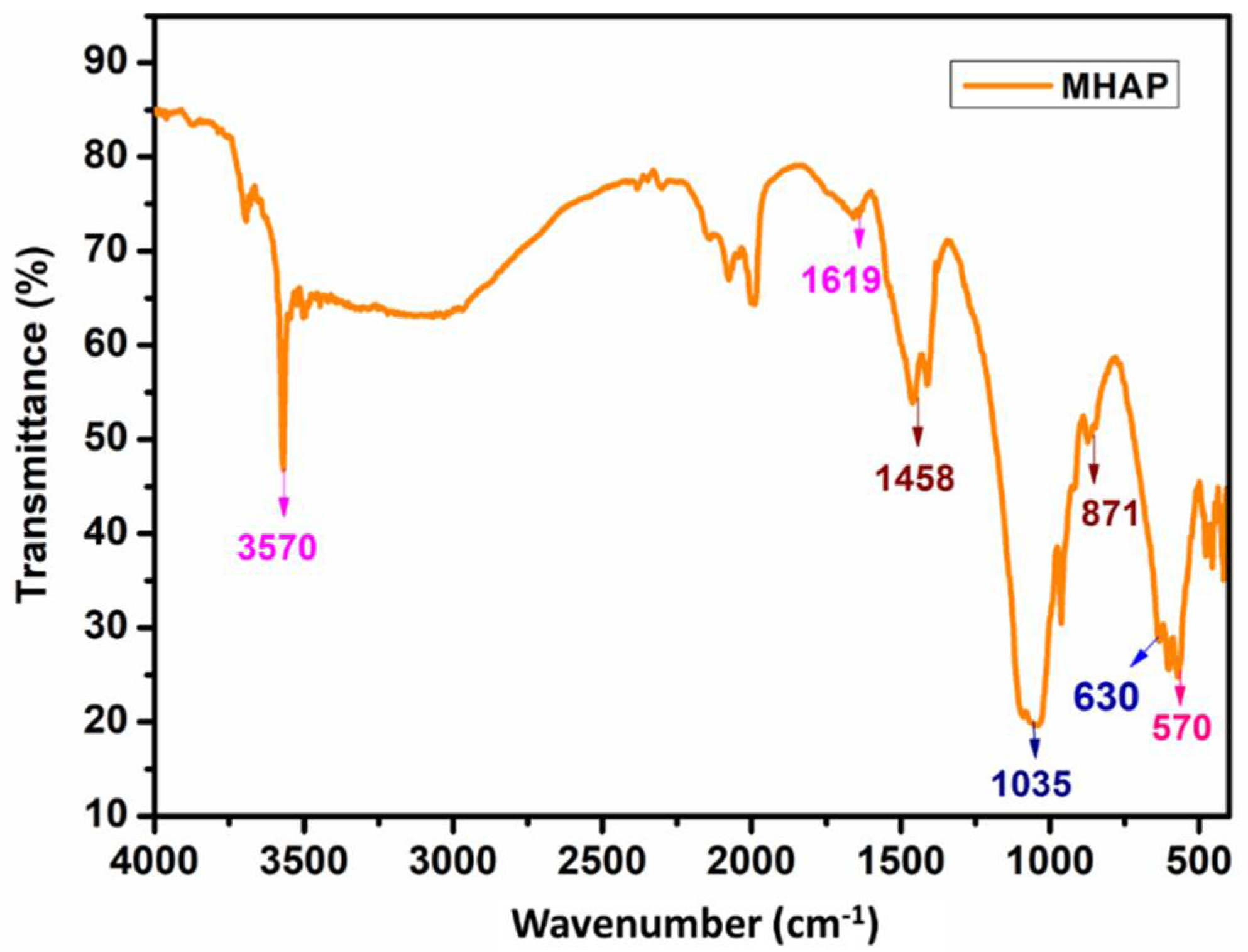
4.2. FT-IR of Magnetic Hydroxyapatite Nanoparticles
4.3. Magnetic Behavior of MHAP Nanoparticles
4.4. Coating Properties
4.5. Chemical Resistance Study
4.6. Anticorrosive Performance by Immersion Method
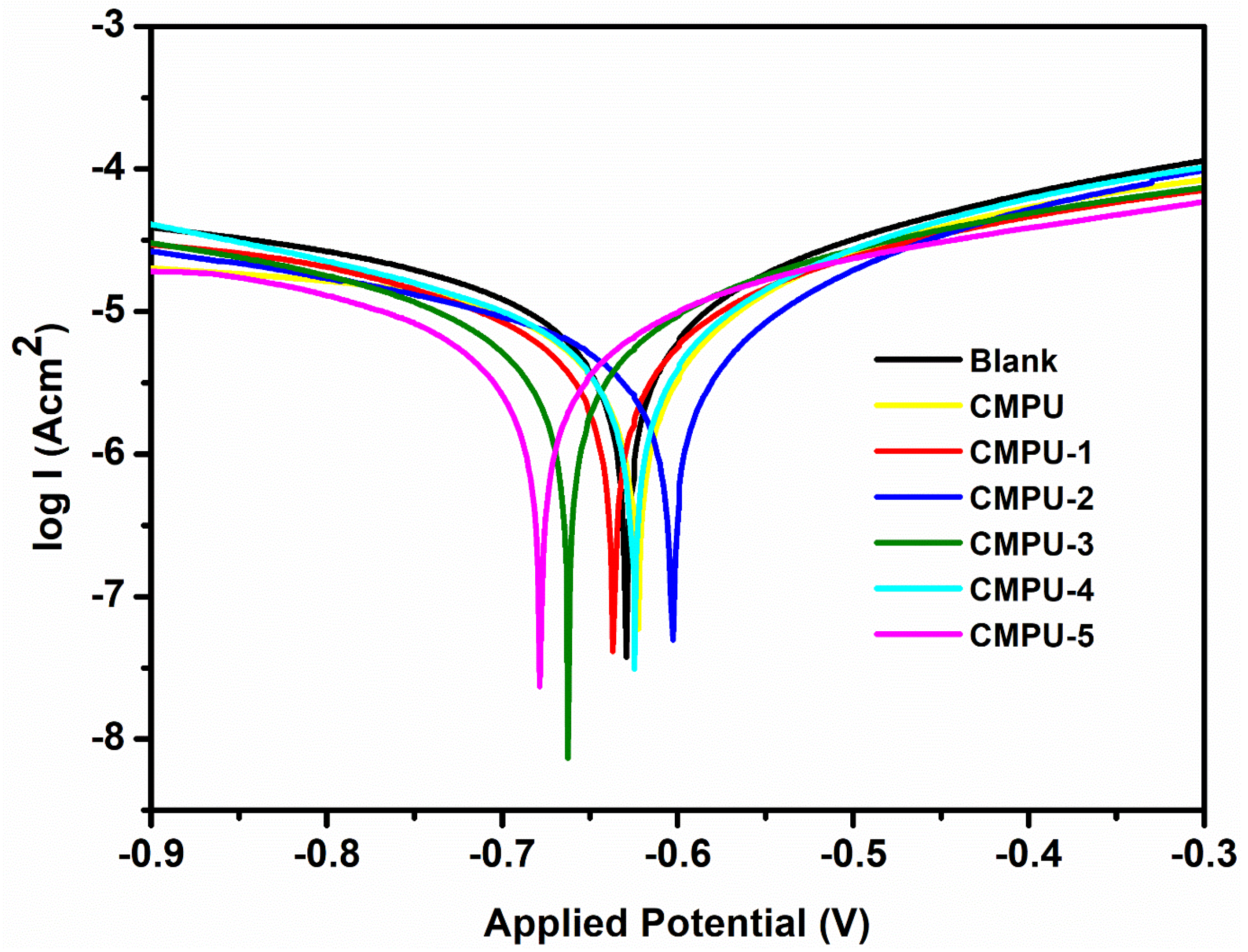
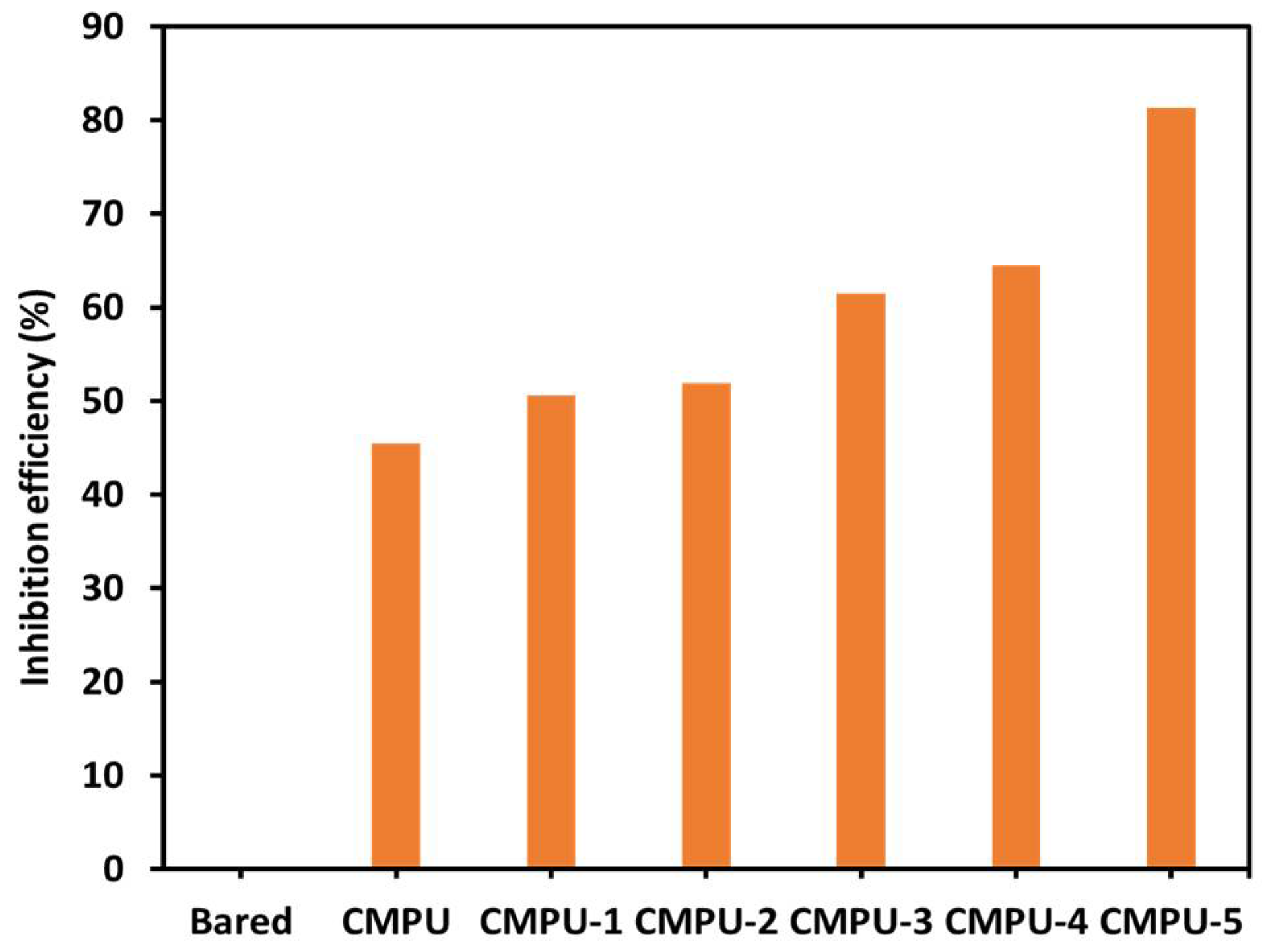
4.7. Anticorrosive Study by Electrochemical Method
4.8. Contact Angle
4.9. Thermogravimetric Analysis
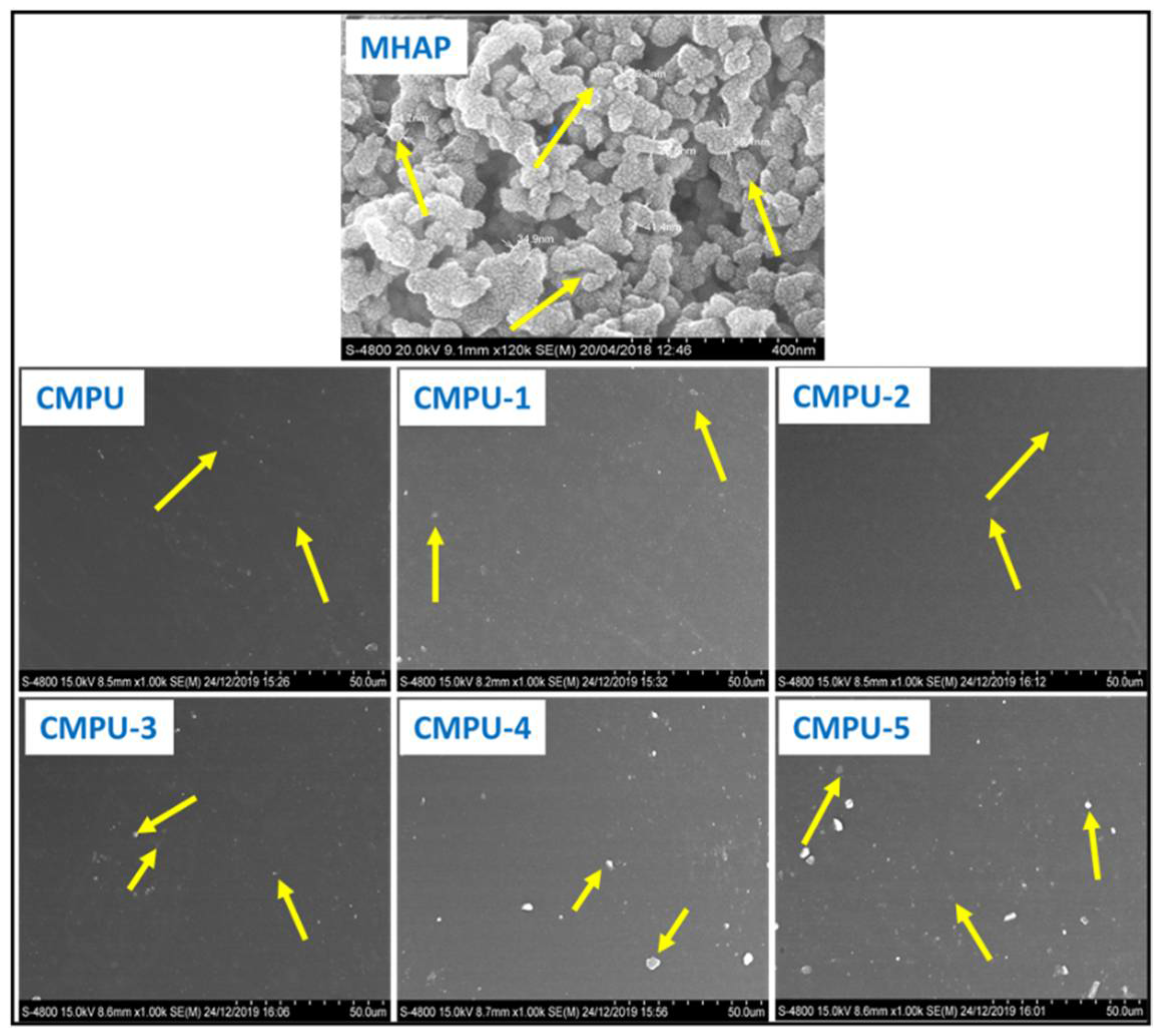
4.10. Surface Morphology of MHAP Composite Coating
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bastidas, D.M. Corrosion and protection of metals. Metals 2020, 10, 458. [Google Scholar] [CrossRef] [Green Version]
- Melchers, R.E. A review of trends for corrosion loss and pit depth in longer-term exposures. Corros. Mater. Degrad. 2020, 1, 42–58. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente, D.; Díaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef] [Green Version]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [Green Version]
- Paraskar, P.M.; Prabhudesai, M.S.; Hatkar, V.M.; Kulkarni, R.D. Vegetable oil based polyurethane coatings—A sustainable approach: A review. Prog. Org. Coat. 2021, 156, 106267. [Google Scholar] [CrossRef]
- Khanderay, J.C.; Gite, V.V. Fully biobased polyester polyols derived from renewable resources toward preparation of polyurethane and their application for coatings. J. Appl. Polym. Sci. 2019, 136, 47558. [Google Scholar] [CrossRef]
- Huang, H.; Sheng, X.; Tian, Y.; Zhang, L.; Chen, Y.; Zhang, X. Two-Dimensional Nanomaterials for Anticorrosive Polymeric Coatings: A Review. Ind. Eng. Chem. Res. 2020, 59, 15424–15446. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.-A.T. Environmental impact of bioplastic use: A review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef] [PubMed]
- Komartin, R.S.; Balanuca, B.; Necolau, M.I.; Cojocaru, A.; Stan, R. Composite Materials from Renewable Resources as Sustainable Corrosion Protection Coatings. Polymers 2021, 13, 3792. [Google Scholar] [CrossRef]
- Patil, D.M.; Phalak, G.A.; Mhaske, S.T. Enhancement of anti-corrosive performances of cardanol based amine functional benzoxazine resin by copolymerizing with epoxy resins. Prog. Org. Coat. 2017, 105, 18–28. [Google Scholar] [CrossRef]
- Chen, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-modified polysiloxanes as effective anticorrosion additives for epoxy resin coatings. RSC Adv. 2017, 7, 55967–55976. [Google Scholar] [CrossRef] [Green Version]
- Lochab, B.; Shukla, S.; Varma, I.K. Naturally occurring phenolic sources: Monomers and polymers. RSC Adv. 2014, 4, 21712–21752. [Google Scholar] [CrossRef]
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162. [Google Scholar] [CrossRef]
- Darroman, E.; Durand, N.; Boutevin, B.; Caillol, S. Improved cardanol derived epoxy coatings. Prog. Org. Coat. 2016, 91, 9–16. [Google Scholar] [CrossRef]
- TabkhPaz, M.; Park, D.-Y.; Lee, P.C.; Hugo, R.; Park, S.S. Development of nanocomposite coatings with improved mechanical, thermal, and corrosion protection properties. J. Compos. Mater. 2018, 52, 1045–1060. [Google Scholar] [CrossRef]
- Peng, T.; Xiao, R.; Rong, Z.; Liu, H.; Hu, Q.; Wang, S.; Li, X.; Zhang, J. Polymer Nanocomposite-based Coatings for Corrosion Protection. Chem. Asian J. 2020, 15, 3915–3941. [Google Scholar] [CrossRef] [PubMed]
- Vaithylingam, R.; Ansari, M.; Shanks, R.A. Recent advances in polyurethane-based nanocomposites: A review. Polym. Plast. Technol. Eng. 2017, 56, 1528–1541. [Google Scholar] [CrossRef]
- Jirimali, H.D.; Chaudhari, B.C.; Khanderay, J.C.; Joshi, S.A.; Singh, V.; Patil, A.M.; Gite, V.V. Waste eggshell-derived calcium oxide and nanohydroxyapatite biomaterials for the preparation of LLDPE polymer nanocomposite and their thermomechanical study. Polym. Plast. Technol. Eng. 2018, 57, 804–811. [Google Scholar] [CrossRef]
- Zhou, C.; Lu, X.; Xin, Z.; Liu, J.; Zhang, Y. Polybenzoxazine/SiO2 nanocomposite coatings for corrosion protection of mild steel. Corros. Sci. 2014, 80, 269–275. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, X.; Liu, W.; Fu, F.; Yang, D. A novel lignin/ZnO hybrid nanocomposite with excellent UV-absorption ability and its application in transparent polyurethane coating. Ind. Eng. Chem. Res. 2017, 56, 11133–11141. [Google Scholar] [CrossRef]
- Kumar, K.; Ghosh, P.; Kumar, A. Improving mechanical and thermal properties of TiO2-epoxy nanocomposite. Compos. Part B Eng. 2016, 97, 353–360. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of Poly(Titanium Oxide) on the Viscoelastic and Thermophysical Properties of Interpenetrating Polymer Networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Gang, L.; Yan-Feng, L.; Lin-Cheng, Z.; Bo-Nian, L.; Xue-Hu, M.; Xian-Zhen, L. Photo-Heat Transition of Coatings Derived from Furfural Resin and Fe2 O3 Nanoparticles. High Perform. Polym. 2005, 17, 469–481. [Google Scholar] [CrossRef]
- Aksimentyeva, O.; Savchyn, V.; Dyakonov, V.; Piechota, S.; Horbenko, Y.Y.; Opainych, I.Y.; Demchenko, P.Y.; Popov, A.; Szymczak, H. Modification of polymer-magnetic nanoparticles by luminescent and conducting substances. Mol. Cryst. Liq. Cryst. 2014, 590, 35–42. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, L. Phase separation and properties of UV-curable polyurethane/zirconia nanocomposite coatings. Macromol. Chem. Phys. 2008, 209, 1170–1181. [Google Scholar] [CrossRef]
- Wang, Y.; Lim, S.; Luo, J.; Xu, Z. Tribological and corrosion behaviors of Al2O3/polymer nanocomposite coatings. Wear 2006, 260, 976–983. [Google Scholar] [CrossRef]
- Khatoon, H.; Ahmad, S. Vanadium pentoxide-enwrapped polydiphenylamine/polyurethane nanocomposite: High-performance anticorrosive coating. ACS Appl. Mater. Interfaces 2018, 11, 2374–2385. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Xing, W.; Yang, H.; Song, L.; Hu, Y. UV-curable functionalized graphene oxide/polyurethane acrylate nanocomposite coatings with enhanced thermal stability and mechanical properties. Ind. Eng. Chem. Res. 2012, 51, 14629–14636. [Google Scholar] [CrossRef]
- Savchyn, V.; Popov, A.; Aksimentyeva, O.; Klym, H.; Horbenko, Y.Y.; Serga, V.; Moskina, A.; Karbovnyk, I. Cathodoluminescence characterization of polystyrene-BaZrO3 hybrid composites. Low Temp. Phys. 2016, 42, 597–600. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Behzadnasab, M.; Mirabedini, S.M.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Sadasivuni, K.K.; Ponnamma, D.; Al-Maadeed, M.A.A. Oleic acid-grafted chitosan/graphene oxide composite coating for corrosion protection of carbon steel. Carbohydr. Polym. 2016, 151, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.K.; Jirimali, H.D.; Paradeshi, J.S.; Chaudhari, B.L.; Gite, V.V. Functional antimicrobial and anticorrosive polyurethane composite coatings from algae oil and silver doped egg shell hydroxyapatite for sustainable development. Prog. Org. Coat. 2019, 128, 127–136. [Google Scholar] [CrossRef]
- Ku, J.-K.; Kim, I.-H.; Shim, J.H.; Kim, Y.H.; Kim, B.H.; Kim, Y.-K.; Yun, P.-Y. The Effect of Whitlockite as an Osteoconductive Synthetic Bone Substitute Material in Animal Bony Defect Model. Materials 2022, 15, 1921. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-M.; Liu, S.-M.; Ko, C.-L.; Chen, W.-C. Advances of Hydroxyapatite Hybrid Organic Composite Used as Drug or Protein Carriers for Biomedical Applications: A Review. Polymers 2022, 14, 976. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Chuev, V.; Klyukin, B.; Kuskov, A.; Mezhuev, Y.; Chistyakov, E. Polymeric Dental Nanomaterials: Antimicrobial Action. Polymers 2022, 14, 864. [Google Scholar] [CrossRef] [PubMed]
- El-Maghrabi, H.H.; Younes, A.A.; Salem, A.R.; Rabie, K.; El-Shereafy, E.-S. Magnetically modified hydroxyapatite nanoparticles for the removal of uranium (VI): Preparation, characterization and adsorption optimization. J. Hazard. Mater. 2019, 378, 120703. [Google Scholar] [CrossRef] [PubMed]
- Safatian, F.; Doago, Z.; Torabbeigi, M.; Rahmani Shams, H.; Ahadi, N. Lead ion removal from water by hydroxyapatite nanostructures synthesized from egg sells with microwave irradiation. Appl. Water Sci. 2019, 9, 108. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Gao, X.; Wei, W.; Zhang, Y.; Zhang, Y.; Ma, L.; Liu, H.; Han, R.; Lin, J. Combined performance of hydroxyapatite adsorption and magnetic separation processes for Cd (II) removal from aqueous solution. J. Dispers. Sci. Technol. 2021, 42, 664–676. [Google Scholar] [CrossRef]
- Thanh, D.N.; Novák, P.; Vejpravova, J.; Vu, H.N.; Lederer, J.; Munshi, T. Removal of copper and nickel from water using nanocomposite of magnetic hydroxyapatite nanorods. J. Magn. Magn. Mater. 2018, 456, 451–460. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, L.; Zhang, G.; Yan, T.; Yan, L.; Wei, Q.; Du, B. Removal of Pb (II) and methylene blue from aqueous solution by magnetic hydroxyapatite-immobilized oxidized multi-walled carbon nanotubes. J. Colloid Interface Sci. 2017, 494, 380–388. [Google Scholar] [CrossRef] [PubMed]
- de Luca Bossa, F.; Verdolotti, L.; Russo, V.; Campaner, P.; Minigher, A.; Lama, G.C.; Boggioni, L.; Tesser, R.; Lavorgna, M. Upgrading sustainable polyurethane foam based on greener polyols: Succinic-based polyol and Mannich-based polyol. Materials 2020, 13, 3170. [Google Scholar] [CrossRef]
- Chandra, V.S.; Elayaraja, K.; Arul, K.T.; Ferraris, S.; Spriano, S.; Ferraris, M.; Asokan, K.; Kalkura, S.N. Synthesis of magnetic hydroxyapatite by hydrothermal–microwave technique: Dielectric, protein adsorption, blood compatibility and drug release studies. Ceram. Int. 2015, 41, 13153–13163. [Google Scholar] [CrossRef]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Nguyen, V.T.; Kim, H.H.; Nam, S.Y.; Lee, K.D.; Oh, J. Hydroxyapatite coated iron oxide nanoparticles: A promising nanomaterial for magnetic hyperthermia cancer treatment. Nanomaterials 2017, 7, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Chen, J.; Hu, E.; Zeng, F. A reactive molecular dynamics simulation study on corrosion behaviors of carbon steel in salt spray. Comput. Mater. Sci. 2022, 203, 111142. [Google Scholar] [CrossRef]
- Sherif, E.-S.M. Corrosion behavior of magnesium in naturally aerated stagnant seawater and 3.5% sodium chloride solutions. Int. J. Electrochem. Sci 2012, 7, 4235–4249. [Google Scholar]
- Mushtaq, A.; Zhao, R.; Luo, D.; Dempsey, E.; Wang, X.; Iqbal, M.Z.; Kong, X. Magnetic hydroxyapatite nanocomposites: The advances from synthesis to biomedical applications. Mater. Des. 2021, 197, 109269. [Google Scholar] [CrossRef]
- Mahajan, M.S.; Mahulikar, P.P.; Gite, V.V. Eugenol based renewable polyols for development of 2K anticorrosive polyurethane coatings. Prog. Org. Coat. 2020, 148, 105826. [Google Scholar] [CrossRef]
- Ionescu, M.; Wan, X.; Bilić, N.; Petrović, Z.S. Polyols and rigid polyurethane foams from cashew nut shell liquid. J. Polym. Environ. 2012, 20, 647–658. [Google Scholar] [CrossRef]
- Gandhi, T.S.; Patel, M.R.; Dholakiya, B.Z. Synthesis of cashew Mannich polyol via a three step continuous route and development of PU rigid foams with mechanical, thermal and fire studies. J. Polym. Eng. 2015, 35, 533–544. [Google Scholar] [CrossRef]
- Gupta, R.; Ionescu, M.; Wan, X.; Radojcic, D.; Petroviƈ, Z. Synthesis of a novel limonene based mannich polyol for rigid polyurethane foams. J. Polym. Environ. 2015, 23, 261–268. [Google Scholar] [CrossRef]
- Du, K.; Liu, X.; Li, S.; Qiao, L.; Ai, H. Synthesis of Cu2+ chelated cellulose/magnetic hydroxyapatite particles hybrid beads and their potential for high specific adsorption of histidine-rich proteins. ACS Sustain. Chem. Eng. 2018, 6, 11578–11586. [Google Scholar] [CrossRef]
- Ch, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Phalak, G.A.; Patil, D.M.; Mhaske, S. Synthesis and characterization of thermally curable guaiacol based poly (benzoxazine-urethane) coating for corrosion protection on mild steel. Eur. Polym. J. 2017, 88, 93–108. [Google Scholar] [CrossRef]





| Element Composition | Amount in Steel (%) |
|---|---|
| Carbon | 0.16 |
| Aluminum | 0.07 |
| Silicon | 0.168 |
| Manganese | 0.18 |
| Phosphorous | 0.025 |
| Copper | 0.09 |
| Iron | Balance |
| Wavenumber (cm−1) | Vibration Type |
|---|---|
| Cardanol and cardanol Mannich polyol | |
| 3371 | -OH bending vibration |
| 2854–2924 | vibration of symmetric and asymmetric -CH2 group |
| 3009 | -C-H stretching vibration |
| 1651 | C=C-bond |
| 1041 | -C-N- stretching |
| Magnetic hydroxyapatite nanoparticles | |
| 3570 | -OH bending vibration |
| 1458, and 871 | -C=O stretching vibration |
| 1035 and 630 | phosphate group |
| 1619 and 570 | Fe-O bond stretching |
| PUs Code | Gloss 60° | Cross-Cut Adhesion (%) | Pencil Hardness | Flexibility | Methyl Ethyl Ketone Double Rub Test |
|---|---|---|---|---|---|
| CMPU | 121 | 100 | 3H | Pass | 200 |
| CMPU-1 | 96 | 100 | 3H | Pass | 200 |
| CMPU-2 | 85 | 100 | 4H | Pass | 200 |
| CMPU-3 | 84 | 100 | 4H | Pass | 200 |
| CMPU-4 | 74 | 100 | 5H | Pass | 200 |
| CMPU-5 | 73 | 100 | 5H | Pass | 200 |
| Sample Code | Water | HCl | NaOH | Xylene |
|---|---|---|---|---|
| Bared | F | F | F | F |
| CMPU | B | D | A | A |
| CMPU-1 | B | B | A | A |
| CMPU-2 | C | C | A | A |
| CMPU-3 | B | C | A | A |
| CMPU-4 | B | B | A | A |
| CMPU-5 | B | B | A | A |
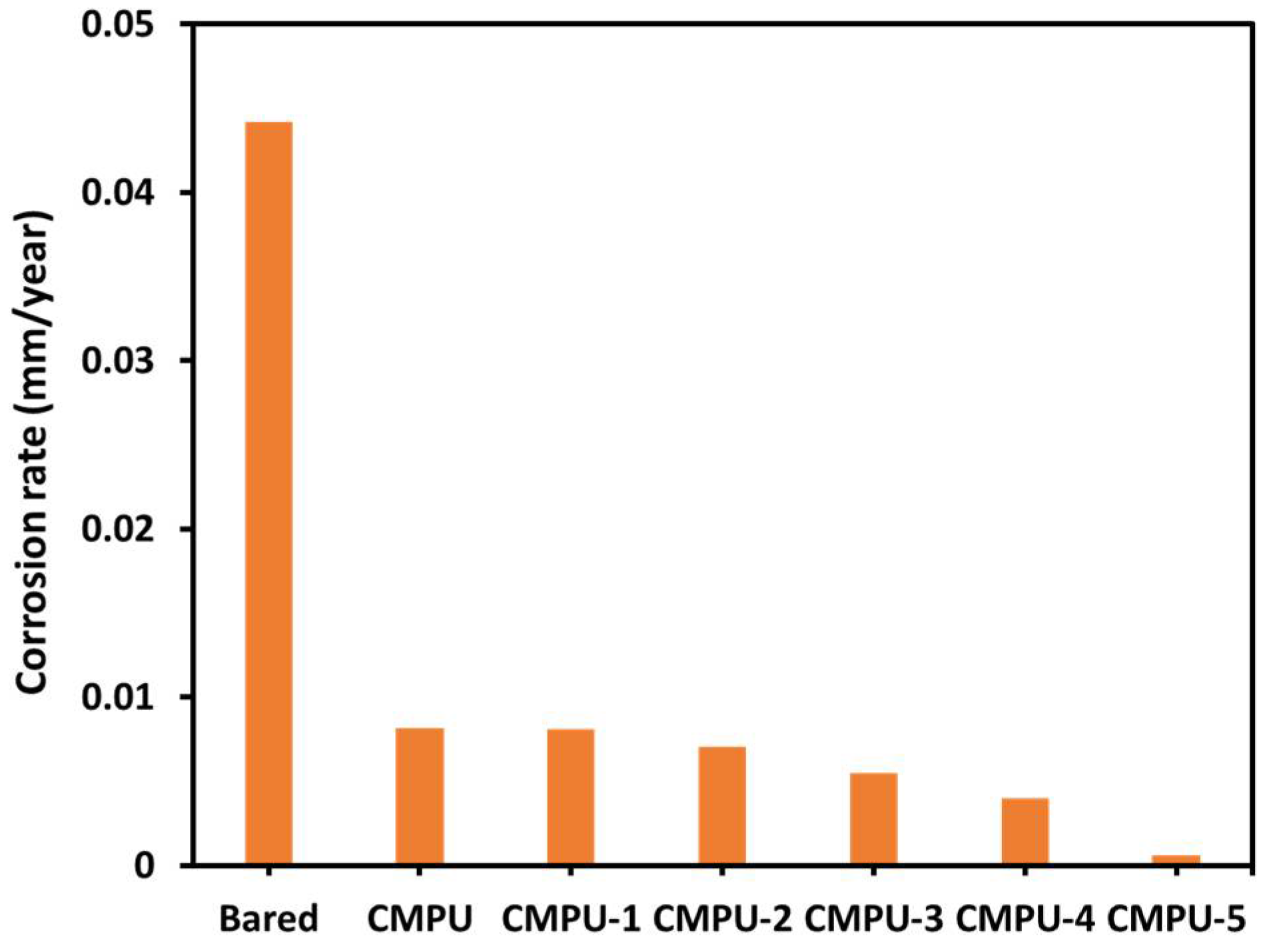
| Sample Code | Ecorr (mV) | Icorr (nA) | Rp (kΩ) | Corrosion Rate (CR) (mm/Year) | Inhibition Efficiency (% IE) |
|---|---|---|---|---|---|
| Bare (Uncoated) | −656.47 | 968.44 | 4.929 | 0.0442 | 0.00 |
| CMPU | −618.24 | 528.12 | 8.994 | 0.00814 | 45.47 |
| CMPU-1 | −602.31 | 479.09 | 8.954 | 0.00812 | 50.52 |
| CMPU-2 | −544.68 | 465.71 | 10.343 | 0.00709 | 51.91 |
| CMPU-3 | −554.76 | 372.97 | 23.280 | 0.005483 | 61.48 |
| CMPU-4 | −547.84 | 345.10 | 16.059 | 0.00401 | 64.47 |
| CMPU-5 | −535.07 | 181.35 | 116.520 | 0.000582 | 81.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asif, A.H.; Mahajan, M.S.; Sreeharsha, N.; Gite, V.V.; Al-Dhubiab, B.E.; Kaliyadan, F.; Nanjappa, S.H.; Meravanige, G.; Aleyadhy, D.M. Enhancement of Anticorrosive Performance of Cardanol Based Polyurethane Coatings by Incorporating Magnetic Hydroxyapatite Nanoparticles. Materials 2022, 15, 2308. https://doi.org/10.3390/ma15062308
Asif AH, Mahajan MS, Sreeharsha N, Gite VV, Al-Dhubiab BE, Kaliyadan F, Nanjappa SH, Meravanige G, Aleyadhy DM. Enhancement of Anticorrosive Performance of Cardanol Based Polyurethane Coatings by Incorporating Magnetic Hydroxyapatite Nanoparticles. Materials. 2022; 15(6):2308. https://doi.org/10.3390/ma15062308
Chicago/Turabian StyleAsif, Afzal Haq, Mahendra S. Mahajan, Nagaraja Sreeharsha, Vikas V. Gite, Bandar E. Al-Dhubiab, Feroze Kaliyadan, Shivakumar H. Nanjappa, Girish Meravanige, and Dalal Mishary Aleyadhy. 2022. "Enhancement of Anticorrosive Performance of Cardanol Based Polyurethane Coatings by Incorporating Magnetic Hydroxyapatite Nanoparticles" Materials 15, no. 6: 2308. https://doi.org/10.3390/ma15062308
APA StyleAsif, A. H., Mahajan, M. S., Sreeharsha, N., Gite, V. V., Al-Dhubiab, B. E., Kaliyadan, F., Nanjappa, S. H., Meravanige, G., & Aleyadhy, D. M. (2022). Enhancement of Anticorrosive Performance of Cardanol Based Polyurethane Coatings by Incorporating Magnetic Hydroxyapatite Nanoparticles. Materials, 15(6), 2308. https://doi.org/10.3390/ma15062308







