Development of Y2O3 Dispersion-Strengthened Copper Alloy by Sol-Gel Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
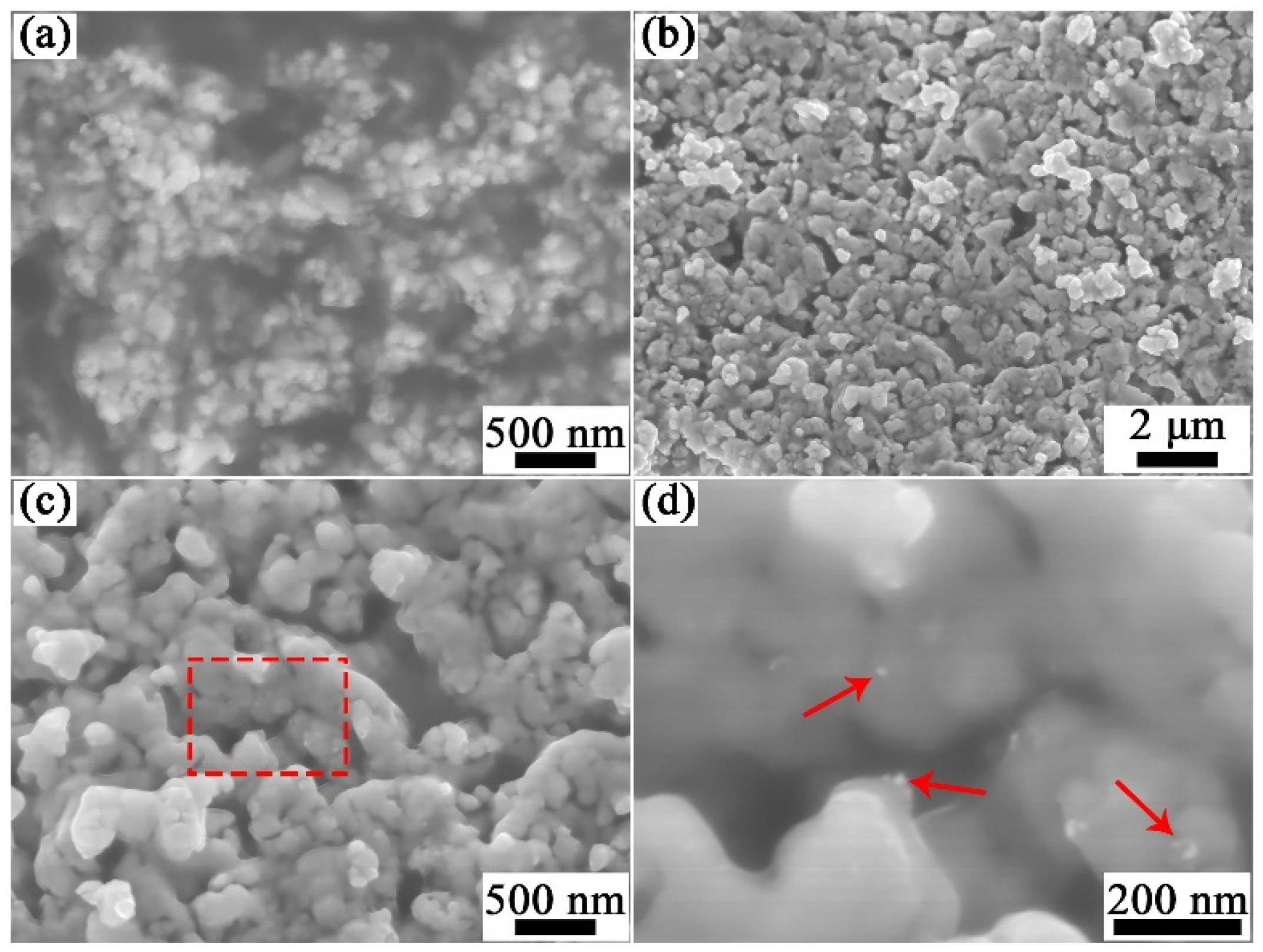
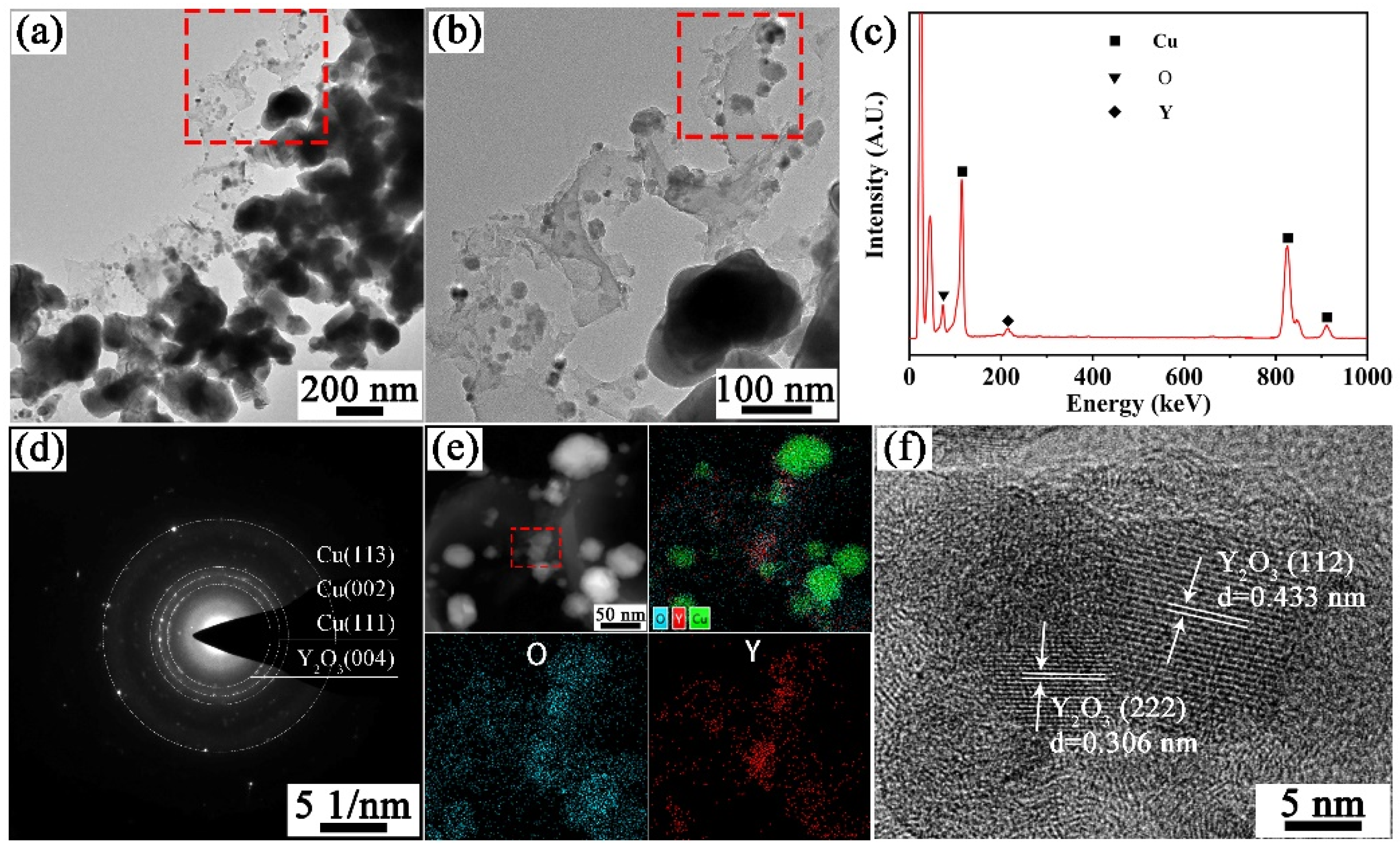
3.1. Characteristics of Powders
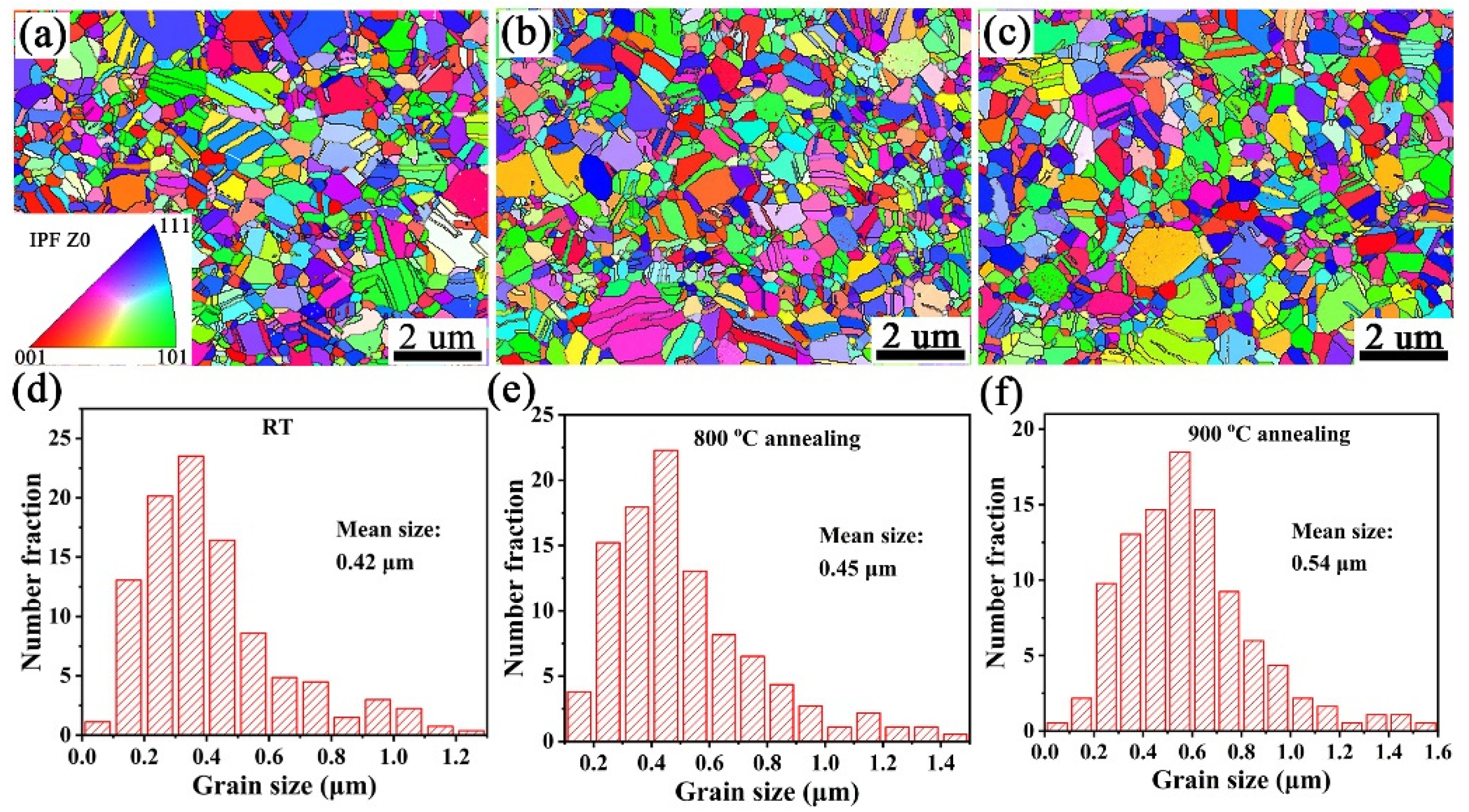
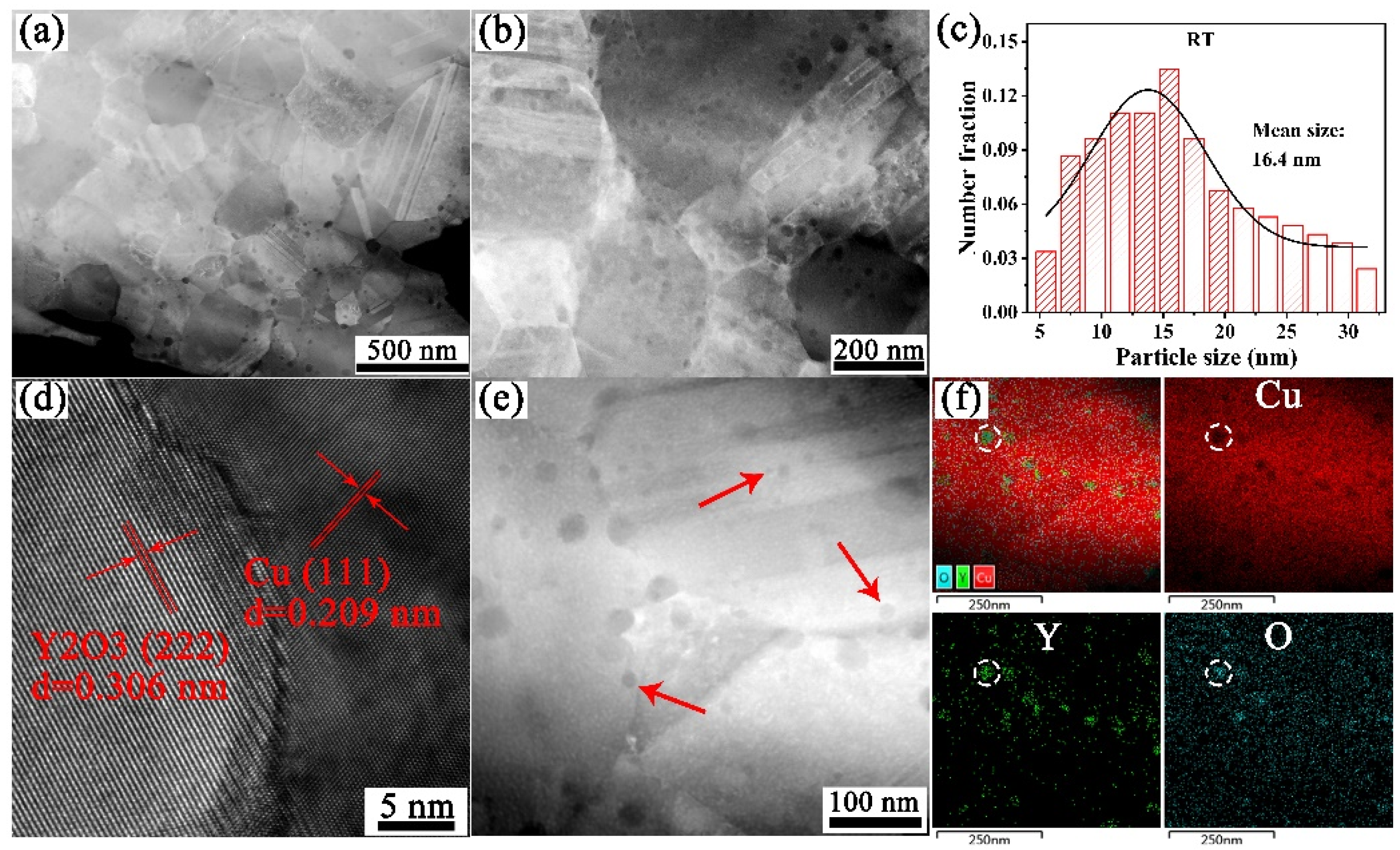
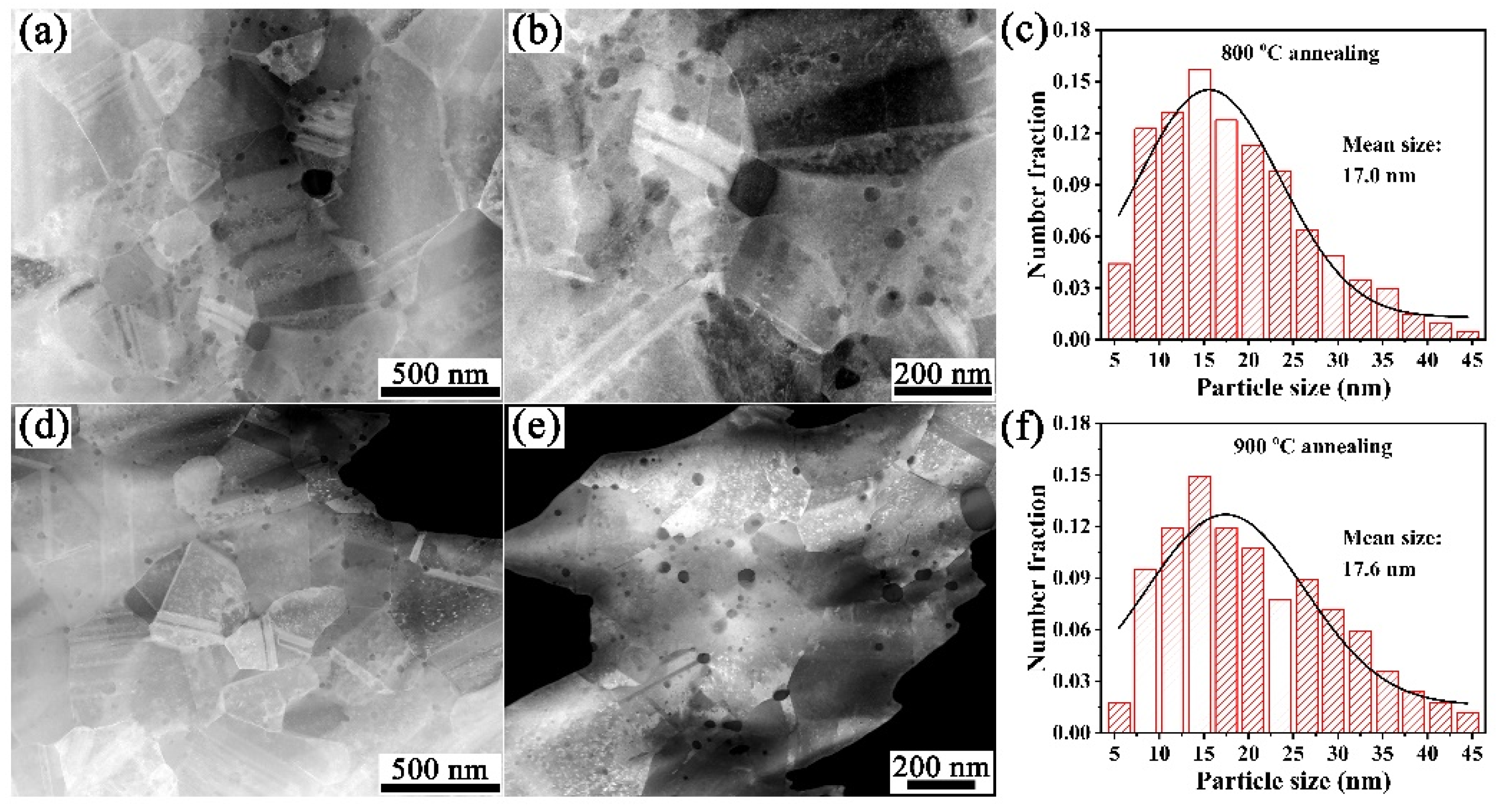
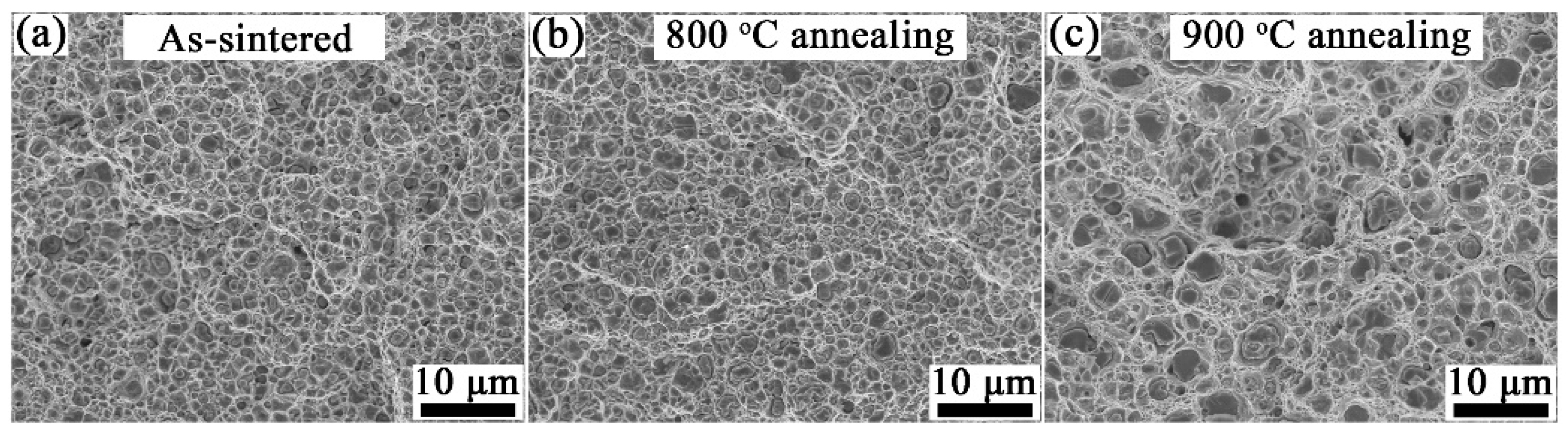
3.2. Microstructure Characterization
3.3. Mechanical Properties
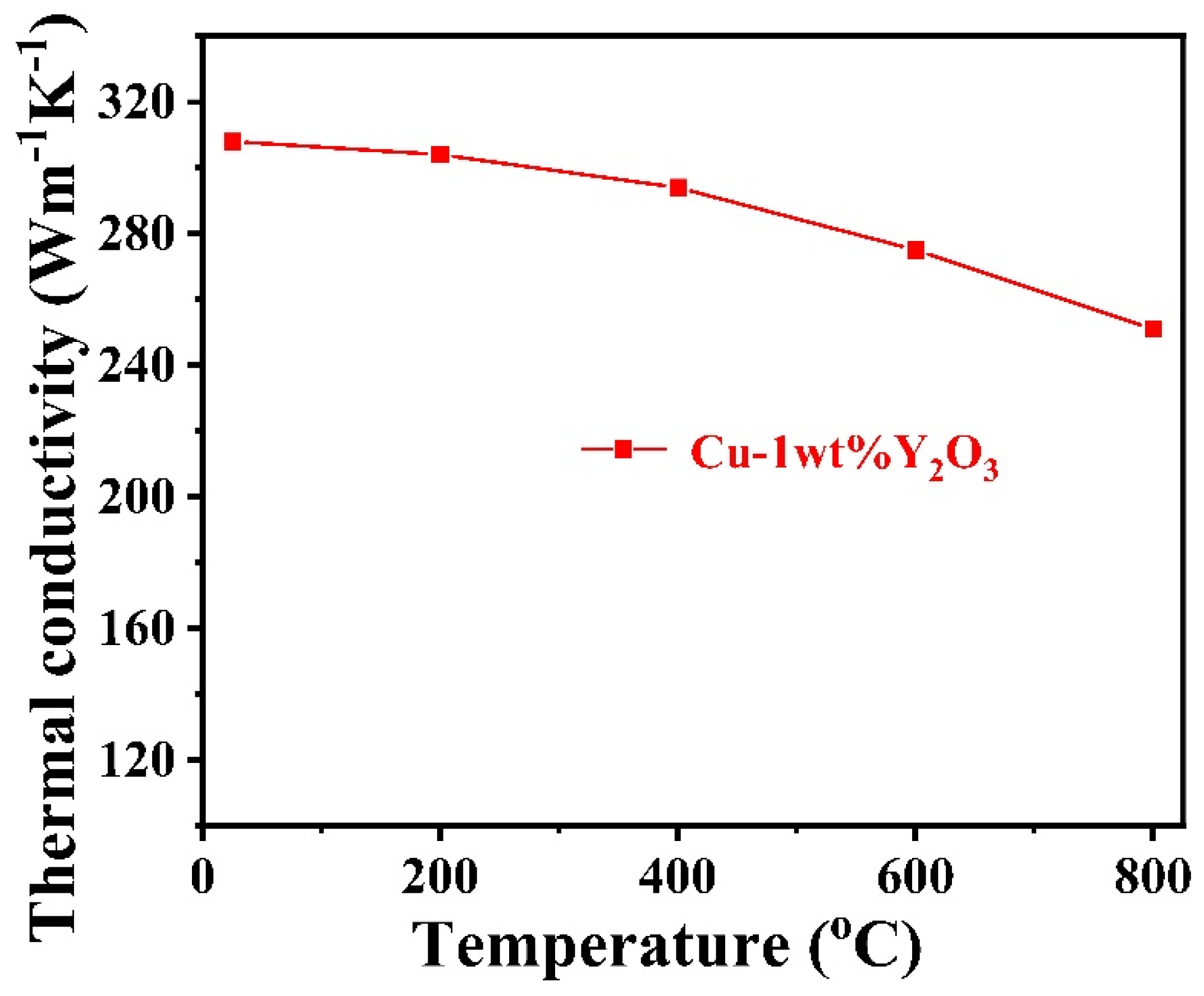
3.4. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davis, J.W.; Kalinin, G.M. Material properties and design requirements for copper alloys used in ITER. J. Nucl. Mater. 1998, 258, 323–328. [Google Scholar] [CrossRef]
- Kalinin, G.; Barabash, V.; Cardella, A.; Dietz, J.; Ioki, K.; Matera, R.; Santoro, R.T.; Tivey, R. Assessment and selection of materials for ITER in-vessel components. J. Nucl. Mater. 2000, 283–287, 10–19. [Google Scholar] [CrossRef]
- Bolt, H.; Barabash, V.; Krauss, W.; Linke, J.; Neu, R.; Suzuki, S.; Yoshida, N.; ASDEX Upgrade Team. Materials for the plasma-facing components of fusion reactors. J. Nucl. Mater. 2004, 329–333, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Minneci, R.; Lass, E.; Bunn, J.; Choo, H.; Rawn, C. Copper-based alloys for structural high-heat-flux applications: A review of development, properties, and performance of Cu-rich Cu–Cr–Nb alloys. Int. Mater. Rev. 2020, 66, 394–425. [Google Scholar] [CrossRef]
- Fabritsiev, S.A.; Zinkle, S.J.; Singh, B.N. Evaluation of copper alloys for fusion reactor divertor and first wall components. J. Nucl. Mater. 1995, 233, 127–137. [Google Scholar] [CrossRef]
- Carro, G.; Muñoz, A.; Monge, M.; Savoini, B.; Pareja, R. Microstructural and mechanical characterization of Cu-0.8wt.%Y. Fusion Eng. Des. 2015, 98–99, 1941–1944. [Google Scholar] [CrossRef]
- Carro, G.; Muñoz, A.; Monge, M.A.; Savoini, B.; Pareja, R.; Ballesteros, C.; Adeva, P. Fabrication and characterization of Y2O3 dispersion strengthened copper alloys. J. Nucl. Mater. 2014, 455, 655–659. [Google Scholar] [CrossRef]
- Visca, E.; Cacciotti, E.; Komarov, A.; Libera, S.; Litunovsky, N.; Makhankov, A.; Mancini, A.; Merola, M.; Pizzuto, A.; Riccardi, B.; et al. Manufacturing, testing and post-test examination of ITER divertor vertical target W small scale mock-ups. Fusion Eng. Des. 2011, 86, 1591–1594. [Google Scholar] [CrossRef]
- Bucalossi, J.; Missirlian, M.; Moreau, P.; Samaille, F.; Tsitrone, E.; van Houtte, D.; Batal, T.; Bourdelle, C.; Chantant, M.; Corre, Y.; et al. The WEST project: Testing ITER divertor high heat flux component technology in a steady state tokamak environment. Fusion Eng. Des. 2014, 89, 907–912. [Google Scholar] [CrossRef]
- Zhang, K.; Gaganidze, E.; Gorley, M. Development of the material property handbook and database of CuCrZr. Fusion Eng. Des. 2019, 144, 148–153. [Google Scholar] [CrossRef]
- Zhang, X.H.; Li, X.X.; Chen, H.; Li, T.B.; Su, W.; Guo, S.D. Investigation on microstructure and properties of Cu–Al2O3 composites fabricated by a novel in-situ reactive synthesis. Mater. Des. 2016, 92, 58–63. [Google Scholar] [CrossRef]
- You, J.-H. Copper matrix composites as heat sink materials for water-cooled divertor target. Nucl. Mater. Energy 2015, 5, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, S.B.; Sudhakara Sarma, S.; Ramakrishna, M.; Suresh Babu, P.; Rao, T.N.; Kashyap, B.P. Microstructure and properties of hot extruded Cu-1wt.%Al2O3 nano-composites synthesized by various techniques. Mater. Sci. Eng. A 2014, 591, 46–53. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Wang, X.; Koizumi, Y.; Kenta, Y.; Chiba, A. In-Situ fabrication and characterization of ultrafine structured Cu–TiC composites with high strength and high conductivity by mechanical milling. J. Alloys Compd. 2016, 657, 122–132. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Yamanaka, K.; Wakon, K.; Harata, K.; Chiba, A. Influence of two-step ball-milling condition on electrical and mechanical properties of TiC-dispersion-strengthened Cu alloys. Mater. Des. 2014, 64, 441–449. [Google Scholar] [CrossRef]
- Zeng, W.; Xie, J.; Zhou, D.; Fu, Z.; Zhang, D.; Lavernia, E.J. Bulk Cu-NbC nanocomposites with high strength and high electrical conductivity. J. Alloys Compd. 2018, 745, 55–62. [Google Scholar] [CrossRef]
- Ma, B.; Hishinuma, Y.; Noto, H.; Shimada, Y.; Muroga, T. Development of Y2O3 dispersion strengthened Cu alloy using Cu6Y and Cu2O addition through the MA-HIP process. Fusion Eng. Des. 2020, 161, 112045. [Google Scholar] [CrossRef]
- Zhou, D.; Geng, H.; Zeng, W.; Zheng, D.; Pan, H.; Kong, C.; Munroe, P.; Sha, G.; Suryanarayana, C.; Zhang, D. High temperature stabilization of a nanostructured Cu-Y2O3 composite through microalloying with Ti. Mater. Sci. Eng. A 2018, 712, 80–87. [Google Scholar] [CrossRef]
- Zhuo, H.; Tang, J.; Ye, N. A novel approach for strengthening Cu–Y2O3 composites by in situ reaction at liquidus temperature. Mater. Sci. Eng. A 2013, 584, 1–6. [Google Scholar] [CrossRef]
- Liang, S.; Fan, Z.; Xu, L.; Fang, L. Kinetic analysis on Al2O3/Cu composite prepared by mechanical activation and internal oxidation. Compos. Part Appl. Sci. Manuf. 2004, 35, 1441–1446. [Google Scholar] [CrossRef]
- Rowcliffe, A.F.; Zinkle, S.J.; Stubbins, J.F.; Edwards, D.J.; Alexander, D.J. Austenitic stainless steels and high strength copper alloys for fusion components. J. Nucl. Mater. 1998, 258–263, 183–192. [Google Scholar] [CrossRef]
- Groza, J.R.; Gibeling, J.C. Principles of particle selection for dispersion-strengthened copper. Mater. Sci. Eng. A 1993, 171, 115–125. [Google Scholar] [CrossRef]
- Aghamiri, S.M.S.; Oono, N.; Ukai, S.; Kasada, R.; Noto, H.; Hishinuma, Y.; Muroga, T. Microstructure and mechanical properties of mechanically alloyed ODS copper alloy for fusion material application. Nucl. Mater. Energy 2018, 15, 17–22. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, X.; Zeng, W.; Yang, C.; Pan, H.; Li, C.; Liu, Y.; Zhang, D. Doping Ti to achieve microstructural refinement and strength enhancement in a high volume fraction Y2O3 dispersion strengthened Cu. J. Alloys Compd. 2018, 753, 18–27. [Google Scholar] [CrossRef]
- Ma, B.; Hishinuma, Y.; Shimada, Y.; Noto, H.; Kasada, R.; Oono, N.; Ukai, S.; Muroga, T. The size dependence of microstructure and hardness on the MA powders for the MA-HIP processed Cu-Y2O3 dispersion-strengthened alloys. Nucl. Mater. Energy 2020, 24, 100773. [Google Scholar] [CrossRef]
- Ma, B.; Hishinuma, Y.; Noto, H.; Muroga, T. Influence of Cu-Y compound content on the microstructure of Cu-Y2O3 dispersion strengthened alloys synthesized by MA and HIP process. Plasma Fusion Res. 2021, 16, 2405053. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Ke, J.G.; Xie, Z.M.; Liu, R.; Gao, R.; Wang, X.P.; Wu, X.B.; Jing, K.; Wang, L.; Zhao, B.L.; Fang, Q.F.; et al. Strengthening of copper with homogeneous dispersion of nanoscale tungsten particles fabricated by spark plasma sintering. Mater. Sci. Eng. A 2021, 818, 141438. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, G.J.; Jiang, F.; Ding, X.D.; Sun, Y.J.; Sun, J.; Ma, E. Nanostructured high-strength molybdenum alloys with unprecedented tensile ductility. Nat. Mater. 2013, 12, 344–350. [Google Scholar] [CrossRef]
- Tejado, E.; Müller, A.v.; You, J.-H.; Pastor, J.Y. The thermo-mechanical behaviour of W-Cu metal matrix composites for fusion heat sink applications: The influence of the Cu content. J. Nucl. Mater. 2018, 498, 468–475. [Google Scholar] [CrossRef] [Green Version]
- Kalinin, G.; Matera, R. Comparative analysis of copper alloys for the heat sink of plasma facing components in ITER. J. Nucl. Mater. 1998, 258–263, 345–350. [Google Scholar] [CrossRef]
- Muller, A.V.; Ewert, D.; Galatanu, A.; Milwich, M.; Neu, R.; Pastor, J.Y.; Siefken, U.; Tejado, E.; You, J.H. Melt infiltrated tungsten–copper composites as advanced heat sink materials for plasma facing components of future nuclear fusion devices. Fusion Eng. Des. 2017, 124, 455–459. [Google Scholar] [CrossRef]


| Raw Materials | Molecular Formula | Purity |
|---|---|---|
| Citric acid monohydrate | C6H8O7·H2O | ≥99.5% |
| Copper nitrate trihydrate | Cu(NO3)2·3H2O | ≥99% |
| Yttrium nitrate hexahydrate | YN3O9·6H2O | ≥99.99% |
| Polyethylene glycol | HO(CH2CH2O)nH | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, J.; Xie, Z.; Liu, R.; Jing, K.; Cheng, X.; Wang, H.; Wang, X.; Wu, X.; Fang, Q.; Liu, C. Development of Y2O3 Dispersion-Strengthened Copper Alloy by Sol-Gel Method. Materials 2022, 15, 2416. https://doi.org/10.3390/ma15072416
Ke J, Xie Z, Liu R, Jing K, Cheng X, Wang H, Wang X, Wu X, Fang Q, Liu C. Development of Y2O3 Dispersion-Strengthened Copper Alloy by Sol-Gel Method. Materials. 2022; 15(7):2416. https://doi.org/10.3390/ma15072416
Chicago/Turabian StyleKe, Jiangang, Zhuoming Xie, Rui Liu, Ke Jing, Xiang Cheng, Hui Wang, Xianping Wang, Xuebang Wu, Qianfeng Fang, and Changsong Liu. 2022. "Development of Y2O3 Dispersion-Strengthened Copper Alloy by Sol-Gel Method" Materials 15, no. 7: 2416. https://doi.org/10.3390/ma15072416
APA StyleKe, J., Xie, Z., Liu, R., Jing, K., Cheng, X., Wang, H., Wang, X., Wu, X., Fang, Q., & Liu, C. (2022). Development of Y2O3 Dispersion-Strengthened Copper Alloy by Sol-Gel Method. Materials, 15(7), 2416. https://doi.org/10.3390/ma15072416







