Evolution of Superhydrophilic Aluminum Alloy Properties in Contact with Water during Cyclic Variation in Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Thermal Cycling
2.3. Hydrophobic Modification of Samples after Thermal Cycling
2.4. Surface Characterization
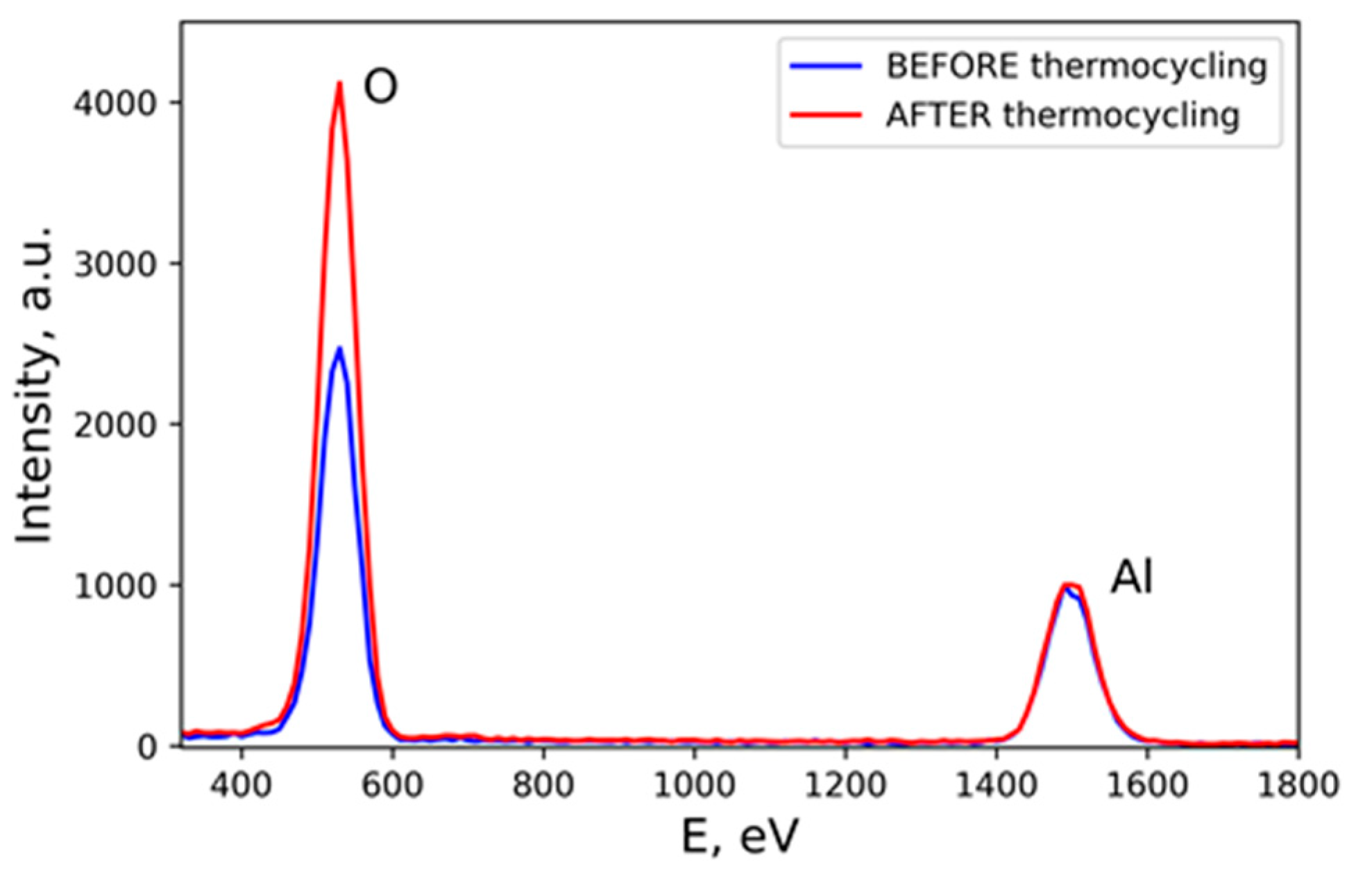
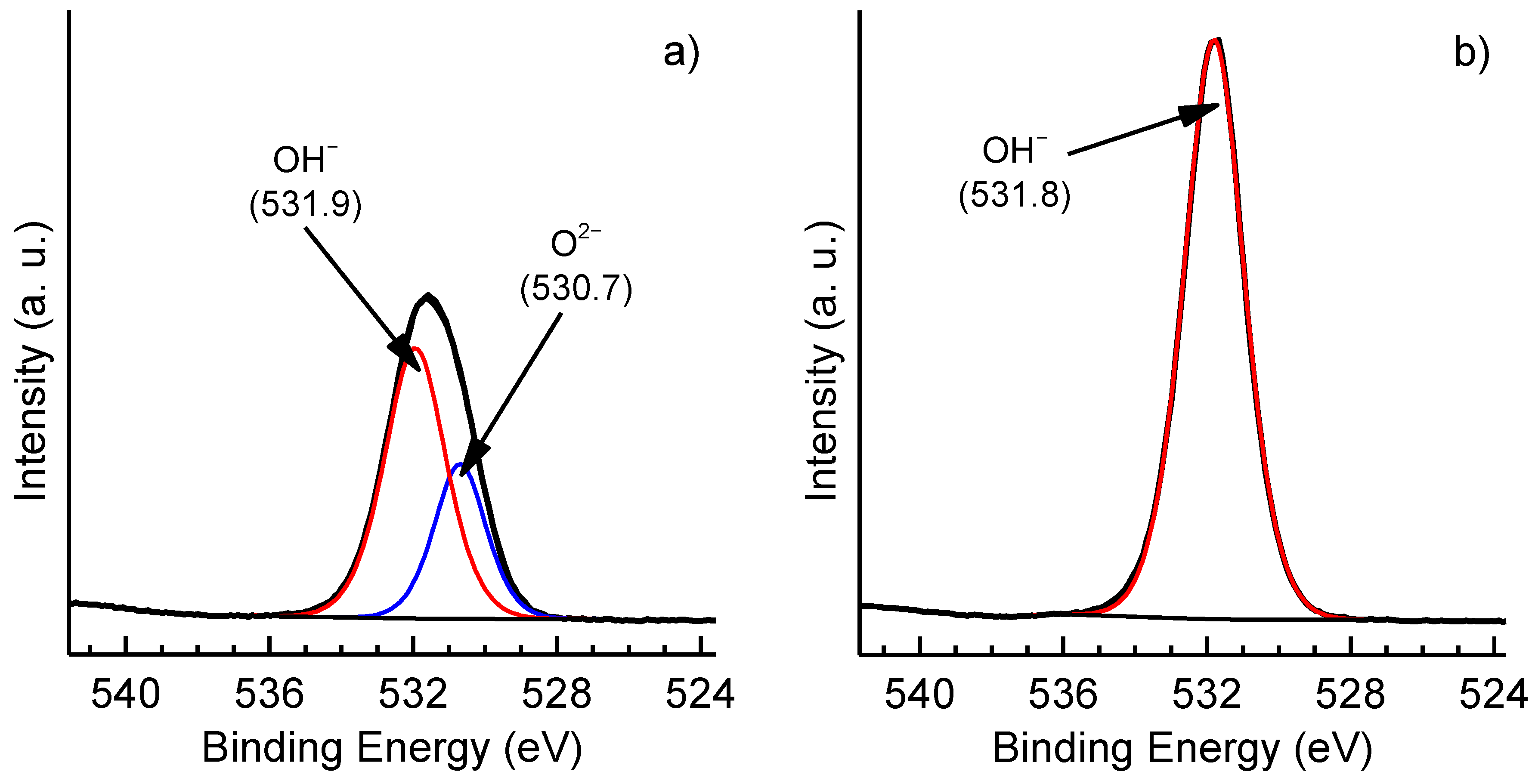
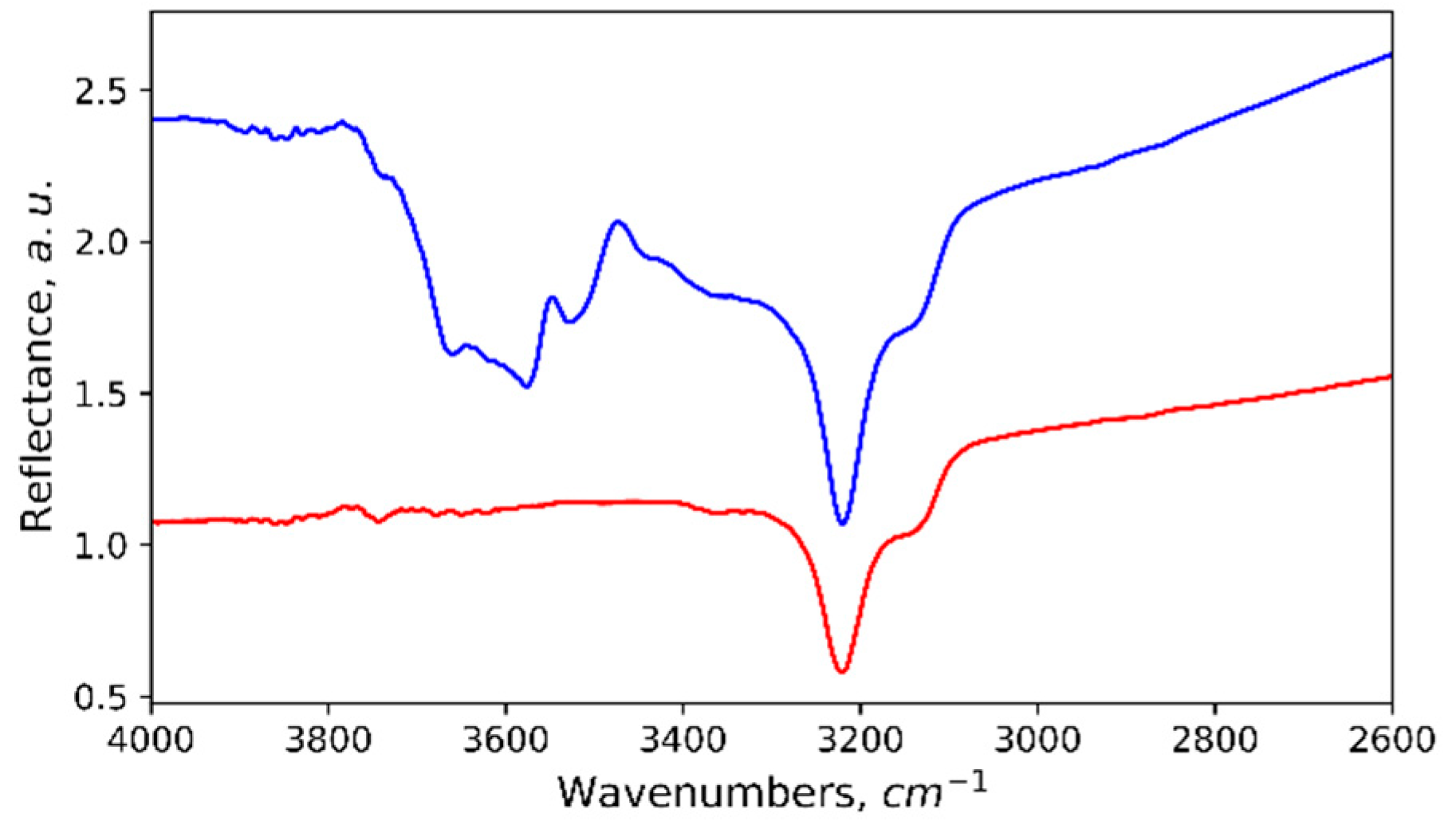
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emelyanenko, K.A.; Emelyanenko, A.M.; Boinovich, L.B. Water and Ice Adhesion to Solid Surfaces: Common and Specific, the Impact of Temperature and Surface Wettability. Coatings 2020, 10, 648. [Google Scholar] [CrossRef]
- Chen, D.; Gelenter, M.D.; Hong, M.; Cohen, R.E.; McKinley, G.H. Icephobic Surfaces Induced by Interfacial Nonfrozen Water. ACS Appl. Mater. Interfaces 2017, 9, 4202–4214. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.; Chen, J.; Zhang, Y.; Wang, X.; Cui, D.; Song, Y.; Jiang, L.; Wang, J. Anti-Icing Coating with an Aqueous Lubricating Layer. ACS Appl. Mater. Interfaces 2014, 6, 6998–7003. [Google Scholar] [CrossRef]
- Heydari, G.; Tyrode, E.; Visnevskij, C.; Makuska, R.; Claesson, P.M. Temperature-Dependent Deicing Properties of Electrostatically Anchored Branched Brush Layers of Poly(Ethylene Oxide). Langmuir 2016, 32, 4194–4202. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Zhang, G.; Zhong, Z.; Huang, Y.; Su, Z. Superhydrophilic Anti-Icing Coatings Based on Polyzwitterion Brushes. Langmuir 2019, 35, 1294–1301. [Google Scholar] [CrossRef]
- Schreiber, A.; Ketelsen, I.; Findenegg, G.H. Melting and Freezing of Water in Ordered Mesoporous Silica Materials. Phys. Chem. Chem. Phys. 2001, 3, 1185–1195. [Google Scholar] [CrossRef]
- Findenegg, G.H.; Jähnert, S.; Akcakayiran, D.; Schreiber, A. Freezing and Melting of Water Confined in Silica Nanopores. ChemPhysChem 2008, 9, 2651–2659. [Google Scholar] [CrossRef]
- Sataeva, N.E.; Boinovich, L.B.; Emelyanenko, K.A.; Domantovsky, A.G.; Emelyanenko, A.M. Laser-Assisted Processing of Aluminum Alloy for the Fabrication of Superhydrophobic Coatings Withstanding Multiple Degradation Factors. Surf. Coat. Technol. 2020, 397, 125993. [Google Scholar] [CrossRef]
- Boinovich, L.; Emelyanenko, A. A Wetting Experiment as a Tool to Study the Physicochemical Processes Accompanying the Contact of Hydrophobic and Superhydrophobic Materials with Aqueous Media. Adv. Colloid Interface Sci. 2012, 179–182, 133–141. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS Study of the Major Minerals in Bauxite: Gibbsite, Bayerite and (Pseudo-)Boehmite. J. Colloid Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef] [Green Version]
- Emel’yanenko, A.M.; Boinovich, L.B. Analysis of Wetting as an Efficient Method for Studying the Characteristics of Coatings and Surfaces and the Processes That Occur on Them: A Review. Inorg. Mater. 2011, 47, 1667–1675. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Domantovskiy, A.G.; Emelyanenko, A.M.; Pashinin, A.S.; Ionin, A.A.; Kudryashov, S.I.; Saltuganov, P.N. Femtosecond Laser Treatment for the Design of Electro-Insulating Superhydrophobic Coatings with Enhanced Wear Resistance on Glass. ACS Appl. Mater. Interfaces 2014, 6, 2080–2085. [Google Scholar] [CrossRef] [PubMed]
- Rotole, J.A.; Sherwood, P.M.A. Boehmite (γ-AlOOH) by XPS. Surf. Sci. Spectra 1998, 5, 53–59. [Google Scholar] [CrossRef]
- Ingram-Jones, V.J.; Slade, R.C.T.; Davies, T.W.; Southern, J.C.; Salvador, S. Dehydroxylation Sequences of Gibbsite and Boehmite: Study of Differences between Soak and Flash Calcination and of Particle-Size Effects. J. Mater. Chem. 1996, 6, 73. [Google Scholar] [CrossRef]
- Balan, E.; Blanchard, M.; Hochepied, J.-F.; Lazzeri, M. Surface Modes in the Infrared Spectrum of Hydrous Minerals: The OH Stretching Modes of Bayerite. Phys. Chem. Miner. 2008, 35, 279–285. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Buldakov, D.A.; Tishkin, A.A.; Lukashin, A.V.; Eliseev, A.A. Liquid Permeation and Chemical Stability of Anodic Alumina Membranes. Beilstein J. Nanotechnol. 2017, 8, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Carrier, X.; Marceau, E.; Lambert, J.-F.; Che, M. Transformations of γ-Alumina in Aqueous Suspensions. J. Colloid Interface Sci. 2007, 308, 429–437. [Google Scholar] [CrossRef]
- Lefèvre, G.; Duc, M.; Lepeut, P.; Caplain, R.; Fédoroff, M. Hydration of γ-Alumina in Water and Its Effects on Surface Reactivity. Langmuir 2002, 18, 7530–7537. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Modin, E.B.; Sayfutdinova, A.R.; Emelyanenko, K.A.; Vasiliev, A.L.; Emelyanenko, A.M. Combination of Functional Nanoengineering and Nanosecond Laser Texturing for Design of Superhydrophobic Aluminum Alloy with Exceptional Mechanical and Chemical Properties. ACS Nano 2017, 11, 10113–10123. [Google Scholar] [CrossRef]
- Chen, Y.; Hyldtoft, J.; Jacobsen, C.J.H.; Nielsen, O.F. NIR FT Raman Spectroscopic Studies of η-Al2O3 and Mo/η-Al2O3 Catalysts. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 1995, 51, 2161–2169. [Google Scholar] [CrossRef]
- Abi Aad, J.; Casale, S.; Michau, M.; Courty, P.; Diehl, F.; Marceau, E.; Carrier, X. Chemical Weathering of Alumina in Aqueous Suspension at Ambient Pressure: A Mechanistic Study. ChemCatChem 2017, 9, 2186–2194. [Google Scholar] [CrossRef] [Green Version]
- Vainer, B.G.; Volodin, A.M.; Shepelin, A.V. Hydration-Induced Thermal Behavior of Crystalline and Amorphous Dispersed Alumina. Thermochim. Acta 2021, 706, 179066. [Google Scholar] [CrossRef]
- Dokmai, V.; Methaapanon, R.; Pavarajarn, V. Corrosion of Amorphous Alumina in Deionized Water under Mild Condition. Appl. Surf. Sci. 2020, 499, 143906. [Google Scholar] [CrossRef]
- Sharma, C.S.; Combe, J.; Giger, M.; Emmerich, T.; Poulikakos, D. Growth Rates and Spontaneous Navigation of Condensate Droplets Through Randomly Structured Textures. ACS Nano 2017, 11, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Koichumanova, K.; Sai Sankar Gupta, K.B.; Lefferts, L.; Mojet, B.L.; Seshan, K. An in Situ ATR-IR Spectroscopy Study of Aluminas under Aqueous Phase Reforming Conditions. Phys. Chem. Chem. Phys. 2015, 17, 23795–23804. [Google Scholar] [CrossRef]
- Zhang, J.; Klasky, M.; Letellier, B.C. The Aluminum Chemistry and Corrosion in Alkaline Solutions. J. Nucl. Mater. 2009, 384, 175–189. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Modin, E.B. Modus Operandi of Protective and Anti-Icing Mechanisms Underlying the Design of Longstanding Outdoor Icephobic Coatings. ACS Nano 2019, 13, 4335–4346. [Google Scholar] [CrossRef]


| Sample | Contact Angle ° | Roll-Off Angle ° | |
|---|---|---|---|
| Laser-textured sample subjected to fluorosilane deposition | As prepared | 171.5 ± 0.8 | 2.0 ± 0.6 |
| After 20 min of abrasion load | 159.8 ± 2.5 | 17.1 ± 1.8 | |
| Laser-textured sample, subjected to thermal cycling and fluorosilane deposition | As prepared | 171.2 ± 0.7 | 6.2 ± 2.3 |
| After 20 min of abrasion load | 146.3 ± 1.7 | 43.2 ± 9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domantovsky, A.G.; Chulkova, E.V.; Emelyanenko, K.A.; Maslakov, K.I.; Emelyanenko, A.M.; Boinovich, L.B. Evolution of Superhydrophilic Aluminum Alloy Properties in Contact with Water during Cyclic Variation in Temperature. Materials 2022, 15, 2447. https://doi.org/10.3390/ma15072447
Domantovsky AG, Chulkova EV, Emelyanenko KA, Maslakov KI, Emelyanenko AM, Boinovich LB. Evolution of Superhydrophilic Aluminum Alloy Properties in Contact with Water during Cyclic Variation in Temperature. Materials. 2022; 15(7):2447. https://doi.org/10.3390/ma15072447
Chicago/Turabian StyleDomantovsky, Alexander G., Elizaveta V. Chulkova, Kirill A. Emelyanenko, Konstantin I. Maslakov, Alexandre M. Emelyanenko, and Ludmila B. Boinovich. 2022. "Evolution of Superhydrophilic Aluminum Alloy Properties in Contact with Water during Cyclic Variation in Temperature" Materials 15, no. 7: 2447. https://doi.org/10.3390/ma15072447
APA StyleDomantovsky, A. G., Chulkova, E. V., Emelyanenko, K. A., Maslakov, K. I., Emelyanenko, A. M., & Boinovich, L. B. (2022). Evolution of Superhydrophilic Aluminum Alloy Properties in Contact with Water during Cyclic Variation in Temperature. Materials, 15(7), 2447. https://doi.org/10.3390/ma15072447