Application of the Solute–Solvent Intermolecular Interactions as Indicator of Caffeine Solubility in Aqueous Binary Aprotic and Proton Acceptor Solvents: Measurements and Quantum Chemistry Computations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Calibration Curve
2.3. Preparation of Samples in Aqueous Binary Mixtures of Organic Solvents
2.4. Solubility Measurements
2.5. FTIR–ATR Measurements
2.6. Differential Scanning Calorimetry Measurements
2.7. Affinity Quantum Chemistry Computations
3. Results and Discussion
3.1. Solid State Characteristics
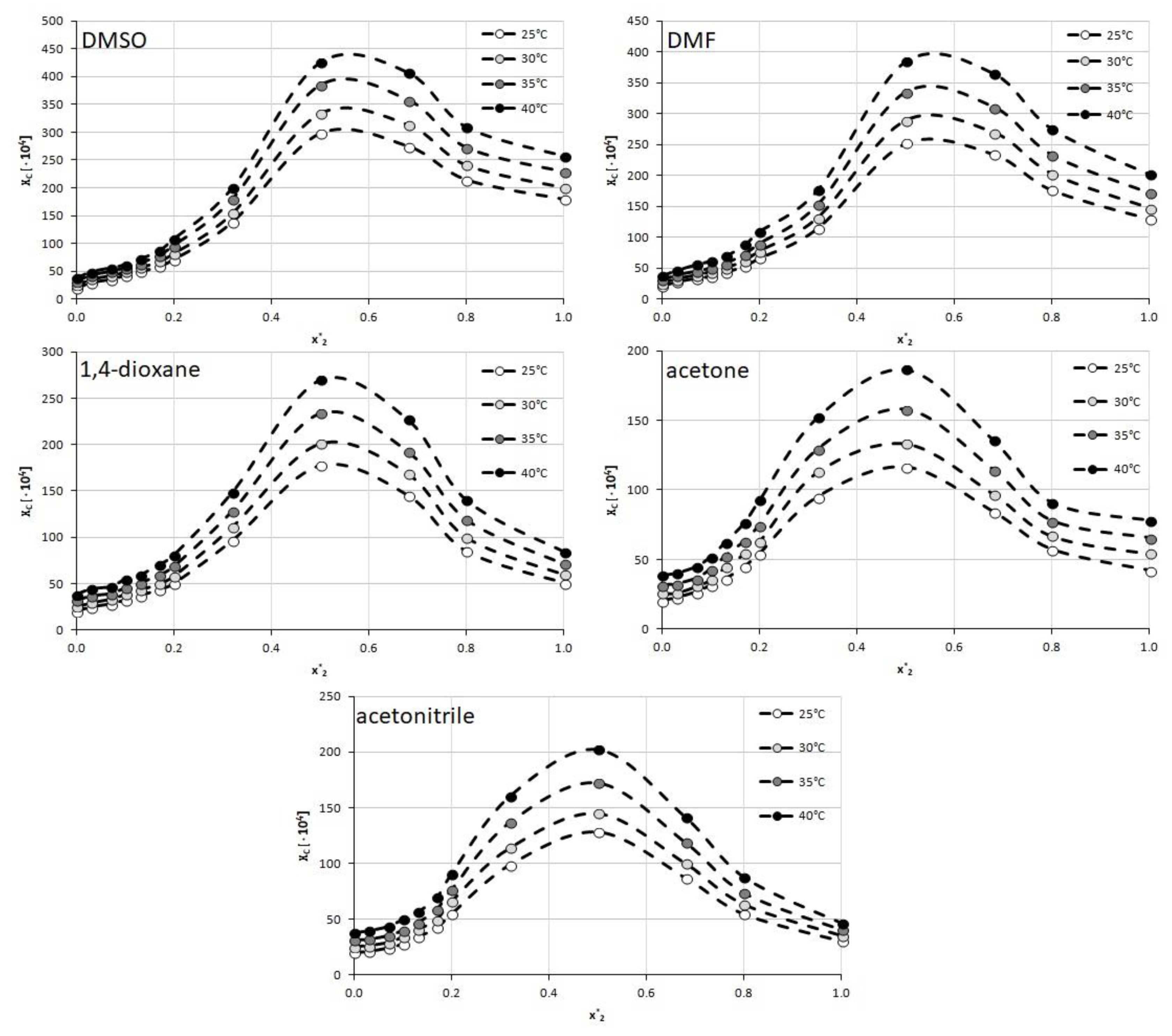
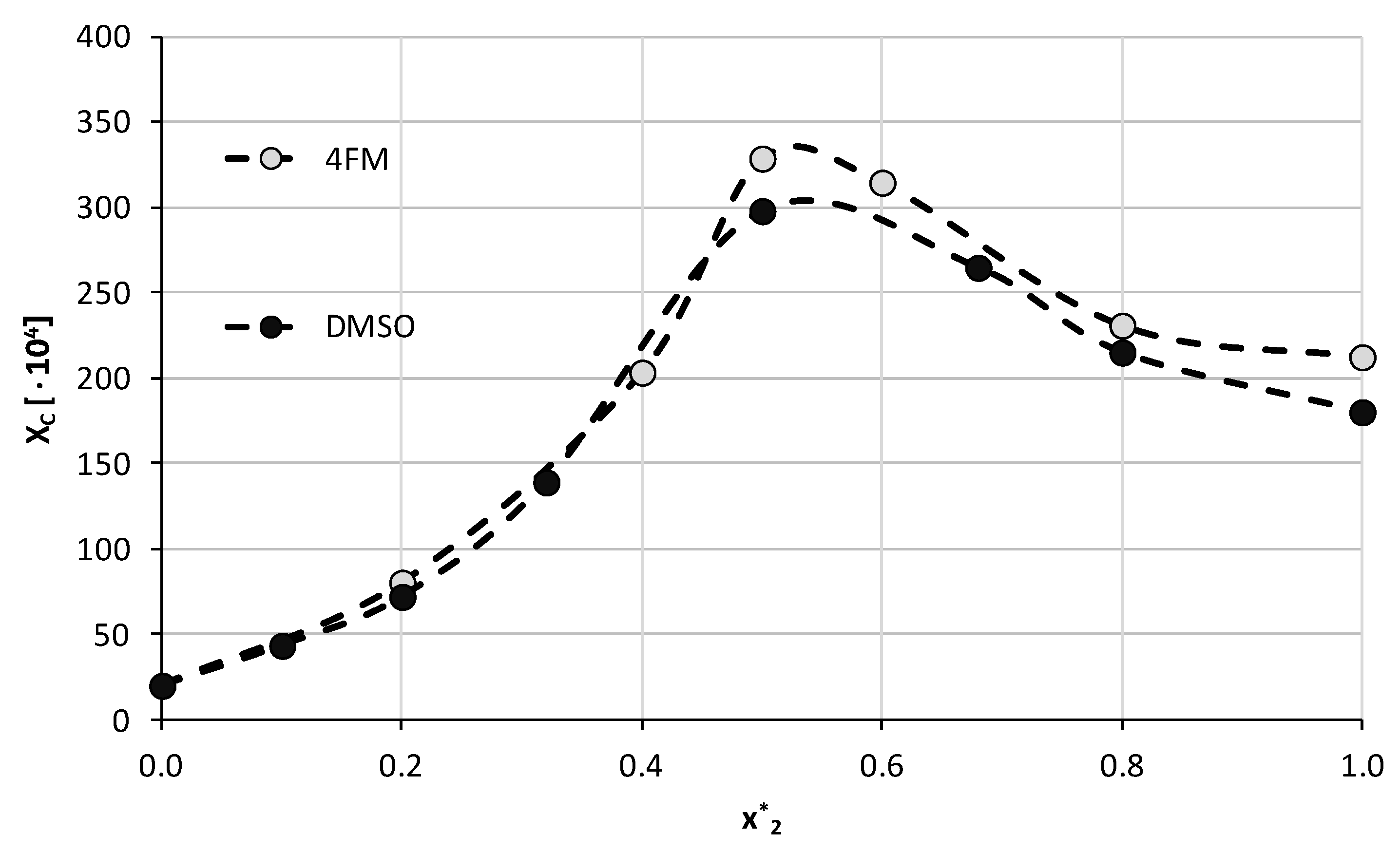
3.2. Experimental Solubility of Caffeine
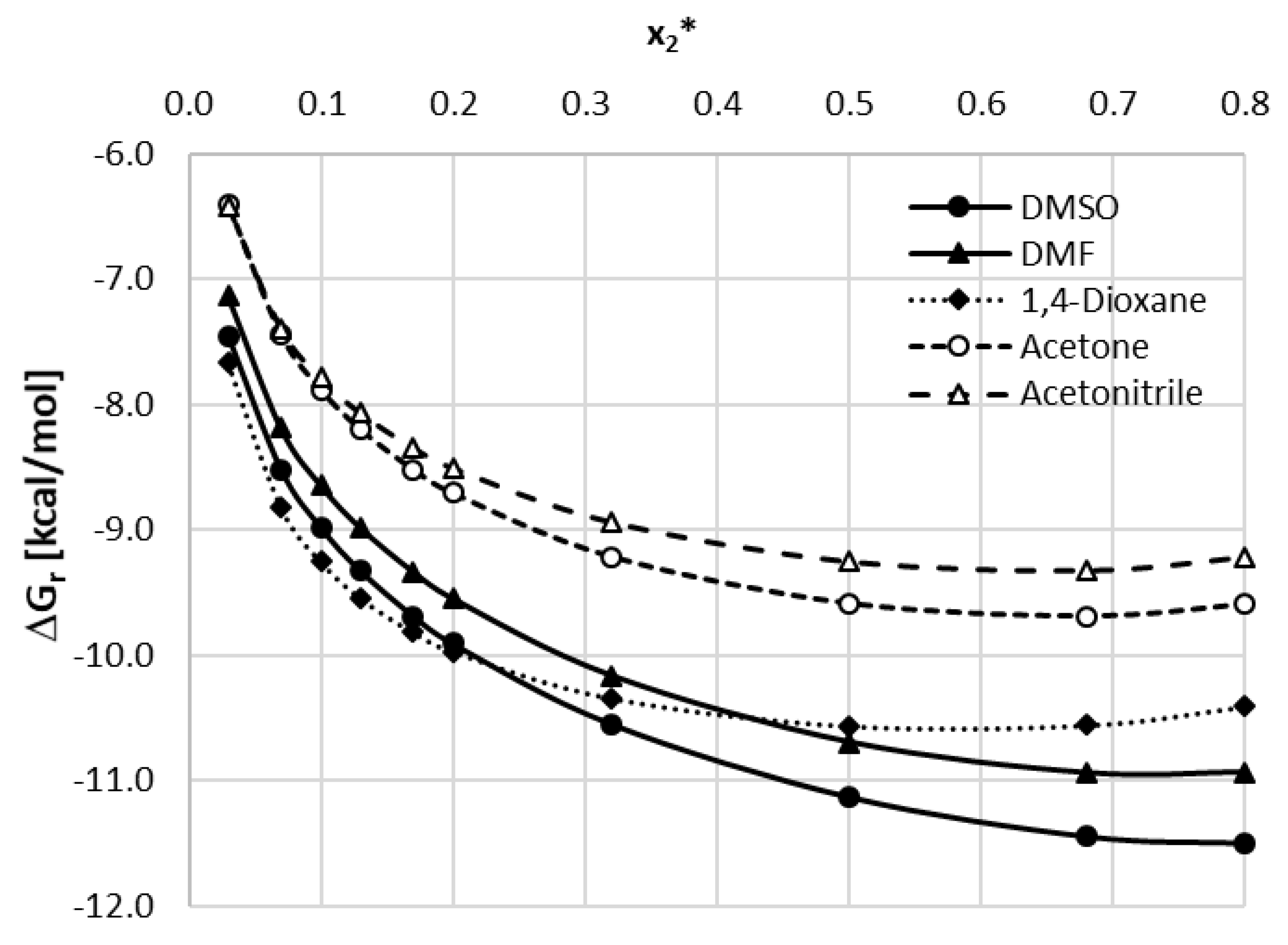
3.3. Caffeine Interactions in Studied Solvents
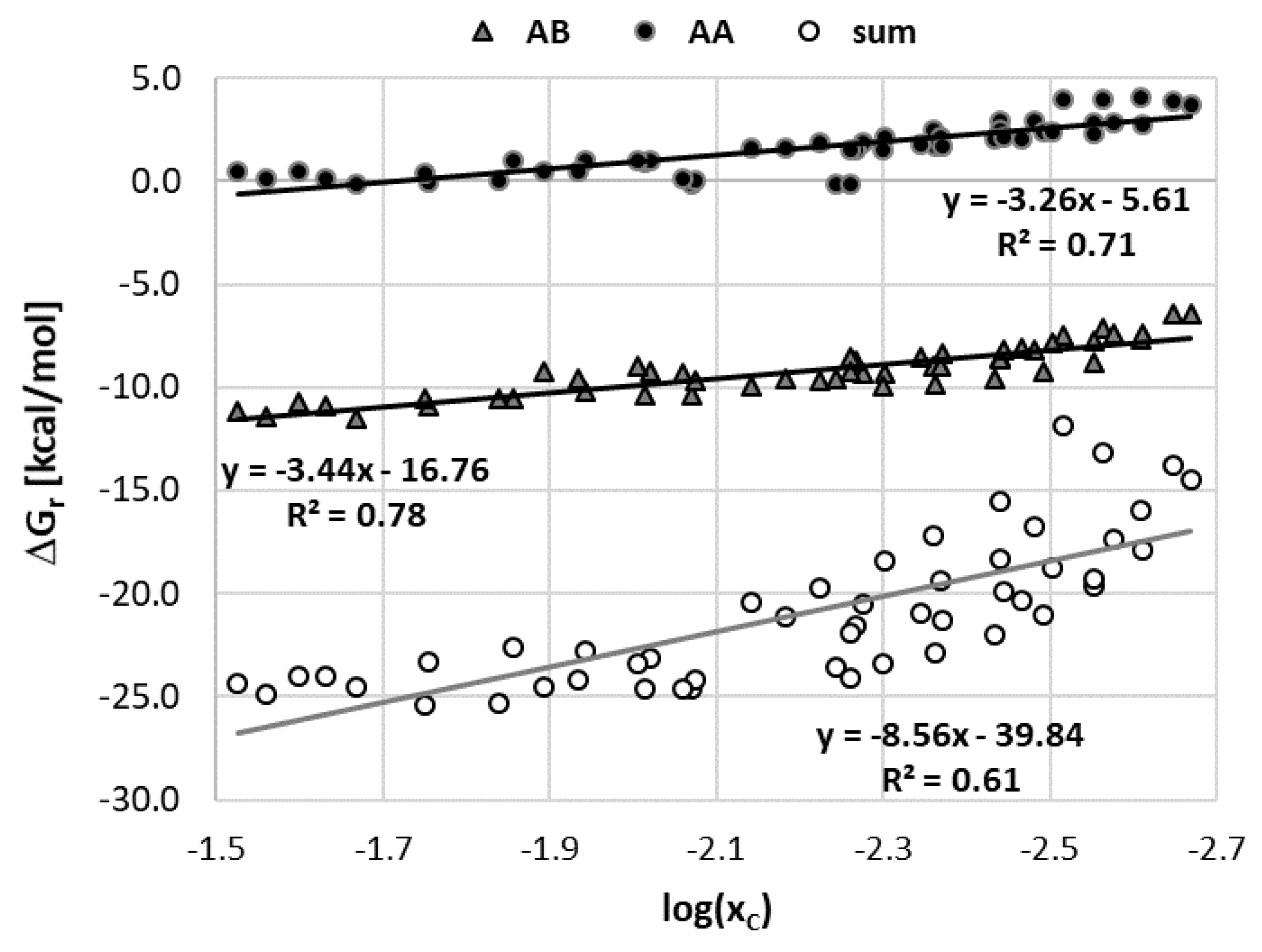
3.4. Solubility Interpretation in Terms of Caffeine Intermolecular Interactions
3.5. Screening of Caffeine Solubility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cysewski, P.; Jeliński, T.; Cymerman, P.; Przybyłek, M. Solvent screening for solubility enhancement of theophylline in neat, binary and ternary NADES solvents: New measurements and ensemble machine learning. Int. J. Mol. Sci. 2021, 22, 7347. [Google Scholar] [CrossRef] [PubMed]
- Jeliński, T.; Stasiak, D.; Kosmalski, T.; Cysewski, P. Experimental and theoretical study on theobromine solubility enhancement in binary aqueous solutions and ternary designed solvents. Pharmaceutics 2021, 13, 1118. [Google Scholar] [CrossRef]
- Dolder, L.K. Methylxanthines: Caffeine, Theobromine, Theophylline. In Small Animal Toxicology, 3rd ed.; Petersen, M.E., Talcott, P.A., Eds.; Saunders: St. Louis, MO, USA, 2013; pp. 647–652. [Google Scholar] [CrossRef]
- Ashihara, H.; Sano, H.; Crozier, A. Caffeine and related purine alkaloids: Biosynthesis, catabolism, function and genetic engineering. Phytochemistry 2008, 69, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Jouyban, A. Handbook of Solubility Data for Pharmaceuticals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Hoekman, D. Exploring QSAR: Volume 2: Hydrophobic, Electronic, and Steric Constants, 1st ed.; American Chemical Society: Washington, WA, USA, 1995. [Google Scholar]
- Andreeva, E.Y.; Dmitrienko, S.G.; Zolotov, Y.A. Methylxanthines: Properties and determination in various objects. Russ. Chem. Rev. 2012, 81, 397–414. [Google Scholar] [CrossRef]
- Craig, C.R.; Stitzel, R.E. Modern Pharmacology with Clinical Applications, 6th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Satoskar, R.S.; Rege, N.; Bhandarkar, S.D. Pharmacology and Pharmacotherapeutics, 24th ed.; Elsevier India: New Delhi, India, 2015. [Google Scholar]
- Essayan, D.M. Cyclic nucleotide phosphodiesterases. J. Allergy Clin. Immunol. 2001, 108, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C.E.; Hide, I.; Daly, J.W.; Rothenhausler, K.; Eger, K. 7-Deaza-2-phenyladenines: Structure-activity relationships of potent A1 selective adenosine receptor antagonists. J. Med. Chem. 1990, 33, 2822–2828. [Google Scholar] [CrossRef] [PubMed]
- Frary, C.D.; Johnson, R.K.; Wang, M.Q. Food sources and intakes of caffeine in the diets of persons in the United States. J. Am. Diet. Assoc. 2005, 105, 110–113. [Google Scholar] [CrossRef]
- Heckman, M.A.; Weil, J.; de Mejia, E.G. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B. Notes on the history of caffeine use. Handb. Exp. Pharmacol. 2011, 200, 1–9. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, J.; Yang, Y.; Yang, X.; Xu, B.; Yang, W.; Tong, T.; Jin, S.; Shen, C.; Rao, H.; et al. Earliest tea as evidence for one branch of the Silk Road across the Tibetan Plateau. Sci. Rep. 2016, 6, 18955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldvogel, S.R. Caffeine—A drug with a surprise. Angew. Chem. Int. Ed. Engl. 2003, 42, 604–605. [Google Scholar] [CrossRef]
- Barone, J.J.; Roberts, H.R. Caffeine consumption. Food Chem. Toxicol. 1996, 34, 119–129. [Google Scholar] [CrossRef]
- Patat, A.; Rosenzweig, P.; Enslen, M.; Trocherie, S.; Miget, N.; Bozon, M.C.; Allain, H.; Gandon, J.M. Effects of a new slow release formulation of caffeine on EEG, psychomotor and cognitive functions in sleep-deprived subjects. Hum. Psychopharmacol. 2000, 15, 153–170. [Google Scholar] [CrossRef]
- Fisone, G.; Borgkvist, A.; Usiello, A. Caffeine as a psychomotor stimulant: Mechanism of action. Cell. Mol. Life Sci. 2004, 61, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.S.; Jetter, A.; Fuhr, U. Assessment of CYP1A2 activity in clinical practice: Why, how, and when? Basic Clin. Pharmacol. Toxicol. 2005, 97, 125–134. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Del Coso, J.; Salinero, J.J.; Lara, B. Effects of caffeine and coffee on human functioning. Nutrients 2020, 12, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlAmmar, W.A.; Albeesh, F.H.; Khattab, R.Y. Food and mood: The corresponsive effect. Curr. Nutr. Rep. 2020, 9, 296–308. [Google Scholar] [CrossRef]
- Drake, C.; Roehrs, T.; Shambroom, J.; Roth, T. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J. Clin. Sleep Med. 2013, 9, 1195–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagarde, D.; Batejat, D.; Sicard, B.; Trocherie, S.; Chassard, D.; Enslen, M.; Chauffard, F. Slow-release caffeine: A new response to the effects of a limited sleep deprivation. Sleep 2000, 23, 651–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The safety of ingested caffeine: A comprehensive review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Göke, K.; Lorenz, T.; Repanas, A.; Schneider, F.; Steiner, D.; Baumann, K.; Bunjes, H.; Dietzel, A.; Finke, J.H.; Glasmacher, B.; et al. Novel strategies for the formulation and processing of poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2018, 126, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Ramachandran, C.; Bermejo, M.; Yamashita, S.; Yu, L.X.; Amidon, G.L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol. Pharm. 2006, 3, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.S.; Dulin, W. A biopharmaceutical classification-based Right-First-Time formulation approach to reduce human pharmacokinetic variability and project cycle time from First-In-Human to clinical Proof-Of-Concept. Pharm. Dev. Technol. 2012, 17, 285–302. [Google Scholar] [CrossRef]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tong, W.-Q. Impact of solid state properties on developability assessment of drug candidates. Adv. Drug Deliv. Rev. 2004, 56, 321–334. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Van den Mooter, G.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75. [Google Scholar] [CrossRef]
- Scholz, A.; Abrahamsson, B.; Diebold, S.M.; Kostewicz, E.; Polentarutti, B.I.; Ungell, A.-L.; Dressman, J.B. Influence of hydrodynamics and particle size on the absorption of felodipine in labradors. Pharm. Res. 2002, 19, 42–46. [Google Scholar] [CrossRef]
- Janssens, S.; Van den Mooter, G. Review: Physical chemistry of solid dispersions. J. Pharm. Pharmacol. 2009, 61, 1571–1586. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Rajewski, R.A.; Stella, V.J. Pharmaceutical applications of cyclodextrins. 2. in vivo drug delivery. J. Pharm. Sci. 1996, 85, 1142–1169. [Google Scholar] [CrossRef]
- Korn, C.; Balbach, S. Compound selection for development—Is salt formation the ultimate answer? Experiences with an extended concept of the “100mg approach. Eur. J. Pharm. Sci. 2014, 57, 257–263. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef]
- Chadha, R.; Bhalla, Y.; Vashisht, M.K.; Chadha, K. Cocrystallization in nutraceuticals. In Recrystallization in Materials Processing, 1st ed.; Glebovsky, V., Ed.; InTech: London, UK, 2015. [Google Scholar] [CrossRef]
- Vishweshwar, P.; McMahon, J.A.; Bis, J.A.; Zaworotko, M.J. Pharmaceutical co-crystals. J. Pharm. Sci. 2006, 95, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.E.; Mazo, R.M. On the theory of solute solubility in mixed solvents. J. Phys. Chem. B 2008, 112, 7875–7884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubino, J.T. Cosolvents and Cosolvency. In Encyclopedia of Pharmaceutical Science and Technology, 4th ed.; Swarbrick, J., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 711–722. [Google Scholar] [CrossRef]
- Jouyban, A. Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures. J. Pharm. Pharm. Sci. 2008, 11, 32–58. [Google Scholar] [CrossRef] [PubMed]
- Seedher, N.; Kanojia, M. Co-solvent solubilization of some poorly-soluble antidiabetic drugs. Pharm. Dev. Technol. 2009, 14, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Paruta, A.N.; Sciarrone, B.J.; Lordi, N.G. Solubility of salicylic acid as a function of dielectric constant. J. Pharm. Sci. 1964, 53, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Fedors, R.F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Millard, J.W.; Alvarez-Núñez, F.A.; Yalkowsky, S.H. Solubilization by cosolvents: Establishing useful constants for the log-linear model. Int. J. Pharm. 2002, 245, 153–166. [Google Scholar] [CrossRef]
- Jain, N.; Yang, G.; Tabibi, S.E.; Yalkowsky, S.H. Solubilization of NSC-639829. Int. J. Pharm. 2001, 225, 41–47. [Google Scholar] [CrossRef]
- Kawakami, K.; Oda, N.; Miyoshi, K.; Funaki, T.; Ida, Y. Solubilization behavior of a poorly soluble drug under combined use of surfactants and cosolvents. Eur. J. Pharm. Sci. 2006, 28, 7–14. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, G.; Ciccarelli, D.; Ortona, O. Effect of glycerol on micelle formation by ionic and nonionic surfactants at 25 degrees C. J. Colloid Interface Sci. 2005, 286, 747–754. [Google Scholar] [CrossRef]
- He, Y.; Li, P.; Yalkowsky, S.H. Solubilization of Fluasterone in cosolvent/cyclodextrin combinations. Int. J. Pharm. 2003, 264, 25–34. [Google Scholar] [CrossRef]
- Miyake, K.; Irie, T.; Arima, H.; Hirayama, F.; Uekama, K.; Hirano, M.; Okamoto, Y. Characterization of itraconazole/2-hydroxypropyl-β-cyclodextrin inclusion complex in aqueous propylene glycol solution. Int. J. Pharm. 1999, 179, 237–245. [Google Scholar] [CrossRef]
- Zhong, J.; Tang, N.; Asadzadeh, B.; Yan, W. Measurement and correlation of solubility of theobromine, theophylline, and caffeine in water and organic solvents at various temperatures. J. Chem. Eng. Data 2017, 62, 2570–2577. [Google Scholar] [CrossRef]
- Shalmashi, A.; Golmohammad, F. Solubility of caffeine in water, ethyl acetate, ethanol, carbon tetrachloride, methanol, chloroform, dichloromethane, and acetone between 298 and 323 K. Lat. Am. Appl. Res. 2010, 40, 283–285. [Google Scholar]
- Dabir, T.O.; Gaikar, V.G.; Jayaraman, S.; Mukherjee, S. Thermodynamic modeling studies of aqueous solubility of caffeine, gallic acid and their cocrystal in the temperature range of 303 K–363 K. Fluid Phase Equilib. 2018, 456, 65–76. [Google Scholar] [CrossRef]
- Rezaei, H.; Rahimpour, E.; Ghafourian, T.; Martinez, F.; Barzegar-Jalali, M.; Jouyban, A. Solubility of caffeine in N-methyl-2-pyrrolidone + ethanol mixture at different temperatures. J. Mol. Liq. 2020, 300, 112354. [Google Scholar] [CrossRef]
- Rezaei, H.; Rahimpour, E.; Martinez, F.; Zhao, H.; Jouyban, A. Study and mathematical modeling of caffeine solubility in N-methyl-2-pyrrolidone + ethylene glycol mixture at different temperatures. J. Mol. Liq. 2021, 341, 117350. [Google Scholar] [CrossRef]
- Golubev, V.A.; Gurina, D.L. Dissolving power of the binary solvent carbon tetrachloride—Methanol. Solubility of caffeine: Experiment, ASL model, and MD simulation. J. Mol. Liq. 2021, 344, 117736. [Google Scholar] [CrossRef]
- Bustamante, P.; Navarro, J.; Romero, S.; Escalera, B. Thermodynamic origin of the solubility profile of drugs showing one or two maxima against the polarity of aqueous and nonaqueous mixtures: Niflumic acid and caffeine. J. Pharm. Sci. 2002, 91, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, Z.J.; Jiménez, D.M.; Almanza, O.A.; Jouyban, A.; Martínez, F.; Acree, W.E. Solubility and preferential solvation of caffeine and theophylline in {methanol + water} mixtures at 298.15 K. J. Solut. Chem. 2017, 46, 1605–1624. [Google Scholar] [CrossRef]
- Adjei, A.; Newburger, J.; Martin, A. Extended hildebrand approach: Solubility of caffeine in dioxane–water mixtures. J. Pharm. Sci. 1980, 69, 659–661. [Google Scholar] [CrossRef]
- Rezaei, H.; Rahimpour, E.; Martinez, F.; Jouyban, A. Solubility of caffeine in carbitol + ethanol mixture at different temperatures. J. Mol. Liq. 2020, 301, 112465. [Google Scholar] [CrossRef]
- Rezaei, H.; Jouyban, A.; Zhao, H.; Martinez, F.; Rahimpour, E. Solubility of caffeine in N-methyl-2-pyrrolidone + 1-propanol mixtures at different temperatures. J. Mol. Liq. 2022, 346, 117067. [Google Scholar] [CrossRef]
- Rezaei, H.; Rahimpour, E.; Zhao, H.; Martinez, F.; Jouyban, A. Solubility measurement and thermodynamic modeling of caffeine in N-methyl-2-pyrrolidone + isopropanol mixtures at different temperatures. J. Mol. Liq. 2021, 336, 116519. [Google Scholar] [CrossRef]
- Rezaei, H.; Rahimpour, E.; Zhao, H.; Martinez, F.; Jouyban, A. Determination and modeling of caffeine solubility in N-methyl-2-pyrrolidone + propylene glycol mixtures. J. Mol. Liq. 2021, 343, 117613. [Google Scholar] [CrossRef]
- Lentz, H.; Gehrig, M.; Schulmeyer, J. Dynamic solubility measurements of caffeine in carbon dioxide and in carbon dioxide saturated with water. Phys. B+C 1986, 139–140, 70–72. [Google Scholar] [CrossRef]
- Johannsen, M.; Brunner, G. Solubilities of the xanthines caffeine, theophylline and theobromine in supercritical carbon dioxide. Fluid Phase Equilib. 1994, 95, 215–226. [Google Scholar] [CrossRef]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Canongia Lopes, J.N.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.M.; Gomes, H.M.D.; Coutinho, J.A.P.; Freire, M.G. Valorization of spent coffee by caffeine extraction using aqueous solutions of cholinium-based ionic liquids. Sustainability 2021, 13, 7509. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Ferreira, A.M.; Freire, M.G.; Coutinho, J.A.P. Enhanced extraction of caffeine from guaraná seeds using aqueous solutions of ionic liquids. Green Chem. 2013, 15, 2002–2010. [Google Scholar] [CrossRef]
- Cai, C.; Li, F.; Liu, L.; Tan, Z. Deep eutectic solvents used as the green media for the efficient extraction of caffeine from Chinese dark tea. Sep. Purif. Technol. 2019, 227, 115723. [Google Scholar] [CrossRef]
- Basaiahgari, A.; Priyanka, V.P.; Ijardar, S.P.; Gardas, R.L. Aqueous biphasic systems of amino acid-based ionic liquids: Evaluation of phase behavior and extraction capability for caffeine. Fluid Phase Equilib. 2020, 506, 112373. [Google Scholar] [CrossRef]
- Yazdabadi, A.; Shahriari, S.; Salehifar, M. Extraction of caffeine using aqueous two-phase systems containing ionic liquid and sorbitol. Fluid Phase Equilib. 2019, 502, 112287. [Google Scholar] [CrossRef]
- Yu, B.; Ren, H.; Piao, X. Towards adsorptive enrichment of flavonoids from honey using h-BN monolayer. Chem. Phys. Chem. 2022, 23, e202100828. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P. Intermolecular interaction as a direct measure of water solubility advantage of meloxicam cocrystalized with carboxylic acids. J. Mol. Model. 2018, 24, 112. [Google Scholar] [CrossRef] [Green Version]
- Cysewski, P.; Jeliński, T. Optimization, thermodynamic characteristics and solubility predictions of natural deep eutectic solvents used for sulfonamide dissolution. Int. J. Pharm. 2019, 570, 118682. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Przybyłek, M.; Rozalski, R. Experimental and theoretical screening for green solvents improving sulfamethizole solubility. Materials 2021, 14, 5915. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Walczak, P.; Ziółkowska, D.; Grela, I.; Przybyłek, M. Experimental and theoretical studies on the Sulfamethazine-Urea and Sulfamethizole-Urea solid-liquid equilibria. J. Drug Deliv. Sci. Technol. 2021, 61, 102186. [Google Scholar] [CrossRef]
- COSMOtherm, version 21.0.0; Dassault Systèmes; Biovia: San Diego, CA, USA, 2020.
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- TURBOMOLE, version 7.5.1; TURBOMOLE GmbH: Frankfurt, Germany, 2020.
- TmoleX, version 21.0.1; Dassault Systèmes; Biovia: San Diego, CA, USA, 2020.
- Hellweg, A.; Eckert, F. Brick by brick computation of the gibbs free energy of reaction in solution using quantum chemistry and COSMO-RS. AIChE J. 2017, 63, 3944–3954. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Witte, J.; Neaton, J.B.; Head-Gordon, M. Effective empirical corrections for basis set superposition error in the def2-SVPD basis: gCP and DFT-C. J. Chem. Phys. 2017, 146, 234105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, K.L.; Beal, G.D. Notes on the water content of crystalline caffeine. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1946, 35, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Bogardus, J.B. Crystalline anhydrous-hydrate phase changes of caffeine and theophylline in solvent-water mixtures. J. Pharm. Sci. 1983, 72, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Pirttimäki, J.; Laine, E. The transformation of anhydrate and hydrate forms of caffeine at 100% RH and 0% RH. Eur. J. Pharm. Sci. 1994, 1, 203–208. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Lawson, E.; Matas, M.d.; Shields, L.; York, P. Metamorphosis of caffeine hydrate and anhydrous caffeine. J. Chem. Soc. Perkin Trans. 2 1997, 1985–1990. [Google Scholar] [CrossRef]
- Dong, J.X.; Li, Q.; Tan, Z.C.; Zhang, Z.H.; Liu, Y. The standard molar enthalpy of formation, molar heat capacities, and thermal stability of anhydrous caffeine. J. Chem. Thermodyn. 2007, 1, 108–114. [Google Scholar] [CrossRef]
- Dichi, E.; Legendre, B.; Sghaier, M. Physico-chemical characterisation of a new polymorph of caffeine. J. Therm. Anal. Calorim. 2014, 115, 1551–1561. [Google Scholar] [CrossRef]
- González-González, J.S.; Zúñiga-Lemus, O.; Hernández-Galindo, M.d.C. Hydrated solid forms of theophylline and caffeine obtained by mechanochemistry. IOSR J. Pharm. 2017, 7, 28–30. [Google Scholar] [CrossRef]
- Harten, P. Paris III—Epa’s Solvent Substitution Software Tool (Program for Assisting the Replacements of Industrial Solvents); EPA Office of Research and Development: Washington, DC, USA, 2015; EPA/600/F-14/408.
- Harten, P.; Martin, T.; Gonzalez, M.; Young, D. The software tool to find greener solvent replacements, PARIS III. Environ. Prog. Sustain. Energy 2020, 39, 13331. [Google Scholar] [CrossRef] [PubMed]
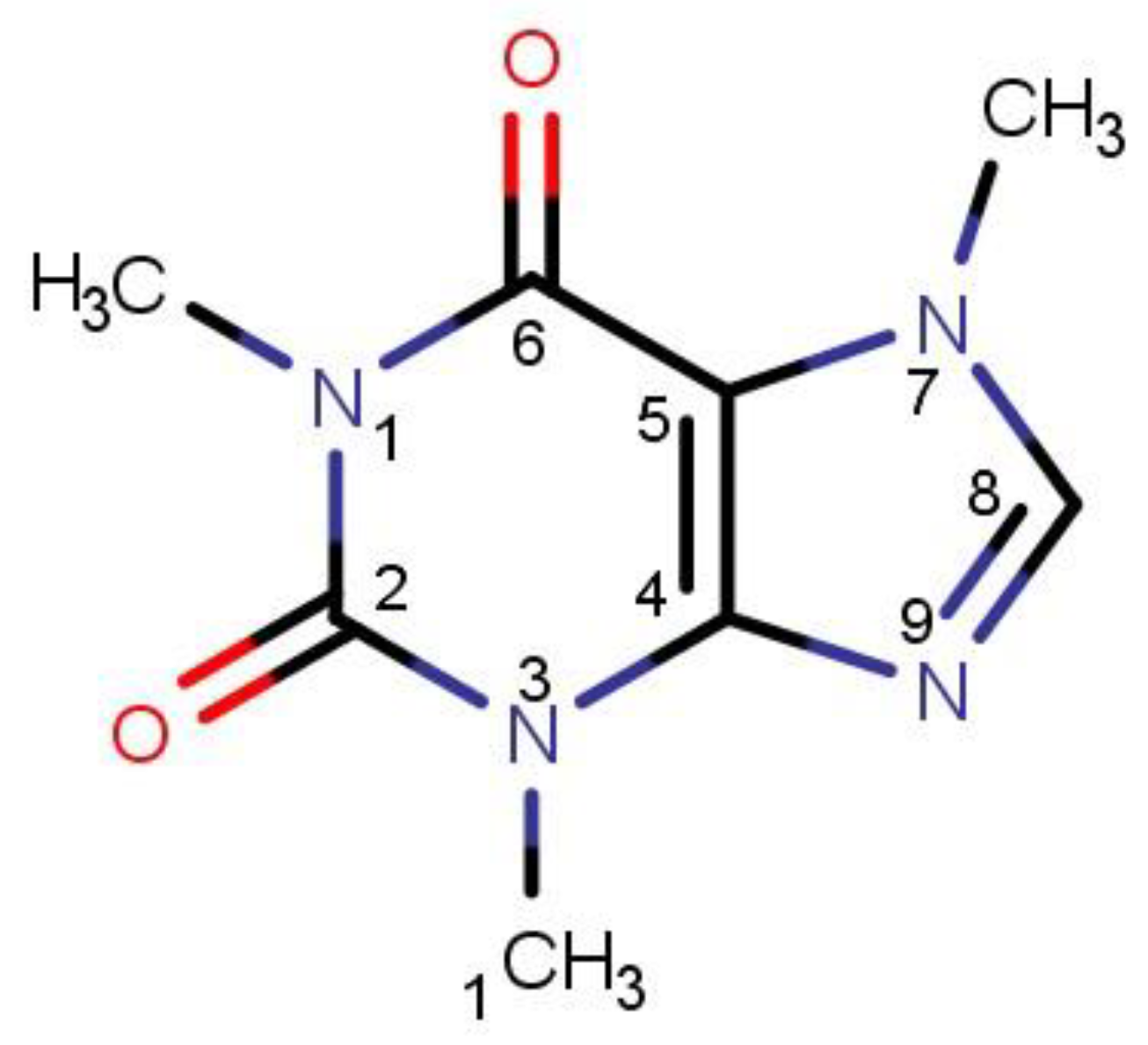
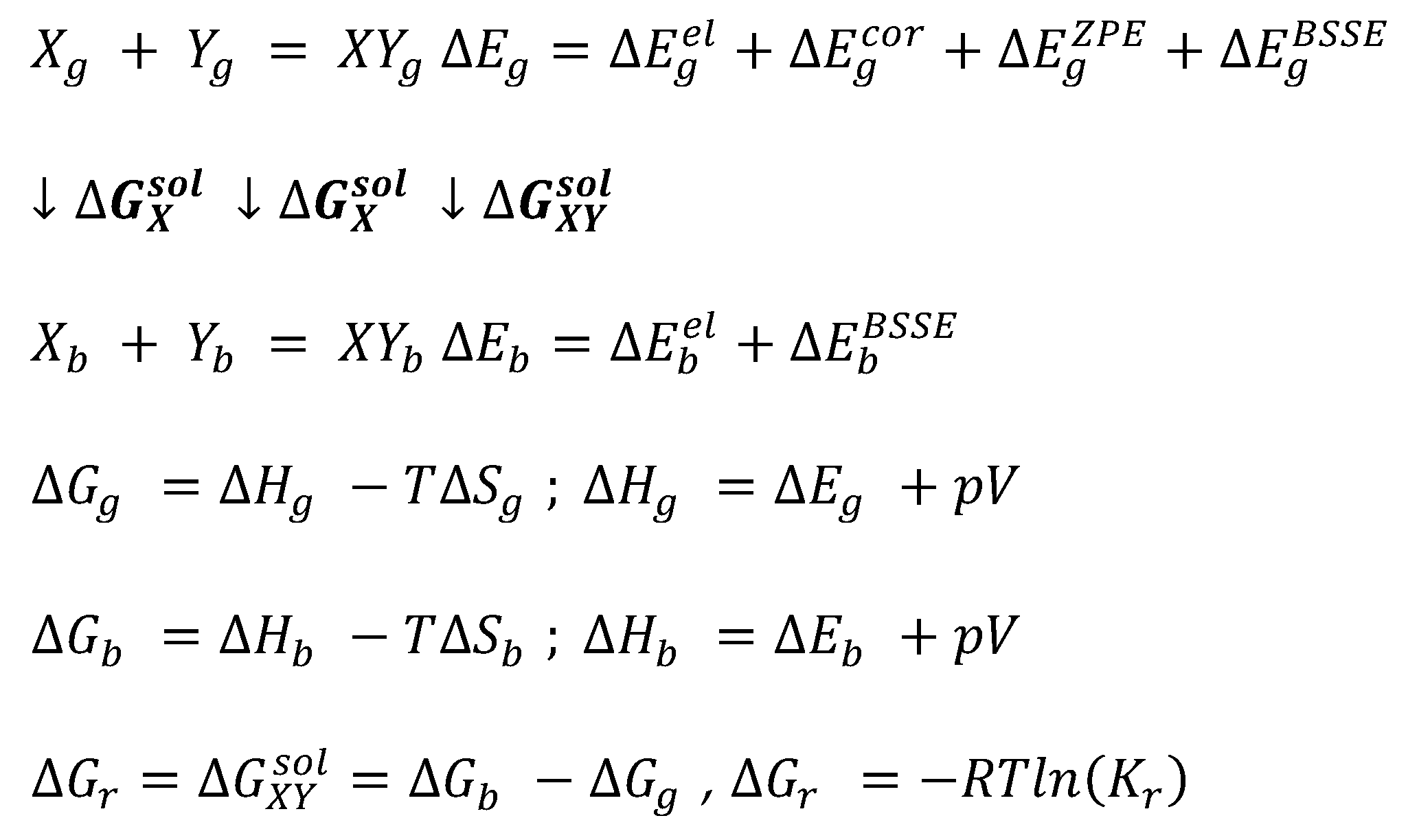
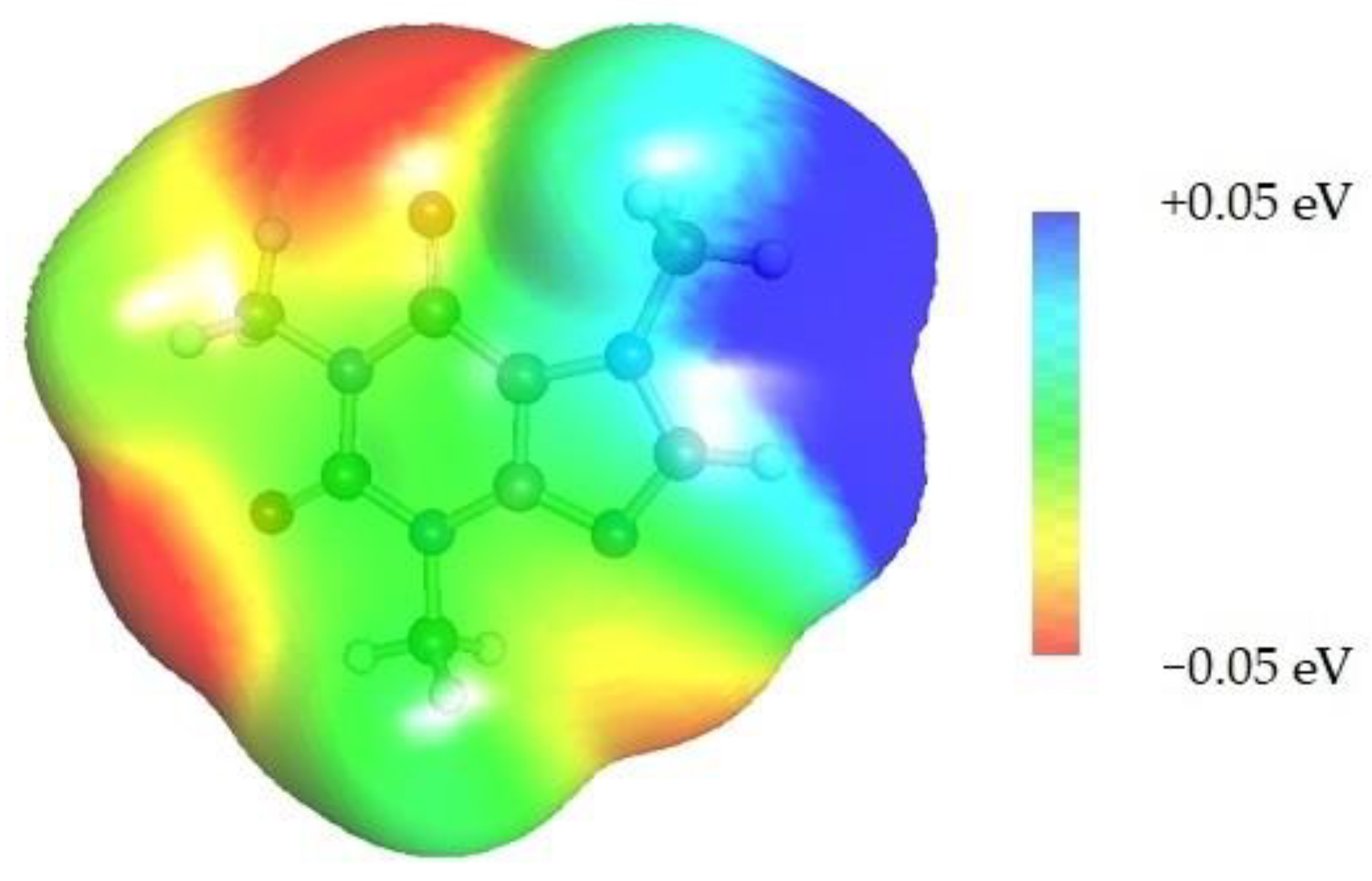
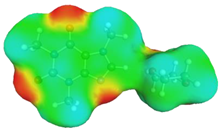
| caffeine–DMF | caffeine–DMSO | ||
 dH⋯O’ = 2.149 Å, αC-H⋯O’ = 176.3° ΔGr(AB) = −5.7 kcal/mol |  dH⋯O’= 2.105 Å, αC-H⋯O’= 179.1° ΔGr(AB) = −6.5 kcal/mol |  | +0.01e2/Å2 −0.01e2/Å2 |
| caffeine–1,4-dioxane | caffeine–acetone | ||
 dH⋯O’ = 2.192 Å, αC-H⋯O’ = 176.5° ΔGr(AB) = −5.0 kcal/mol | 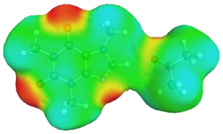 dH⋯O’ = 2.283 Å, αC-H⋯O’ = 175.5° ΔGr(AB) = −4.2 kcal/mol | ||
| caffeine–acetonitrile | caffeine–water | ||
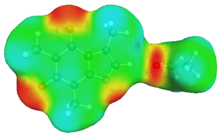 dH⋯O’ = 2.388 Å, αC-H⋯O’ = 175.5° ΔGr(AB) = −3.5 kcal/mol |  dN⋯H’ = 1.852 Å, αC-H⋯O’ = 177.3° ΔGr(AB) = −6.4 kcal/mol | ||
| Solvent | CAS | EI | EI(PCOP=0) | TB [K] |
|---|---|---|---|---|
| DMSO | [67-68-5] | 11.68 | 0.26 | 462 |
| DMF | [68-12-2] | 2.16 | 2.16 | 426 |
| 1,4-dioxane | [123-91-1] | 5.26 | 0.97 | 375 |
| acetone | [67-64-1] | 1.27 | 0.66 | 329 |
| acetonitrile | [75-05-8] | 1.88 | 1.88 | 355 |
| morpholine | [110-91-8] | 2.95 | 2.95 | 401 |
| 4-formylmorpholine | [4394-85-8] | 0.51 | 0.51 | 512 |
| 4-methylmorpholine | [109-02-4] | 1.93 | 1.93 | 389 |
| 4-ethylmorpholine | [100-74-3] | 2.12 | 2.12 | 412 |
| caffeine–morpholine ΔGr(AB) = −5.2 kcal/mol | |||
 dH⋯N’ = 2.227 Å, αC-H⋯N’ = 179.2° |  dH⋯O’ = 2.165 Å, αC-H⋯O’ = 178.7° |  | +0.01e2/Å2 −0.01e2/Å2 |
| caffeine–4-formylmorpholine ΔGr(AB) = −5.8 kcal/mol | |||
 dH⋯O’ = 2.159 Å, αC-H⋯O’ = 177.9° | 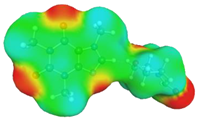 dH⋯O’ = 2.226 Å, αC-H⋯O’ = 173.9° | ||
| caffeine–4-methylmorpholine ΔGr(AB) = −6.7 kcal/mol | |||
 dH⋯N’ = 2.313 Å, αC-H⋯N’ = 172.8° |  dH⋯O’ = 2.221 Å, αC-H⋯O’ = 178.5° | ||
| caffeine–4-ethylmorpholine ΔGr(AB) = −6.8 kcal/mol | |||
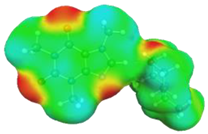 dH⋯O’ = 2.204 Å, αC-H⋯O’ = 175.5° |  dH⋯N’ = 2.358 Å, αC-H⋯N’ = 171.3° | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeliński, T.; Kubsik, M.; Cysewski, P. Application of the Solute–Solvent Intermolecular Interactions as Indicator of Caffeine Solubility in Aqueous Binary Aprotic and Proton Acceptor Solvents: Measurements and Quantum Chemistry Computations. Materials 2022, 15, 2472. https://doi.org/10.3390/ma15072472
Jeliński T, Kubsik M, Cysewski P. Application of the Solute–Solvent Intermolecular Interactions as Indicator of Caffeine Solubility in Aqueous Binary Aprotic and Proton Acceptor Solvents: Measurements and Quantum Chemistry Computations. Materials. 2022; 15(7):2472. https://doi.org/10.3390/ma15072472
Chicago/Turabian StyleJeliński, Tomasz, Maciej Kubsik, and Piotr Cysewski. 2022. "Application of the Solute–Solvent Intermolecular Interactions as Indicator of Caffeine Solubility in Aqueous Binary Aprotic and Proton Acceptor Solvents: Measurements and Quantum Chemistry Computations" Materials 15, no. 7: 2472. https://doi.org/10.3390/ma15072472
APA StyleJeliński, T., Kubsik, M., & Cysewski, P. (2022). Application of the Solute–Solvent Intermolecular Interactions as Indicator of Caffeine Solubility in Aqueous Binary Aprotic and Proton Acceptor Solvents: Measurements and Quantum Chemistry Computations. Materials, 15(7), 2472. https://doi.org/10.3390/ma15072472








