Bacterial Polyglucuronic Acid/Alginate/Carbon Nanofibers Hydrogel Nanocomposite as a Potential Scaffold for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polyglucuronic Acid (PGU) Production
2.3. Preparation of Carbon Nanofiber (CNF)
2.4. CNFs Characterization
2.5. Production of Alg/PGU/CNFs Hydrogels
2.6. Hydrogel Nanocomposite Characterization
2.7. In Vitro Assessments
2.7.1. Hemolysis Induction Assay
2.7.2. Cell Viability
2.8. Statistical Analysis
3. Results
3.1. PAN and CNFs Characteristics
3.2. Hydrogel Nanocomposite Characteristics
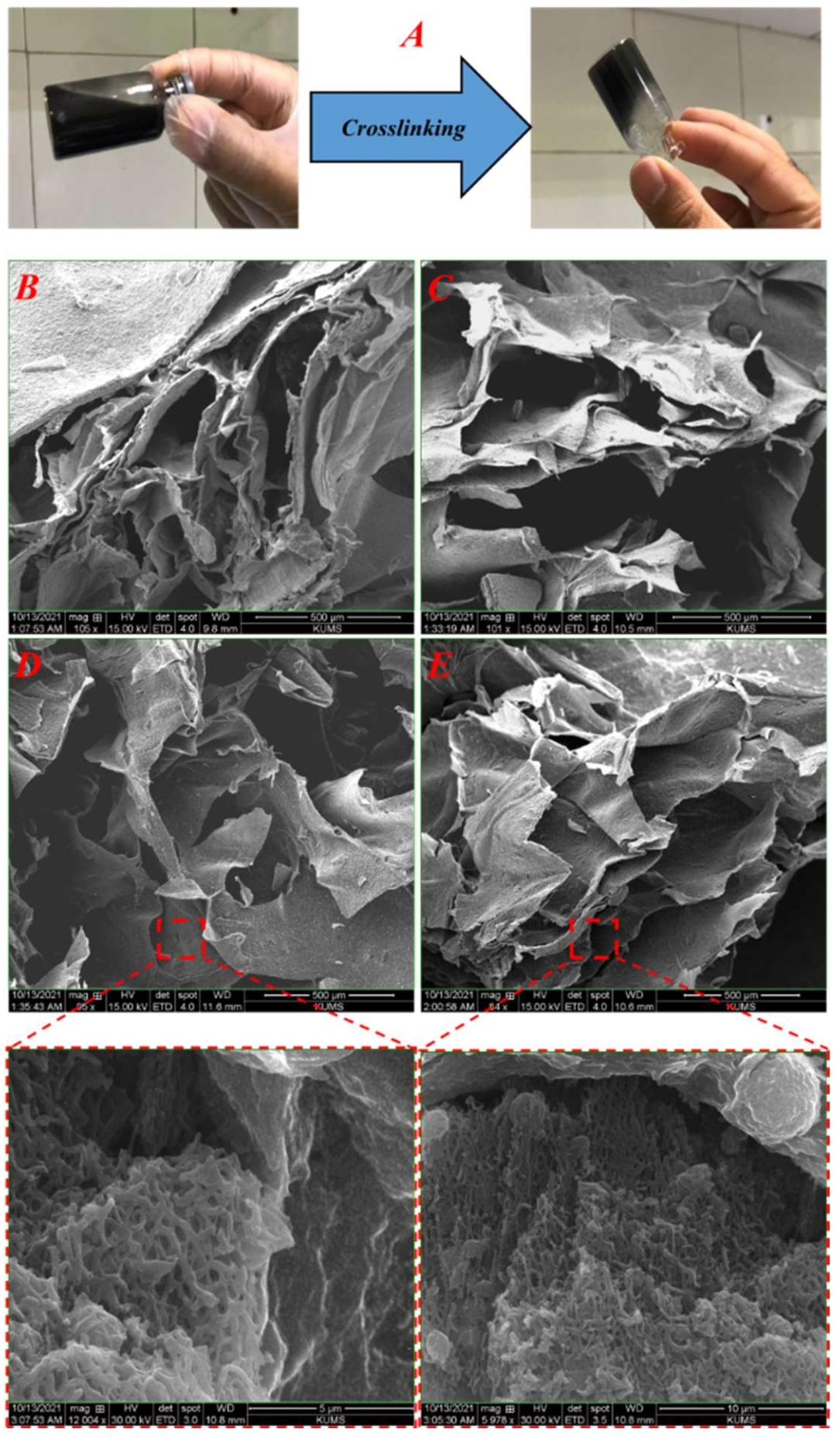
3.2.1. Internal Structure and Morphology
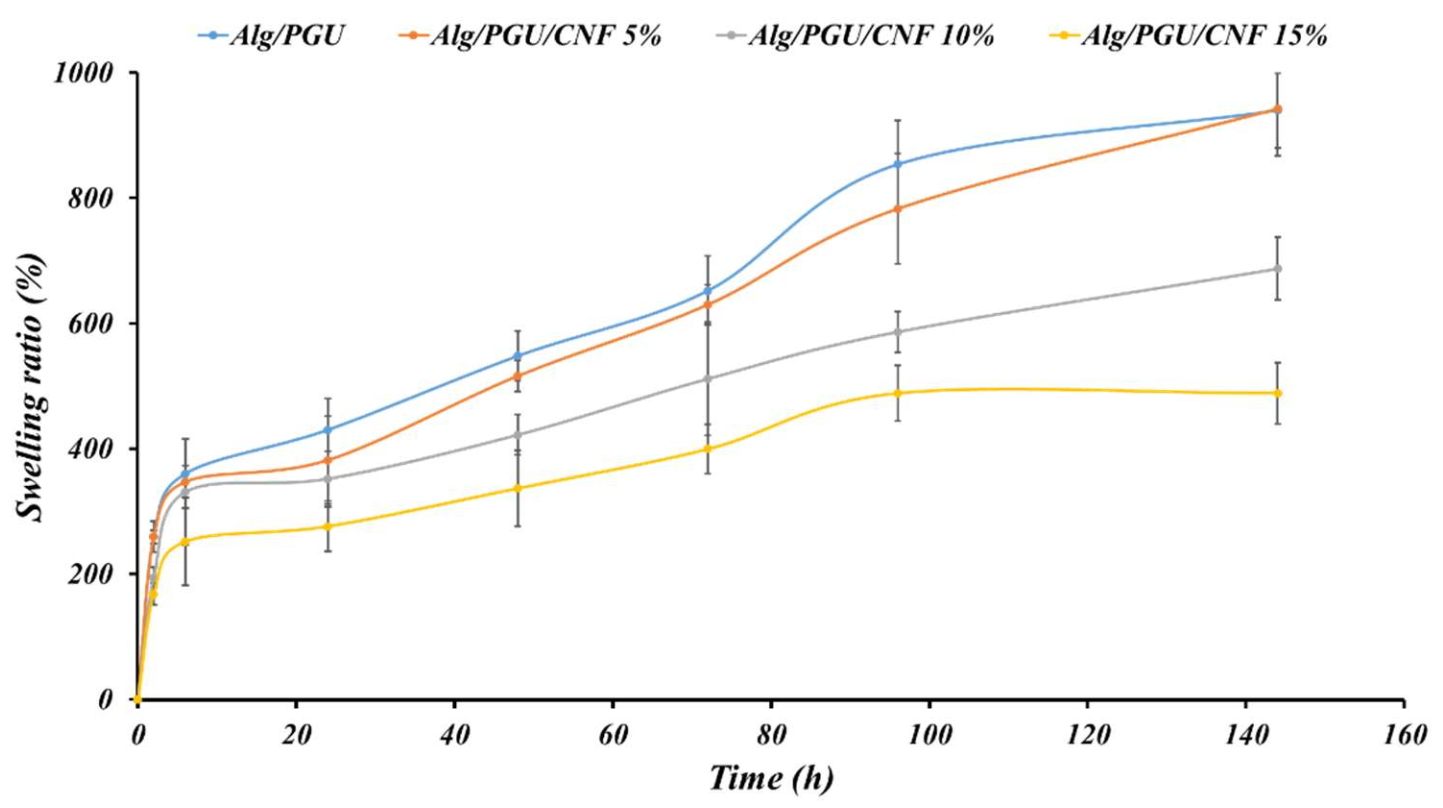
3.2.2. Swelling Kinetics
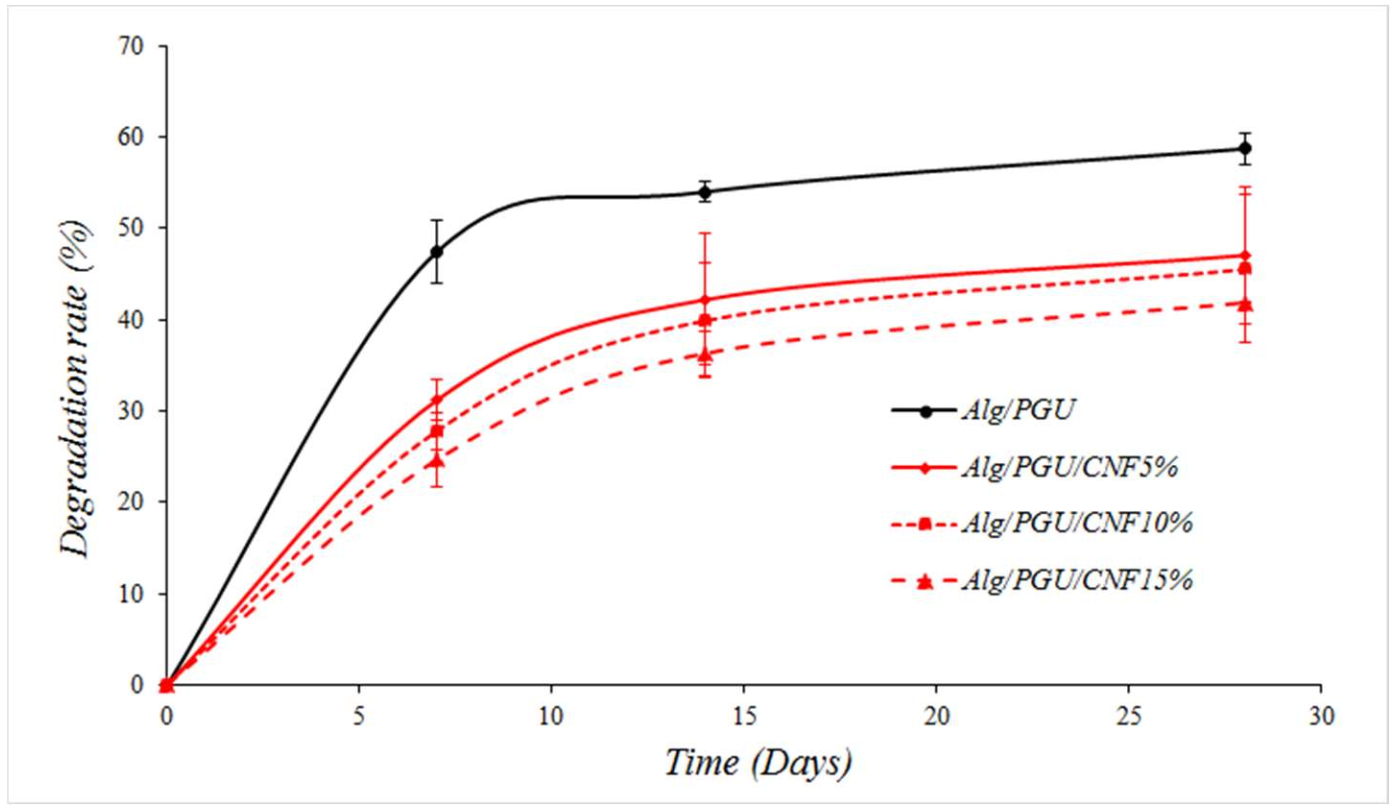
3.2.3. Degradation Results
3.3. In Vitro Assessments Results
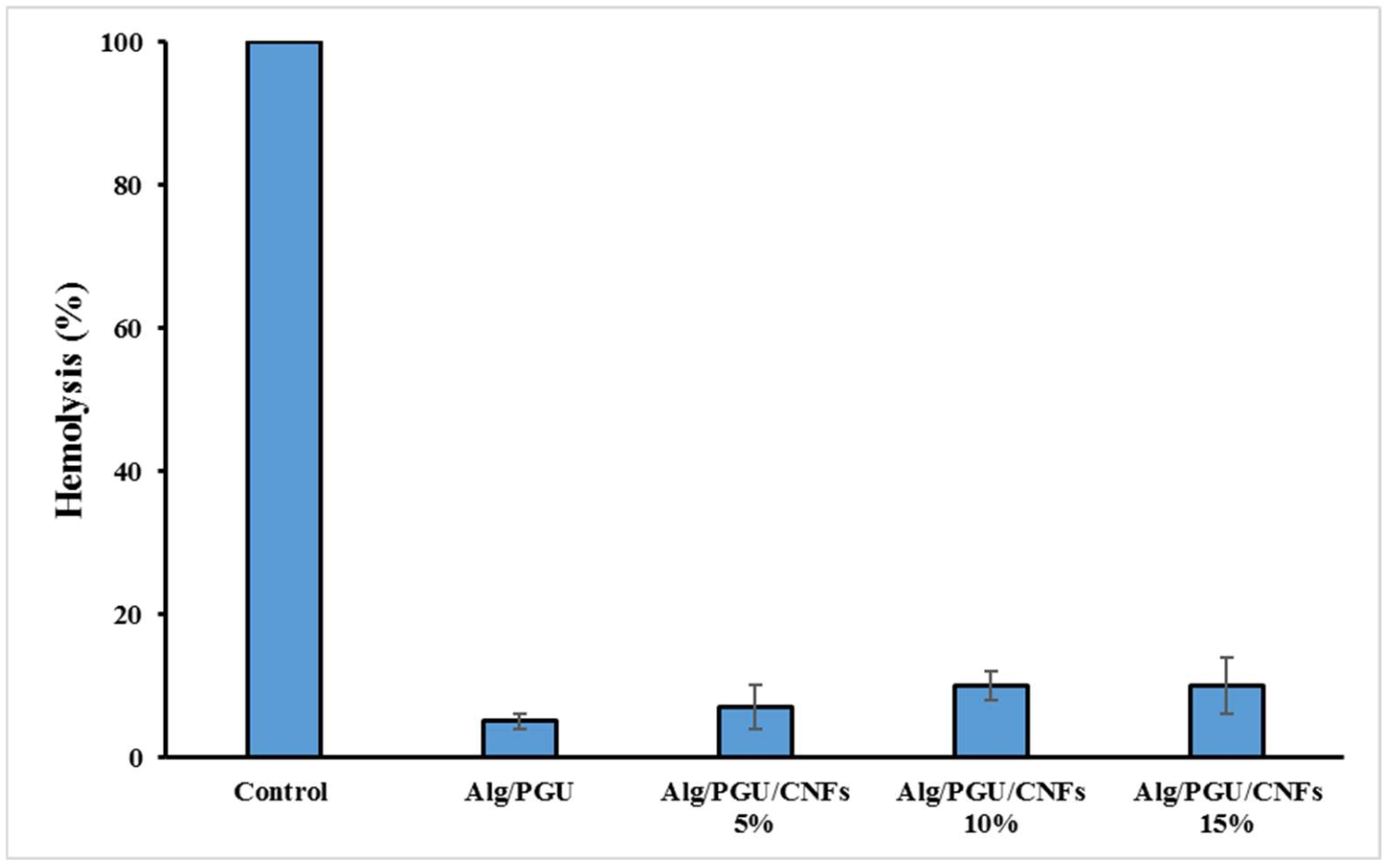
3.3.1. Hemocompatibility
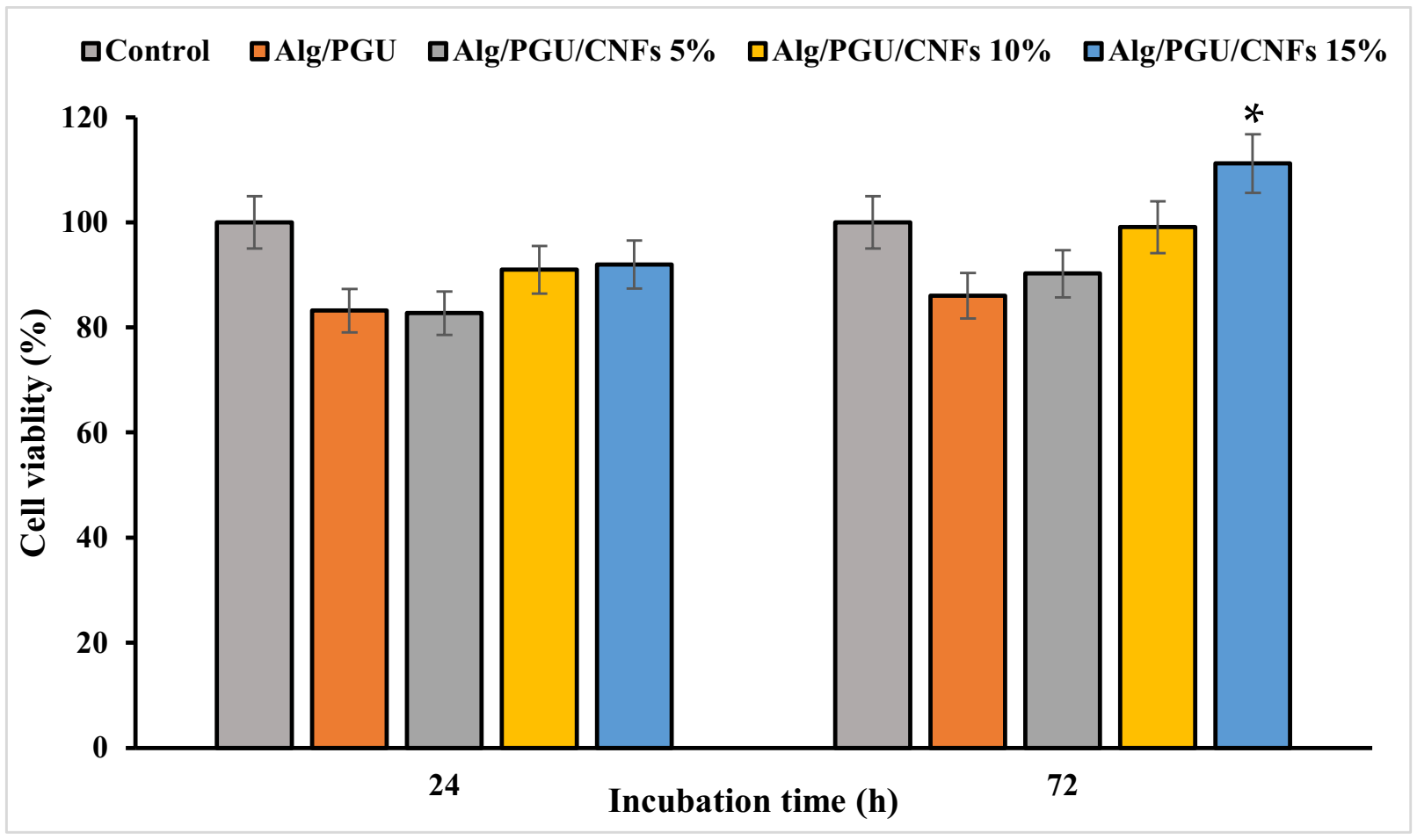
3.3.2. Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M.W. Allograft and alloplastic bone substitutes: A review of science and technology for the craniomaxillofacial surgeon. J. Craniofacial Surg. 2005, 16, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Schlickewei, W.; Schlickewei, C. The use of bone substitutes in the treatment of bone defects—The clinical view and history. Macromol. Symp. 2007, 253, 10–23. [Google Scholar] [CrossRef]
- Griffin, K.S.; Davis, K.M.; McKinley, T.O.; Anglen, J.O.; Chu, T.-M.G.; Boerckel, J.D.; Kacena, M.A. Evolution of bone grafting: Bone grafts and tissue engineering strategies for vascularized bone regeneration. Clin. Rev. Bone Miner. Metab. 2015, 13, 232–244. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Nanostructured scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef] [Green Version]
- Sakai, S.; Kotani, T.; Harada, R.; Goto, R.; Morita, T.; Bouissil, S.; Dubessay, P.; Pierre, G.; Michaud, P.; El Boutachfaiti, R. Development of phenol-grafted polyglucuronic acid and its application to extrusion-based bioprinting inks. Carbohydr. Polym. 2022, 277, 118820. [Google Scholar] [CrossRef]
- Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Hamidi, M.; Valentine Okoro, O.; Eskandani, M.; Jaymand, M. Polysaccharide-based hydrogels: Properties, advantages, challenges, and optimization methods for applications in regenerative medicine. Int. J. Polym. Mater. Polym. Biomater. 2021, 1–15. [Google Scholar] [CrossRef]
- Naranda, J.; Bračič, M.; Vogrin, M.; Maver, U. Recent advancements in 3D printing of polysaccharide hydrogels in cartilage tissue engineering. Materials 2021, 14, 3977. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef]
- Hu, Y.; Jeong, D.; Kim, Y.; Kim, S.; Jung, S. Preparation of Succinoglycan Hydrogel Coordinated with Fe3+ Ions for Controlled Drug Delivery. Polymers 2020, 12, 977. [Google Scholar] [CrossRef]
- Sathain, A.; Monvisade, P.; Siriphannon, P. Bioactive alginate/carrageenan/calcium silicate porous scaffolds for bone tissue engineering. Mater. Today Commun. 2021, 26, 102165. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Bao, C.; Liu, C.; Liu, C.; Li, D.; Yan, H.; Lin, Q. Fabrication and Evaluation of Alginate/Bacterial Cellulose Nanocrystals–Chitosan–Gelatin Composite Scaffolds. Molecules 2021, 26, 5003. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Fukushima, T.; Hayakawa, T.; Nakashima, N.; Inoue, Y.; Takeda, S.; Okamura, K.; Taniguchi, K. Preparation of Carbon Nanotube-alginate Nanocomposite Gel for Tissue Engineering. Dent. Mater. J. 2006, 25, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Kaushik, A. Biomedical applications of nanotechnology and nanomaterials. Micromachines 2017, 8, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, M.S.; Alnazzawi, A.A.; Alrahabi, M.; Fareed, M.A.; Najeeb, S.; Khurshid, Z. Nanotechnology and nanomaterials in dentistry. In Advanced Dental Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 477–505. [Google Scholar]
- Zou, Y.; Huang, B.; Cao, L.; Deng, Y.; Su, J. Tailored mesoporous inorganic biomaterials: Assembly, functionalization, and drug delivery engineering. Adv. Mater. 2021, 33, 2005215. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Kotaki, M.; Zhang, Y.; Mo, X.; Ramakrishna, S. Recent advances in polymer nanofibers. J. Nanosci. Nanotechnol. 2004, 4, 52–65. [Google Scholar]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Mohammad-Rezaei, R.; Massoumi, B.; Abbasian, M.; Rezaei, A.; Samadian, H.; Jaymand, M. Conducting polymer-based electrically conductive adhesive materials: Design, fabrication, properties, and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 10947–10961. [Google Scholar] [CrossRef]
- Saberi, A.; Jabbari, F.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Electrically Conductive Materials: Opportunities and Challenges in Tissue Engineering. Biomolecules 2019, 9, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Salami, M.S.; Jaymand, M.; Azarnezhad, A.; Najafi, M.; Barabadi, H.; Ahmadi, A. Genotoxicity assessment of carbon-based nanomaterials; Have their unique physicochemical properties made them double-edged swords? Mutat. Res./Rev. Mutat. Res. 2020, 783, 108296. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Mobasheri, H.; Azami, M.; Faridi-Majidi, R. Osteoconductive and electroactive carbon nanofibers/hydroxyapatite nanocomposite tailored for bone tissue engineering: In vitro and in vivo studies. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Hammel, E.; Tang, X.; Trampert, M.; Schmitt, T.; Mauthner, K.; Eder, A.; Pötschke, P. Carbon nanofibers for composite applications. Carbon 2004, 42, 1153–1158. [Google Scholar] [CrossRef]
- Feng, L.; Xie, N.; Zhong, J. Carbon nanofibers and their composites: A review of synthesizing, properties and applications. Materials 2014, 7, 3919–3945. [Google Scholar] [CrossRef]
- Pirhanov, A.; Bridges, C.M.; Goodwin, R.A.; Guo, Y.-S.; Furrer, J.; Shor, L.M.; Gage, D.J.; Cho, Y.K. Optogenetics in Sinorhizobium meliloti enables spatial control of exopolysaccharide production and biofilm structure. ACS Synth. Biol. 2021, 10, 345–356. [Google Scholar] [CrossRef]
- Okoro, O.V.; Gholipour, A.R.; Sedighi, F.; Shavandi, A.; Hamidi, M. Optimization of Exopolysaccharide (EPS) Production by Rhodotorula mucilaginosa sp. GUMS16. ChemEngineering 2021, 5, 39. [Google Scholar] [CrossRef]
- Delattre, C.; Michaud, P.; Lion, J.; Courtois, B.; Courtois, J. Production of glucuronan oligosaccharides using a new glucuronan lyase activity from a Trichoderma sp. strain. J. Biotechnol. 2005, 118, 448–457. [Google Scholar] [CrossRef]
- Elboutachfaiti, R.; Delattre, C.; Petit, E.; Michaud, P. Polyglucuronic acids: Structures, functions and degrading enzymes. Carbohydr. Polym. 2011, 84, 1–13. [Google Scholar] [CrossRef]
- Park, J.K.; Khan, T.; Jung, J.Y. Structural studies of the glucuronic acid oligomers produced by Gluconacetobacter hansenii strain. Carbohydr. Polym. 2006, 63, 482–486. [Google Scholar] [CrossRef]
- Tai, C.; Bouissil, S.; Gantumur, E.; Carranza, M.S.; Yoshii, A.; Sakai, S.; Pierre, G.; Michaud, P.; Delattre, C. Use of anionic polysaccharides in the development of 3D bioprinting technology. Appl. Sci. 2019, 9, 2596. [Google Scholar] [CrossRef] [Green Version]
- Heyraud, A.; Courtois, J.; Dantas, L.; Colin-Morel, P.; Courtois, B. Structural characterization and rheological properties of an extracellular glucuronan produced by a Rhizobium meliloti M5N1 mutant strain. Carbohydr. Res. 1993, 240, 71–78. [Google Scholar] [CrossRef]
- Courtois, J.; Seguin, J.-P.; Roblot, C.; Heyraud, A.; Gey, C.; Dantas, L.; Barbotin, J.-N.; Courtois, B. Exopolysaccharide production by the Rhizobium meliloti M5N1 CS strain. Location and quantitation of the sites of O-acetylation. Carbohydr. Polym. 1994, 25, 7–12. [Google Scholar] [CrossRef]
- Michaud, P.; Courtois, J.; Courtois, B.; Heyraud, A.; Colin-Morel, P.; Seguin, J.; Barbotin, J. Physicochemical properties of extracellular (1 → 4)-β-D-glucuronan produced by the Rhizobium meliloti M5N1CS strain during fermentation: Evidence of degradation by an exoenzyme activated by Mg2+. Int. J. Biol. Macromol. 1994, 16, 301–305. [Google Scholar] [CrossRef]
- Franceschini, G.; Visconti, G.; Terribile, D.; Fabbri, C.; Magno, S.; Di Leone, A.; Salgarello, M.; Masetti, R. The role of oxidized regenerate cellulose to prevent cosmetic defects in oncoplastic breast surgery. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 966–971. [Google Scholar]
- Kumar, V.; Dang, Y. Biodegradable Oxidized Cellulose Esters. US Patent 7,662,801, 16 February 2010. [Google Scholar]
- Samadian, H.; Mobasheri, H.; Hasanpour, S.; Faridi-Majid, R. Needleless electrospinning system, an efficient platform to fabricate carbon nanofibers. J. Nano Res. 2017, 50, 78–89. [Google Scholar] [CrossRef]
- Khademolqorani, S.; Tavanai, H.; Chronakis, I.S.; Boisen, A.; Ajalloueian, F. The determinant role of fabrication technique in final characteristics of scaffolds for tissue engineering applications: A focus on silk fibroin-based scaffolds. Mater. Sci. Eng. C 2021, 122, 111867. [Google Scholar] [CrossRef]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Bruggeman, K.F.; Williams, R.J.; Nisbet, D.R. Dynamic and responsive growth factor delivery from electrospun and hydrogel tissue engineering materials. Adv. Healthc. Mater. 2018, 7, 1700836. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, H.-W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018, 9, 2041731418768285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17, 20190505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.D.; Mura, C.; Lampe, K.J. Stimuli-responsive, pentapeptide, nanofiber hydrogel for tissue engineering. J. Am. Chem. Soc. 2019, 141, 4886–4899. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Tamburaci, S.; Tihminlioglu, F. Biosilica incorporated 3D porous scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2018, 91, 274–291. [Google Scholar] [CrossRef]

| XRD Results | |||||||
|---|---|---|---|---|---|---|---|
| Lc (nm) | d (002) Å | FWHM | 2θ | ||||
| 4.7 | 3.26 | 0.19 | 26.32 | ||||
| Raman Results | |||||||
| D Position (cm−1) | G Position (cm−1) | ID/IG (integrated areas) | Crystallite Parameters | Defect Parameters | R(ID/IG) | ||
| Size La (nm) | Area La2 (nm2) | Average Distanse LD(nm) | Density nD(cm−2 × 1011) | ||||
| 1372.14 | 1582.42 | 3.41 | 5.63 | 31.69 | 2.05 | 7.65 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibazar, Z.E.; Mohammadpour, M.; Samadian, H.; Zare, S.; Azizi, M.; Hamidi, M.; Elboutachfaiti, R.; Petit, E.; Delattre, C. Bacterial Polyglucuronic Acid/Alginate/Carbon Nanofibers Hydrogel Nanocomposite as a Potential Scaffold for Bone Tissue Engineering. Materials 2022, 15, 2494. https://doi.org/10.3390/ma15072494
Dibazar ZE, Mohammadpour M, Samadian H, Zare S, Azizi M, Hamidi M, Elboutachfaiti R, Petit E, Delattre C. Bacterial Polyglucuronic Acid/Alginate/Carbon Nanofibers Hydrogel Nanocomposite as a Potential Scaffold for Bone Tissue Engineering. Materials. 2022; 15(7):2494. https://doi.org/10.3390/ma15072494
Chicago/Turabian StyleDibazar, Zahra Ebrahimvand, Mahnaz Mohammadpour, Hadi Samadian, Soheila Zare, Mehdi Azizi, Masoud Hamidi, Redouan Elboutachfaiti, Emmanuel Petit, and Cédric Delattre. 2022. "Bacterial Polyglucuronic Acid/Alginate/Carbon Nanofibers Hydrogel Nanocomposite as a Potential Scaffold for Bone Tissue Engineering" Materials 15, no. 7: 2494. https://doi.org/10.3390/ma15072494
APA StyleDibazar, Z. E., Mohammadpour, M., Samadian, H., Zare, S., Azizi, M., Hamidi, M., Elboutachfaiti, R., Petit, E., & Delattre, C. (2022). Bacterial Polyglucuronic Acid/Alginate/Carbon Nanofibers Hydrogel Nanocomposite as a Potential Scaffold for Bone Tissue Engineering. Materials, 15(7), 2494. https://doi.org/10.3390/ma15072494









