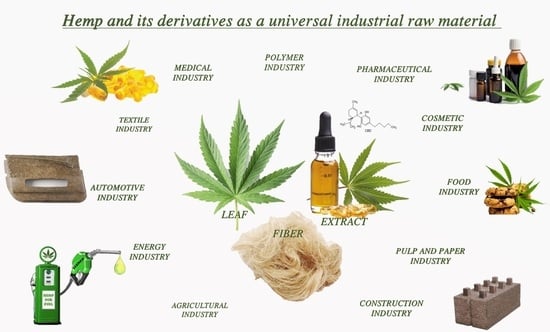
Hemp and Its Derivatives as a Universal Industrial Raw Material (with Particular Emphasis on the Polymer Industry)—A Review
Abstract
:1. Introduction
2. Characteristics of Hemp Plants
2.1. Structure and Composition
2.1.1. Hemp Fibres
- Homopolymer that comes from natural sources;
- It has a zero-carbon balance for the environment due to its use in its synthesis, carbon dioxide;
- It is highly pure and non-toxic [18];
- It is characterized by good mechanical strength, which is why it is used as one of the basic natural construction materials [19].
- Primary-CH2-OH;
- Two secondary hydroxyl groups-OH.
- An intact fiber-containing crystalline and amorphous regions, with frayed ends at the periphery consisting of a paracrystalline region of cellulose, lignocellulosic or hemicellulose;
- Initial attack on regions with an amorphous structure;
- There will remain residual microcrystallites and decomposition of the remaining free short chain fragments;
- Attack on a crystalline region.
- Iα
- with the structure of a triclinic unit cell;
- Iβ
- monoclinic unit cell structure.
- A710 absorption intensity at the wavenumber of 710 cm−1;
- A750 absorption intensity at the wavenumber of 750 cm−1.
2.1.2. Extract
2.1.3. Waxes
2.2. Sectors of the Economy Using Cannabis
2.2.1. Agriculture and Energetic
2.2.2. Food Industry
2.2.3. Textile Industry
2.2.4. Pulp and Paper Industry
2.2.5. Construction
2.2.6. Automotive Industry
2.2.7. Cosmetics, Pharmaceutical and Medical Industries
2.2.8. Polymer Industry
2.2.9. Other Uses
3. Hemp and Derivatives in the Polymer Industry
3.1. Thermoplastics
3.2. Elastomers
3.3. Duroplasts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPPH (ORAC) | Oxygen Radical Absorbance Capacity |
| ABTS | 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate) |
| CBC | Cannabichromene |
| CBD | Cannabidiol |
| CBDA | Cannabinoid acid |
| CBG | Cannabigerol |
| CBN | Cannabinol |
| C-NMR | Carbon-13 (C13) nuclear magnetic resonance |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl. |
| FT-IR | Infrared Spectroscopy with Fourier Transformation |
| NaOH | Sodium hydroxide |
| THC | Delta-9-tetrahydrocannabinol |
| THCV | Tetrahydrocannabivarin |
References
- Lambert, S.; Sinclair, C.; Boxall, A. Occurrence, Degradation, and Effect of Polymer-Based Materials in the Environment. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer International Publishing: Cham, Switzerland, 2014; Volume 227, pp. 1–53. ISBN 978-3-319-01327-5. [Google Scholar]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Dominguiano, O.; Chandra, R.; Rustgi, R. Related papers Polyet hylene and biodegradable mulches for agricult ural applicat ions: A review Nguyen Van Biological degradat ion of plast ics: A comprehensive review biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers—A Review on Recent Trends and Emerging Perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Molina, A.C.; Castro-Vargas, H.I.; Garzón-Méndez, W.F.; Martínez Ramírez, J.A.; Rivera Monroy, Z.J.; King, J.W.; Parada-Alfonso, F. Extraction, isolation and purification of tetrahydrocannabinol from the Cannabis sativa L. plant using supercritical fluid extraction and solid phase extraction. J. Supercrit. Fluids 2019, 146, 208–216. [Google Scholar] [CrossRef]
- Long, T.; Wagner, M.; Demske, D.; Leipe, C.; Tarasov, P.E. Cannabis in Eurasia: Origin of human use and Bronze Age trans-continental connections. Veg. Hist. Archaeobot. 2017, 26, 245–258. [Google Scholar] [CrossRef]
- Fike, J. The history of hemp. In Industrial Hemp as a Modern Commodity Crop; Wiley: Hoboken, NJ, USA, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Fike, J. Industrial Hemp: Renewed Opportunities for an Ancient Crop. CRC Crit. Rev. Plant Sci. 2016, 35, 406–424. [Google Scholar] [CrossRef]
- Shibata, T. Cellulose and its derivatives in medical use. In Renewable Resources for Functional Polymers and Biomaterials; RCS Royal Chemistry Society: London, UK, 2011; pp. 48–87. [Google Scholar] [CrossRef]
- Szymański, Ł.; Grabowska, B.; Kurleto, Ż.; Kaczmarska, K. Celuloza i jej pochodne—Zastosowanie w przemyśle. Arch. Foundry Eng. 2015, 15, 129–132. [Google Scholar]
- Samyn, P.; Nordell, P. Photosynthesis—Gigantic factory of cellulose. Przegląd Pap. 2015, 48, 6455–6498. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi Júnior, H.L.; Zattera, A.J. Native cellulose: Structure, characterization and thermal properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Keshk, S.M.A.S.; Gouda, M. Natural biodegradable medical polymers: Cellulose. In Science and Principles of Biodegradable and Bioresorbable Medical Polymers; Woodhead Publishing: Sawston, UK, 2017; pp. 279–294. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J.; Lewiñski, P. Polimery z odnawialnych surowców, polimery biodegradowalne. Polimery 2013, 58, 835–846. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Bajer, K. Badanie przebiegu biodegradacji kompozytów poli(chlorek winylu)/celuloza. Polimery 2008, 53, 631–638. [Google Scholar] [CrossRef]
- Kubiak, K.; Kalinowska, H.; Peplińska, M.; Bielecki, S. Celuloza bakteryjna jako nanobiomateriał. Postępy Biol. Komórki 2009, 36, 85–98. [Google Scholar]
- Wasilewska, A.W.; Pietruszka, D.B.L. Materiały naturalne w ekobudownictwie. Przegląd Bud. 2017, 88, 50–53. [Google Scholar]
- Antczak, T.; Wietecha, J.; Kazimierczak, J.; Bloda, A.; Ciechańska, D. Nanowłókna celulozowe wytwarzane z biomasy roślinnej. Chemik 2014, 68, 755–760. [Google Scholar]
- Lee, Y.-H.; Fan, L.T. Properties and mode of action of cellulase. Adv. Biochem. Eng. 1980, 17, 101–129. [Google Scholar]
- Seidl, P.R.; Freire, E.; Borschiver, S. Non-fuel applications of sugars in Brazil. In Biomass Sugars for Non-Fuel Applications; Royal Society of Chemistry: London, UK, 2016; pp. 228–257. [Google Scholar] [CrossRef]
- Henriksson, G.; Lennholm, H. Cellulose and carbohydrate chemistry. Wood Chem. Wood Biotechnol. 2009, 71–100. [Google Scholar] [CrossRef]
- Nishino, T. Natural fibre sources. Green Compos. 2005, 49–80. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Urbaniak, M.; Jaszkiewicz, A.; Feldmann, M. Cellulose fibres as an alternative for glass fibres in polymer composites. Polimery 2014, 59, 372–382. [Google Scholar] [CrossRef]
- Gardner, K.H.; Blackwell, J. The hydrogen bonding in native cellulose. BBA Gen. Subj. 1974, 343, 232–237. [Google Scholar] [CrossRef]
- Cousins, S.K.; Brown, R.M. Cellulose I microfibril assembly: Computational molecular mechanics energy analysis favours bonding by van der Waals forces as the initial step in crystallization. Polymer 1995, 36, 3885–3888. [Google Scholar] [CrossRef]
- Tanasă, F.; Zănoagă, M.; Teacă, C.A.; Nechifor, M.; Shahzad, A. Modified hemp fibers intended for fiber-reinforced polymer composites used in structural applications—A review. I. Methods of modification. Polym. Compos. 2020, 41, 5–31. [Google Scholar] [CrossRef]
- Sołowski, G. Wybrane Zagadnienia z Zakresu Ochrony Środowiska i Energii Odnawialnej; Wydawnictwo Uniwersytetu Przyrodniczego w Lublinie: Lublin, Poland, 2016; ISBN 9788365598165. [Google Scholar]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Maréchal, Y.; Chanzy, H. The hydrogen bond network in I(β) cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Chundawat, S.P.S.; Bellesia, G.; Uppugundla, N.; Da Costa Sousa, L.; Gao, D.; Cheh, A.M.; Agarwal, U.P.; Bianchetti, C.M.; Phillips, G.N.; Langan, P.; et al. Restructuring the crystalline cellulose hydrogen bond network enhances its depolymerization rate. J. Am. Chem. Soc. 2011, 133, 11163–11174. [Google Scholar] [CrossRef]
- Kondo, T.; Sawatari, C. A Fourier transform infra-red spectroscopic analysis of the character of hydrogen bonds in amorphous cellulose. Polymer (Guildf.) 1996, 37, 393–399. [Google Scholar] [CrossRef]
- French, A.D.; Miller, D.P.; Aabloo, A. Miniature crystal models of cellulose polymorphs and other carbohydrates. Int. J. Biol. Macromol. 1993, 15, 30–36. [Google Scholar] [CrossRef]
- Marrinan, H.J.; Mann, J. A study by infra-red spectroscopy of hydrogen bonding in cellulose. J. Appl. Chem. 2007, 4, 204–211. [Google Scholar] [CrossRef]
- Dri, F.L.; Hector, L.G., Jr.; Moon, R.J.; Zavattieri, P.D. Anisotropy of the elastic properties of crystalline cellulose Iβ from first principles density functional theory with Van der Waals interactions. Cellulose 2013, 20, 2703–2718. [Google Scholar] [CrossRef]
- Rees, D.A.; Skerrett, R.J. Conformational analysis of cellobiose, cellulose, and xylan. Carbohydr. Res. 1968, 7, 334–348. [Google Scholar] [CrossRef]
- Pizzi, A.; Eaton, N. The Structure of Cellulose by Conformational Analysis. Part 4. Crystalline Cellulose II. J. Macromol. Sci. -Chem. 1984, 24, 901–918. [Google Scholar] [CrossRef]
- Kroon-Batenburg, L.M.; Kroon, J. The crystal and molecular structures of cellulose I and II. Glycoconj. J. 1997, 14, 677–690. [Google Scholar] [CrossRef]
- Manaia, J.P.; Manaia, A.T.; Rodrges, L. Industrial Hemp Fibres: An Overview. Fibers 2019, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Väisänen, T.; Batello, P.; Lappalainen, R.; Tomppo, L. Modification of hemp fibers (Cannabis Sativa L.) for composite applications. Ind. Crops Prod. 2018, 111, 422–429. [Google Scholar] [CrossRef]
- Marrot, L.; Lefeuvre, A.; Pontoire, B.; Bourmaud, A.; Baley, C. Analysis of the hemp fiber mechanical properties and their scattering (Fedora 17). Ind. Crops Prod. 2013, 51, 317–327. [Google Scholar] [CrossRef]
- Müssig, J.; Amaducci, S.; Bourmaud, A.; Beaugrand, J.; Shah, D.U. Transdisciplinary top-down review of hemp fibre composites: From an advanced product design to crop variety selection. Compos. Part C Open Access 2020, 2, 100010. [Google Scholar] [CrossRef]
- Duval, A.; Bourmaud, A.; Augier, L.; Baley, C. Influence of the sampling area of the stem on the mechanical properties of hemp fibers. Mater. Lett. 2011, 65, 797–800. [Google Scholar] [CrossRef]
- Kaczmar, J.W.; Pach, J.; Burgstaller, C. The chemically treated hemp fibres to reinforce polymers. Polimery 2011, 56, 817–822. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Patel, H.A.; Somani, R.S.; Bajaj, H.C.; Jasra, R.V. Preparation and characterization of phosphonium montmorillonite with enhanced thermal stability. Appl. Clay Sci. 2007, 35, 194–200. [Google Scholar] [CrossRef]
- Salmén, L.; Bergström, E. Cellulose structural arrangement in relation to spectral changes in tensile loading FTIR. Cellulose 2009, 16, 975–982. [Google Scholar] [CrossRef]
- Ołdak, D.; Kaczmarek, H.; Buffeteau, T.; Sourisseau, C. Photo- and bio-degradation processes in polyethylene, cellulose and their blends studied by ATR-FTIR and raman spectroscopies. J. Mater. Sci. 2005, 40, 4189–4198. [Google Scholar] [CrossRef]
- Chwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Chen, C.; Luo, J.; Qin, W.; Tong, Z. Elemental analysis, chemical composition, cellulose crystallinity, and FT-IR spectra of Toona sinensis wood. Mon. Für Chem. -Chem. Mon. 2014, 145, 175–185. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Cellulose fibers hydrophobization via a hybrid chemical modification. Polymers 2019, 11, 1174. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Owen, N.L.; Thomas, D.W. Infrared studies of “hard” and “soft” woods. Appl. Spectrosc. 1989, 43, 451–455. [Google Scholar] [CrossRef]
- Sarkanen, K.; Ludwig, C. Liguins. Occurrence, Formation, Structure, and Reactions; Wiley-Interscience: New York, NY, USA, 1971. [Google Scholar]
- Müller, U.; Rätzsch, M.; Schwanninger, M.; Steiner, M.; Zöbl, H. Yellowing and IR-changes of spruce wood as result of UV-irradiation. J. Photochem. Photobiol. B Biol. 2003, 69, 97–105. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef]
- Lebo, S.E.; Lonsky, W.; McDonough, T.; Medvecz, P. The Occurrence and Light Induced Formation of Ortho Quinonoid Lignin Structures in White Spruce Refiner Mechanical Pulp. In Proceedings of the International Pulp Bleaching Conference in Orlando, Orlando, FL, USA, 5–9 June 1988. [Google Scholar]
- Anderson, E.L.; Owen, N.L.; Feist, W.C.; Pawlak, Z. Infrared Studies of Wood Weathering. Part I: Softwoods. Appl. Spectrosc. 1991, 45, 641–647. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Łojewska, J.; Miśkowiec, P.; Łojewski, T.; Proniewicz, L.M. Cellulose oxidative and hydrolytic degradation: In situ FTIR approach. Polym. Degrad. Stab. 2005, 88, 512–520. [Google Scholar] [CrossRef]
- Ramirez, C.L.; Fanovich, M.A.; Churio, M.S. Cannabinoids: Extraction Methods, Analysis, and Physicochemical Characterization, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 61, ISBN 9780444641830. [Google Scholar]
- Horanin, A.; Bryndal, I. Hemp—Active Ingredients, Medicinal Properties and Using. Pr. Nauk. Uniw. Ekon. we Wrocławiu 2017, 76–84. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Zulfiqar, F.; ElSohly, M.A. Cannabinoid ester constituents from high-potency Cannabis sativa. J. Nat. Prod. 2008, 71, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Radwan, M.M.; Chandra, S.; Gul, S.; Elsohly, M.A. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Ramamurthy, P.C.; Anand, U.; Singh, S.; Singh, A.; Dhanjal, D.S.; Dhaka, V.; Kumar, S.; Kapoor, D.; Nandy, S.; et al. Wonder or evil?: Multifaceted health hazards and health benefits of Cannabis sativa and its phytochemicals. Saudi J. Biol. Sci. 2021, 28, 7290–7313. [Google Scholar] [CrossRef] [PubMed]
- Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.-I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823. [Google Scholar] [CrossRef] [PubMed]
- Filer, C.N. Acidic Cannabinoid Decarboxylation. Cannabis Cannabinoid Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Attard, T.M.; Bainier, C.; Reinaud, M.; Lanot, A.; McQueen-Mason, S.J.; Hunt, A.J. Utilisation of supercritical fluids for the effective extraction of waxes and Cannabidiol (CBD) from hemp wastes. Ind. Crops Prod. 2018, 112, 38–46. [Google Scholar] [CrossRef]
- Rock, E.M.; Parker, L.A. Constituents of Cannabis Sativa. Adv. Exp. Med. Biol. 2021, 1264, 1–13. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-karpowicz, I.; Skrzydlewskas, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa l.): Nutritional quality and potential functionality for human health and nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Lewis, M.M.; Yang, Y.; Wasilewski, E.; Clarke, H.A.; Kotra, L.P. Chemical Profiling of Medical Cannabis Extracts. ACS Omega 2017, 2, 6091–6103. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; ElSohly, M.A.; El-Alfy, A.T.; Ahmed, S.A.; Slade, D.; Husni, A.S.; Manly, S.P.; Wilson, L.; Seale, S.; Cutler, S.J.; et al. Isolation and Pharmacological Evaluation of Minor Cannabinoids from High-Potency Cannabis sativa. J. Nat. Prod. 2015, 78, 1271–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radwan, M.M.; ElSohly, M.A.; Slade, D.; Ahmed, S.A.; Khan, I.A.; Ross, S.A. Biologically active cannabinoids from high-potency Cannabis sativa. J. Nat. Prod. 2009, 72, 906–911. [Google Scholar] [CrossRef] [Green Version]
- Pugazhendhi, A.; Suganthy, N.; Chau, T.P.; Sharma, A.; Unpaprom, Y.; Ramaraj, R.; Karuppusamy, I.; Brindhadevi, K. Cannabinoids as anticancer and neuroprotective drugs: Structural insights and pharmacological interactions—A review. Process Biochem. 2021, 111, 9–31. [Google Scholar] [CrossRef]
- Apostol, L. Studies on using hemp seed as a functional ingredient in the production of functional food products. J. EcoAgri Tour. 2017, 13, 12–17. [Google Scholar]
- Leyva-Gutierrez, F.M.A.; Munafo, J.P.; Wang, T. Characterization of By-Products from Commercial Cannabidiol Production. J. Agric. Food Chem. 2020, 68, 7648–7659. [Google Scholar] [CrossRef] [PubMed]
- Żuk-Gołaszewska, K.; Gołaszewski, J. Cannabis sativa L.—Cultivation and quality of raw material. J. Elem. 2018, 23, 971–984. [Google Scholar] [CrossRef]
- Kraszkiewicz, A.; Kachel, M.; Parafiniuk, S.; Zając, G.; Niedziółka, I.; Sprawka, M. Assessment of the Possibility of Using Hemp Biomass ( Cannabis Sativa L.) for Energy Purposes. Appl. Sci. 2019, 9, 4437. [Google Scholar] [CrossRef] [Green Version]
- Kaniewski, R.; Pniewska, I.; Kubacki, A.; Strzelczyk, M.; Chudy, M.; Oleszak, G. Konopie siewne (Cannabis sativa L.)—wartościowa roślina użytkowa i lecznicza. Postępy Fitoter. 2017, 18, 139–144. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Shamreen Amode, N.; Jeetah, P. Paper Production from Mauritian Hemp Fibres. Waste Biomass Valoriz. 2021, 12, 1781–1802. [Google Scholar] [CrossRef]
- van der Werf, H.M.G.; Harsveld van der Veen, J.E.; Bouma, A.T.M.; ten Cate, M. Quality of hemp (Cannabis sativa L.) stems as a raw material for paper. Ind. Crops Prod. 1994, 2, 219–227. [Google Scholar] [CrossRef]
- Stevulova, N.; Kidalova, L.; Junak, J.; Cigasova, J.; Terpakova, E. Effect of hemp shive sizes on mechanical properties of lightweight fibrous composites. Procedia Eng. 2012, 42, 496–500. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Calabria-Holley, J.; Lawrence, M.; Ansell, M.P.; Jiang, Y.; Schorr, D.; Blanchet, P. Development of novel building composites based on hemp and multi-functional silica matrix. Compos. Part B Eng. 2019, 156, 266–273. [Google Scholar] [CrossRef]
- Seng, B.; Magniont, C.; Lorente, S. Characterization of a precast hemp concrete. Part I: Physical and thermal properties. J. Build. Eng. 2019, 24, 100540. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Smarzewski, P.; Brzyski, P. Properties of Hemp–Flax Composites for Use in the Building Industry. J. Nat. Fibers 2017, 14, 410–425. [Google Scholar] [CrossRef]
- Lekavicius, V.; Shipkovs, P.; Ivanovs, S.; Rucins, A. Thermo-insulation properties of hemp-based products. Latv. J. Phys. Tech. Sci. 2015, 52, 38–51. [Google Scholar] [CrossRef] [Green Version]
- La Rosa, A.D.; Cozzo, G.; Latteri, A.; Mancini, G.; Recca, A.; Cicala, G. A comparative life cycle assessment of a composite component for automotive. Chem. Eng. Trans. 2013, 32, 1723–1728. [Google Scholar] [CrossRef]
- Murugu Nachippan, N.; Alphonse, M.; Bupesh Raja, V.K.; Shasidhar, S.; Varun Teja, G.; Harinath Reddy, R. Experimental investigation of hemp fiber hybrid composite material for automotive application. Mater. Today Proc. 2021, 44, 3666–3672. [Google Scholar] [CrossRef]
- Mastura, M.T.; Sapuan, S.M.; Mansor, M.R.; Nuraini, A.A. Materials selection of thermoplastic matrices for ‘green’ natural fibre composites for automotive anti-roll bar with particular emphasis on the environment. Int. J. Precis. Eng. Manuf. Green Technol. 2018, 5, 111–119. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Sikora, V.; Dincheva, I.; Kačániová, M.; Astatkie, T.; Semerdjieva, I.B.; Latkovic, D. Industrial, CBD, and Wild Hemp: How Different Are Their Essential Oil Profile and Antimicrobial Activity? Molecules 2020, 25, 4631. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, A.; Roda, G.; Casagni, E.; Cristina, C.; Musazzi, U.M.; Franzè, S.; Rocco, P.; Giuliani, C.; Fico, G.; Minghetti, P.; et al. Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations. Planta Med. 2018, 84, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Chi Nguyen, L.; Yang, D.; Nicolaescu, V.; Best, T.J.; Gula, H.; Saxena, D.; Gabbard, J.D.; Chen, S.-N.; Ohtsuki, T.; Brent Friesen, J.; et al. Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. Sci. Adv. 2022, 8, 6110. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Park, J.G.; Cho, K.H.; Choi, P.; Kim, T.; Ham, J.; Lee, J. Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in vitro approaches. Int. J. Biol. Macromol. 2021, 168, 474–485. [Google Scholar] [CrossRef]
- Pappu, A.; Pickering, K.L.; Thakur, V.K. Manufacturing and characterization of sustainable hybrid composites using sisal and hemp fibres as reinforcement of poly (lactic acid) via injection moulding. Ind. Crops Prod. 2019, 137, 260–269. [Google Scholar] [CrossRef]
- Rovetto, L.J.; Aieta, N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Mańkowski, J.; Kubacki, A.; Kołodziej, J.; Pniewska, I.; Teren, Z.; Konin, K.W.B. Rekultywacja Terenów Zdegradowanych w Wyniku Działania Kopalni Odkrywkowych; Biuletyn Informacyjny Polskiej Izby Lnu i Konopi: Poznań, Poland, 2013. [Google Scholar]
- Terzopoulou, Z.N.; Papageorgiou, G.Z.; Papadopoulou, E.; Athanassiadou, E.; Alexopoulou, E.; Bikiaris, D.N. Green composites prepared from aliphatic polyesters and bast fibers. Ind. Crops Prod. 2015, 68, 60–79. [Google Scholar] [CrossRef]
- Sergi, C.; Tirillò, J.; Seghini, M.C.; Sarasini, F.; Fiore, V.; Scalici, T. Durability of basalt/hemp hybrid thermoplastic composites. Polymers 2019, 11, 603. [Google Scholar] [CrossRef] [Green Version]
- Sullins, T.; Pillay, S.; Komus, A.; Ning, H. Hemp fiber reinforced polypropylene composites: The effects of material treatments. Compos. Part B Eng. 2017, 114, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Panaitescu, D.M.; Fierascu, R.C.; Gabor, A.R.; Nicolae, C.A. Effect of hemp fiber length on the mechanical and thermal properties of polypropylene/SEBS/hemp fiber composites. J. Mater. Res. Technol. 2020, 9, 10768–10781. [Google Scholar] [CrossRef]
- Yallew, T.B.; Kumar, P.; Singh, I. Sliding behaviour of woven industrial hemp fabric reinforced thermoplastic polymer composites. Int. J. Plast. Technol. 2015, 19, 347–362. [Google Scholar] [CrossRef]
- Etaati, A.; Pather, S.; Fang, Z.; Wang, H. The study of fibre/matrix bond strength in short hemp polypropylene composites from dynamic mechanical analysis. Compos. Part B Eng. 2014, 62, 19–28. [Google Scholar] [CrossRef]
- Shahzad, A. Hemp fiber and its composites—A review. J. Compos. Mater. 2012, 46, 973–986. [Google Scholar] [CrossRef]
- Oliveira, M.A.S.; Pickering, K.L.; Sunny, T.; Lin, R.J.T. Treatment of hemp fibres for use in rotational moulding. J. Polym. Res. 2021, 28, 53. [Google Scholar] [CrossRef]
- Ziąbka, M.; Szaraniec, B. Polymeric composites with natural fiber additives. Composites 2010, 10, 138–142. [Google Scholar]
- Xiao, X.; Chevali, V.S.; Song, P.; He, D.; Wang, H. Polylactide/hemp hurd biocomposites as sustainable 3D printing feedstock. Compos. Sci. Technol. 2019, 184, 107887. [Google Scholar] [CrossRef]
- Khattab, M.M.; Dahman, Y. Production and recovery of poly-3-hydroxybutyrate bioplastics using agro-industrial residues of hemp hurd biomass. Bioprocess Biosyst. Eng. 2019, 42, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Oza, S.; Ning, H.; Ferguson, I.; Lu, N. Effect of surface treatment on thermal stability of the hemp-PLA composites: Correlation of activation energy with thermal degradation. Compos. Part B Eng. 2014, 67, 227–232. [Google Scholar] [CrossRef]
- Mazzanti, V.; Salzano de Luna, M.; Pariante, R.; Mollica, F.; Filippone, G. Natural fiber-induced degradation in PLA-hemp biocomposites in the molten state. Compos. Part A Appl. Sci. Manuf. 2020, 137, 105990. [Google Scholar] [CrossRef]
- Baghaei, B.; Skrifvars, M.; Berglin, L. Manufacture and characterisation of thermoplastic composites made from PLA/hemp co-wrapped hybrid yarn prepregs. Compos. Part A Appl. Sci. Manuf. 2013, 50, 93–101. [Google Scholar] [CrossRef]
- Mazzanti, V.; Pariante, R.; Bonanno, A.; Ruiz de Ballesteros, O.; Mollica, F.; Filippone, G. Reinforcing mechanisms of natural fibers in green composites: Role of fibers morphology in a PLA/hemp model system. Compos. Sci. Technol. 2019, 180, 51–59. [Google Scholar] [CrossRef]
- Marrot, L.; Alao, P.F.; Mikli, V.; Kers, J. Properties of Frost-Retted Hemp Fibers for the Reinforcement of Composites. J. Nat. Fibers 2021, 18, 1–12. [Google Scholar] [CrossRef]
- Plota, A.; Masek, A. Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials. Int. J. Mol. Sci. 2021, 22, 4012. [Google Scholar] [CrossRef] [PubMed]
- Andriotis, E.G.; Chachlioutaki, K.; Monou, P.K.; Bouropoulos, N.; Tzetzis, D.; Barmpalexis, P.; Chang, M.W.; Ahmad, Z.; Fatouros, D.G. Development of Water-Soluble Electrospun Fibers for the Oral Delivery of Cannabinoids. AAPS PharmSciTech 2021, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Dumitrascu, M. New green polymeric composites based on hemp and natural rubber processed by electron beam irradiation. Sci. World J. 2014, 2014, 684047. [Google Scholar] [CrossRef] [PubMed]
- Moonart, U.; Utara, S. Effect of surface treatments and filler loading on the properties of hemp fiber/natural rubber composites. Cellulose 2019, 26, 7271–7295. [Google Scholar] [CrossRef]
- Koushki, P.; Kwok, T.H.; Hof, L.; Wuthrich, R. Reinforcing silicone with hemp fiber for additive manufacturing. Compos. Sci. Technol. 2020, 194, 108139. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Craciun, G.; Surdu, L. Effects of benzoyl peroxide on some properties of composites based on hemp and natural rubber. Polym. Bull. 2014, 71, 2001–2022. [Google Scholar] [CrossRef]
- Dayo, A.Q.; Gao, B.; Wang, J.; Liu, W.; Derradji, M.; Shah, A.H.; Babar, A.A. Natural hemp fiber reinforced polybenzoxazine composites: Curing behavior, mechanical and thermal properties. Compos. Sci. Technol. 2017, 144, 114–124. [Google Scholar] [CrossRef]
- del Borrello, M.; Mele, M.; Campana, G.; Secchi, M. Manufacturing and characterization of hemp-reinforced epoxy composites. Polym. Compos. 2020, 41, 2316–2329. [Google Scholar] [CrossRef]
- Di Landro, L.; Janszen, G. Composites with hemp reinforcement and bio-based epoxy matrix. Compos. Part B Eng. 2014, 67, 220–226. [Google Scholar] [CrossRef]
- Ribeiro, M.P.; de, M. Neuba, L.; da Silveira, P.H.P.M.; da Luz, F.S.; da S. Figueiredo, A.B.-H.; Monteiro, S.N.; Moreira, M.O. Mechanical, thermal and ballistic performance of epoxy composites reinforced with Cannabis sativa hemp fabric. J. Mater. Res. Technol. 2021, 12, 221–233. [Google Scholar] [CrossRef]
- Khdier, H.M.; Ali, A.H.; Salih, W.M. Manufacturing of Thermal and Acoustic Insulation From (Polymer Blend/Recycled Natural Fibers). Eng. Technol. J. 2020, 38, 1801–1807. [Google Scholar] [CrossRef]
- Gupta, M.K.; Gond, R.K.; Bharti, A. Effects of treatments on the properties of polyester based hemp composite. Indian J. Fibre Text. Res. 2018, 43, 313–319. [Google Scholar]
- Caprino, G.; Carrino, L.; Durante, M.; Langella, A.; Lopresto, V. Low impact behaviour of hemp fibre reinforced epoxy composites. Compos. Struct. 2015, 133, 892–901. [Google Scholar] [CrossRef]
- Singha, A.S.; Rana, A.K. Preparation and characterization of graft copolymerized Cannabis indica L. fiber-reinforced unsaturated polyester matrix-based biocomposites. J. Reinf. Plast. Compos. 2012, 31, 1538–1553. [Google Scholar] [CrossRef]
- Inbakumar, J.P.; Ramesh, S. Mechanical, wear and thermal behaviour of hemp fibre/egg shell particle reinforced epoxy resin bio composite. Trans. Can. Soc. Mech. Eng. 2018, 42, 280–285. [Google Scholar] [CrossRef]
- Scarponi, C. Hemp fiber composites for the design of a Naca cowling for ultra-light aviation. Compos. Part B Eng. 2015, 81, 53–63. [Google Scholar] [CrossRef]
- Vinod, B.; Sudev, L.J. Investigation on Effect of Cryogenic Temperature on Mechanical Behavior of Jute and Hemp Fibers Reinforced Polymer Composites. Appl. Mech. Mater. 2019, 895, 76–82. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A. The impact of hemp shives impregnated with selected plant oils on mechanical, thermal, and insulating properties of polyurethane composite foams. Materials 2020, 13, 4709. [Google Scholar] [CrossRef]
- Sair, S.; Oushabi, A.; Kammouni, A.; Tanane, O.; Abboud, Y.; Oudrhiri Hassani, F.; Laachachi, A.; El Bouari, A. Effect of surface modification on morphological, mechanical and thermal conductivity of hemp fiber: Characterization of the interface of hemp-Polyurethane composite. Case Stud. Therm. Eng. 2017, 10, 550–559. [Google Scholar] [CrossRef]
- Sair, S.; Oushabi, A.; Kammouni, A.; Tanane, O.; Abboud, Y.; El Bouari, A. Mechanical and thermal conductivity properties of hemp fiber reinforced polyurethane composites. Case Stud. Constr. Mater. 2018, 8, 203–212. [Google Scholar] [CrossRef]






| Name of the Function Group | Wavenumber [cm−1] | Bibliographic |
|---|---|---|
| C-OH out-of-plane bending vibrations; C-C | 557 | [46] |
| Stretch vibrations of the glucose ring; C–H stretching vibrations outside the plane of the aromatic ring | 895 | [22,23] |
| -OH; -COO | 900–1200 | [47] |
| CO-O-CO | 1000–1100 | [48] |
| C-O stretching vibrations; deformation of the C-H aromatic plane | 1030–1058 | [49,50] |
| The absorption band of hydroxyl compounds -OH | 1100 | [51,52] |
| C-O stretching vibrations; asymmetric bridge C-O-C stretching vibrations | 1158 | [52,53] |
| C-O; C=O; C-C-; COOH | 1100–1300 | [54] |
| Acyl-oxygen CO-OR stretching vibrations in hemicelluloses; -CH3 | 1245 | [50] |
| C-H deformation vibrations; -OH bending vibrations | 1325 | [51] |
| C-H bending vibrations related to the structure of cellulose and hemicellulose | 1369 | [53,55] |
| CH2 stretching vibrations related to the cellulose structure, vibrations of the bonds of the aromatic backbone | 1425–1426 | [52,53,56,57] |
| CH deformation vibrations; asymmetric bending vibrations from -CH2 and -CH3 groups | 1426–1463 | [46] |
| C=C stretching vibrations in aromatic structures | 1508 | [51] |
| C=C stretching of the aromatic ring | 1550 | [45] |
| C=C unsaturated bonds; | 1592 | [51] |
| COO− (pectin) | 1650 | [45] |
| -OH from absorbed water; C=C | 1653 | [50,51,56,58] |
| C=O stretching vibrations in uncoupled ketones and free aldehydes | 1736; 1718 | [55,56,57,59,60] |
| CH stretching vibrations in methyl and methylene groups | 2896 | [53,55,61] |
| -OH stretching vibrations (hydrogen bonds) | 3331 | [53,62] |
| Component | Value [%] |
|---|---|
| The content of the oily fraction in the entire mass of the hemp seed | 28.7 |
| Saturated Fatty Acid | |
| Palmitic acid | 6.96 |
| Stearic acid | 2.74 |
| Arachidic acid | 0.77 |
| Total saturated fatty acid | 10.47 |
| Unsaturated Fatty Acid | |
| Oleic acid | 13.64 |
| Linoleic acid | 56.35 |
| Gamma-linoleic acid | 1.35 |
| Alpha-linoleic acid | 17.30 |
| Stearidonic acid | 0.50 |
| Eicosenoic acid | 0.39 |
| Total unsaturated fatty acid | 89.53 |
| Component | Value [%] |
|---|---|
| Alkanes 27.02–28.85 | |
| pentacosane | 1.92–2.17 |
| heptacosane | 6.96–7.55 |
| octacosane | 0.75–5.56 |
| nonacosane | 9.92–10.51 |
| triacontane | 0.44–0.58 |
| dotriacontane | 0.49 |
| tritriacontane | 1.58–2.06 |
| pentatriacontane | 1.13–1.24 |
| heptatriacontane | 1.18–1.23 |
| Monoterpens | |
| sabinene | 0.31–0.51 |
| p-cymene | 3.32–5.15 |
| Sesquiterpenes | |
| β-cubebene | 0.31–0.40 |
| (−)-trans-caryophyllene | 5.90–7.22 |
| β-copaene | 0.32–0.40 |
| α-humulene | 0.51–0.94 |
| (E,E)-β-farnesene | 0.30–0.33 |
| γ-gurjunene | 0.27 |
| γ-curcumene | 0.59–0.70 |
| valencene | 0.51–0.60 |
| germacrene A | 0.39–0.44 |
| α-7-epi-selinene | 0.42–0.54 |
| α-cadinene | 0.20–0.33 |
| α-bisabolene | 1.63–2.50 |
| (E,E)-α-farnesene | 0.28 |
| Terpenoids 22.92–23.70 | |
| dehydro-1,8-cineole | 1.23–1.99 |
| isoborneol | 0.38 |
| fenchone | 0.26–0.44 |
| cis-thujone | 0.27 |
| endo-fenchol | 0.26–0.28 |
| cis-nerolidol | 2.50–2.84 |
| trans-nerolidol | 0.43 |
| caryophyllene oxide | 0.49–0.89 |
| humulene epoxide II | 0.31–0.37 |
| 10-epi-γ-eudesmol | 0.61–0.82 |
| 1,10-di-epi-cubenol | 0.29–0.36 |
| γ-eudesmol | 0.29–0.47 |
| α-muurolol | 0.25–0.35 |
| β-eudesmol | 0.67–1.01 |
| α-bisabolol | 0.18 |
| (2Z,6Z)-farnesol | 0.49 |
| Cannabinoids 41.67–46.37 | |
| CBD | 4.20–9.67 |
| CBC | 0.11–0.18 |
| Δ8-THC | 0.12–0.13 |
| Δ9-THC | 0.22–0.37 |
| CBG | 0.07–0.22 |
| CBN | 1.20–2.40 |
| CBDA | 22.91–34.56 |
| THCA | 5.78–5.89 |
| Other 1.48–2.09 | |
| heptanal | 0.22–0.61 |
| 2,4-hexadienal | 0.11 |
| nonanal | 0.37 |
| vanillin | 0.27 |
| tridecanoic acid | 0.21–0.31 |
| ethyl tetradecanoate | 0.42 |
| hexadecenoic acid | 0.25–0.27 |
| ethyl hexadecanoate | 0.22–0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutek, K.; Masek, A. Hemp and Its Derivatives as a Universal Industrial Raw Material (with Particular Emphasis on the Polymer Industry)—A Review. Materials 2022, 15, 2565. https://doi.org/10.3390/ma15072565
Tutek K, Masek A. Hemp and Its Derivatives as a Universal Industrial Raw Material (with Particular Emphasis on the Polymer Industry)—A Review. Materials. 2022; 15(7):2565. https://doi.org/10.3390/ma15072565
Chicago/Turabian StyleTutek, Karol, and Anna Masek. 2022. "Hemp and Its Derivatives as a Universal Industrial Raw Material (with Particular Emphasis on the Polymer Industry)—A Review" Materials 15, no. 7: 2565. https://doi.org/10.3390/ma15072565
APA StyleTutek, K., & Masek, A. (2022). Hemp and Its Derivatives as a Universal Industrial Raw Material (with Particular Emphasis on the Polymer Industry)—A Review. Materials, 15(7), 2565. https://doi.org/10.3390/ma15072565