Processability of a Hot Work Tool Steel Powder Mixture in Laser-Based Powder Bed Fusion
Abstract
:1. Introduction
- Is admixing of gas-atomized spherical pure iron powder with aspherical elemental powders and ferroalloys suitable for the PBF-LB/M fabrication of crack-free samples with high density?
- Does utilization of the admixed alloy significantly interfere with the homogeneous powder particle distribution within the powder bed and the chemical homogeneity of the as-built samples?
- To what extent does the admixing of aspherical particles with spherical iron powder influence the laser light reflection capacity of the powder bed? How much does powder particle size reduction decrease the reflectance of the laser light?
2. Materials and Methods
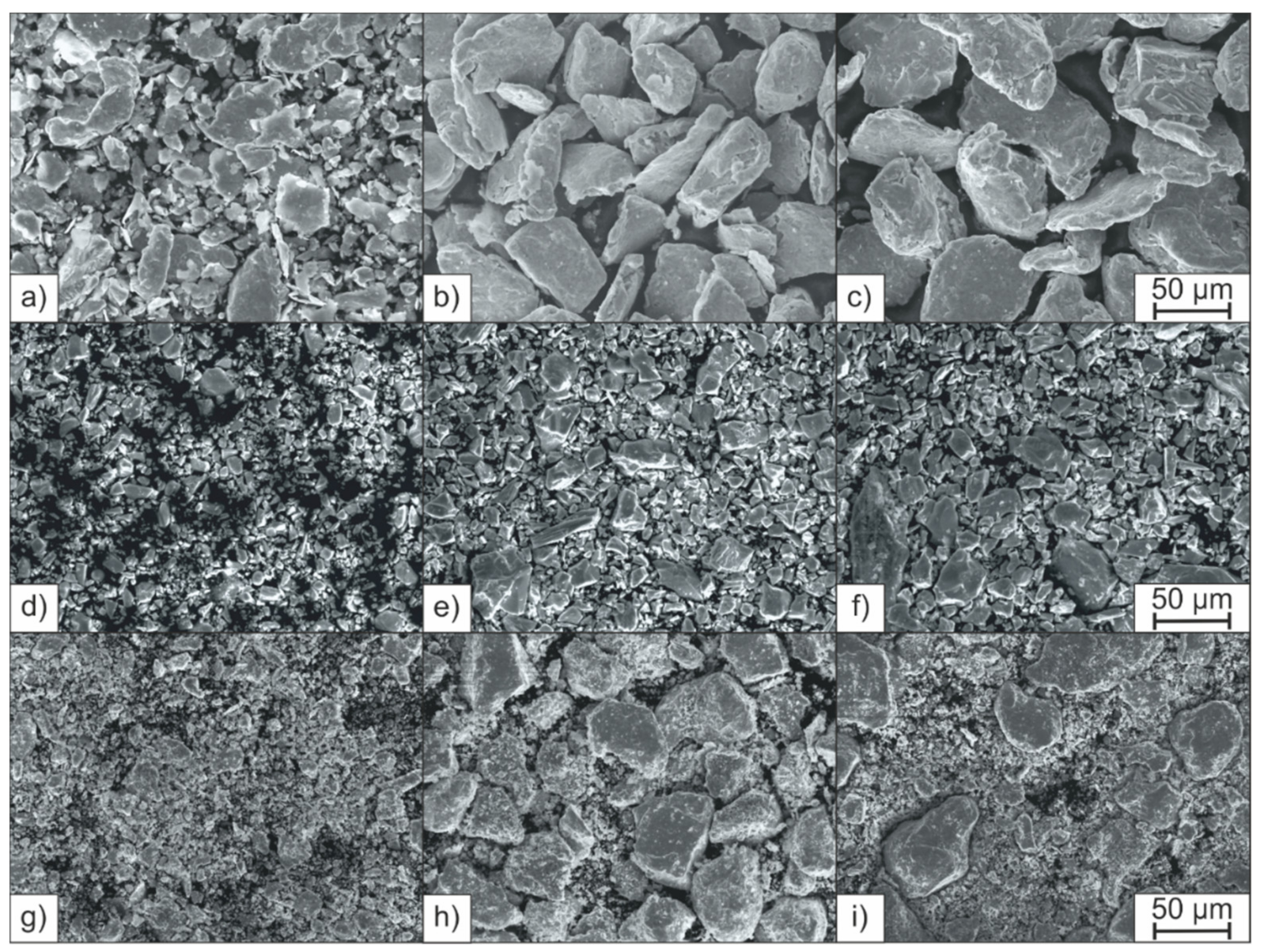
2.1. Alloying Strategy
2.2. Thermodynamic Calculations
2.3. Powder Characterization
2.4. Laser Reflection Measurement
2.5. PBF-LB/M-Fabrication
2.6. Microscopy
2.7. Determination of the Residual Stresses and the Retained Austenite Content
3. Results and Discussion
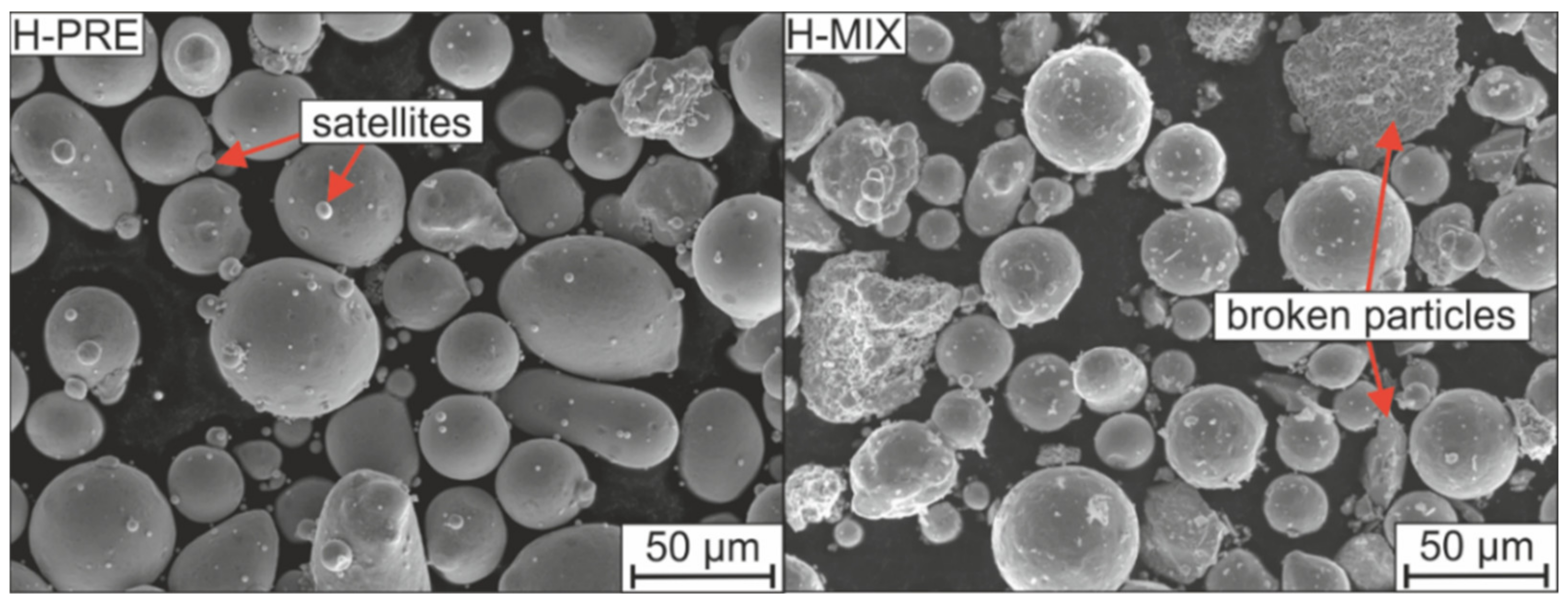
3.1. Powder Morphology and Powder Properties
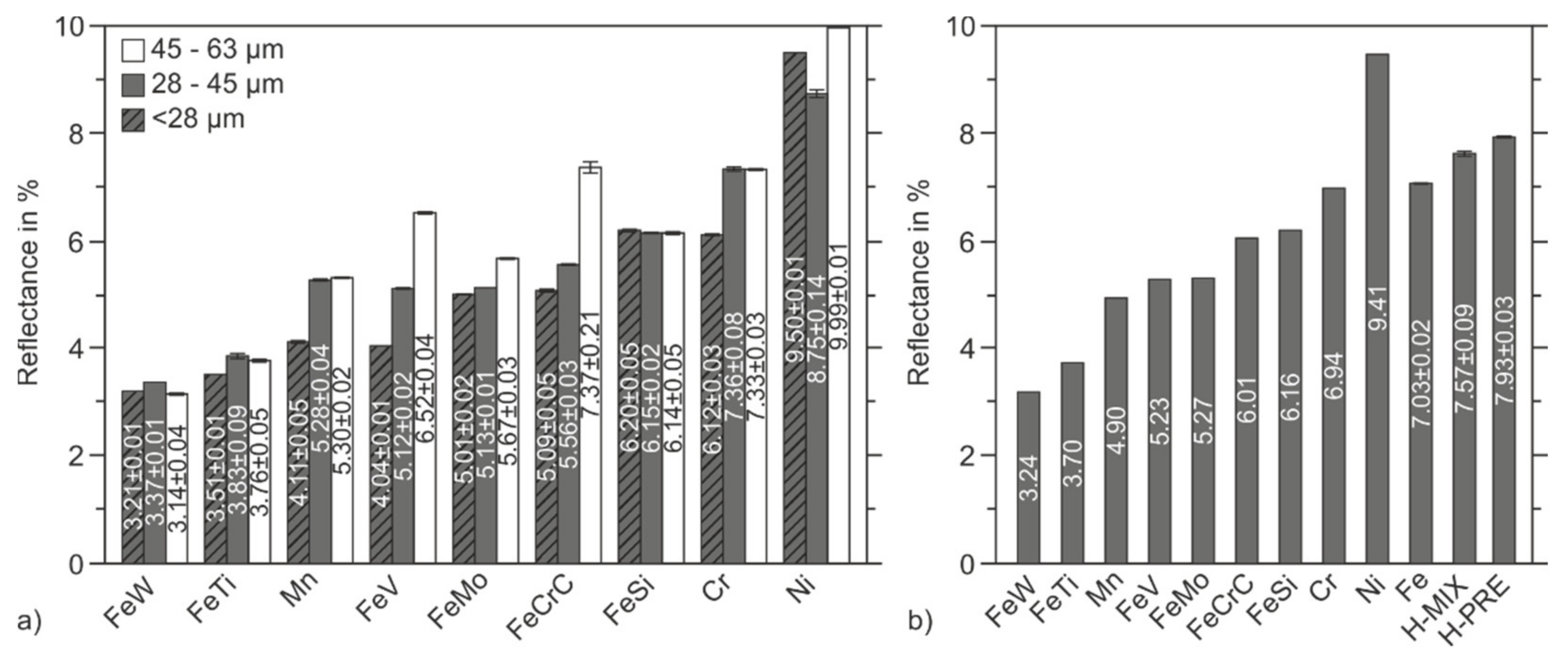
3.2. Laser Reflectance Measurement
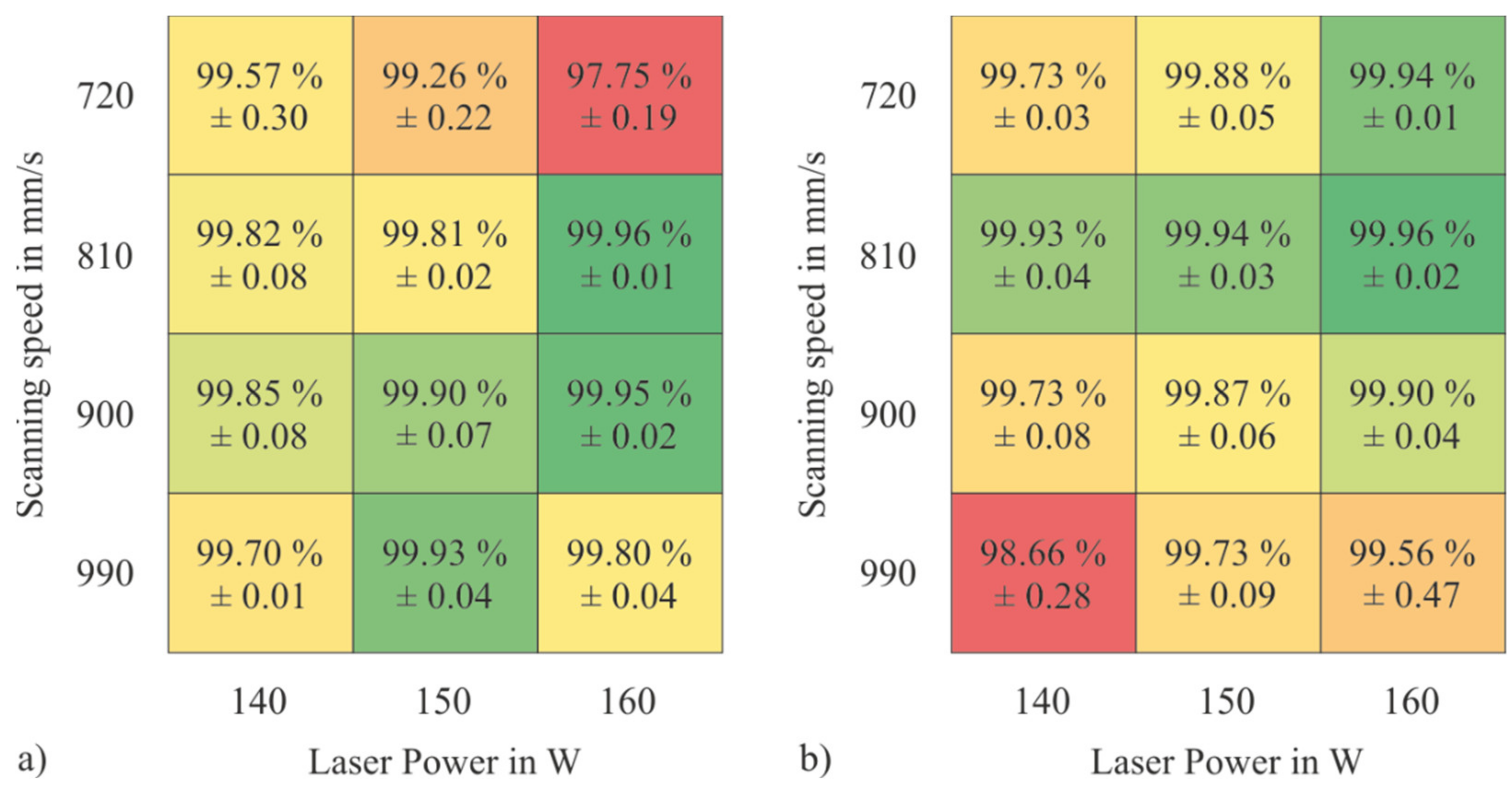
3.3. PBF-LB/M Densification Behavior of the Tool Steel Powders
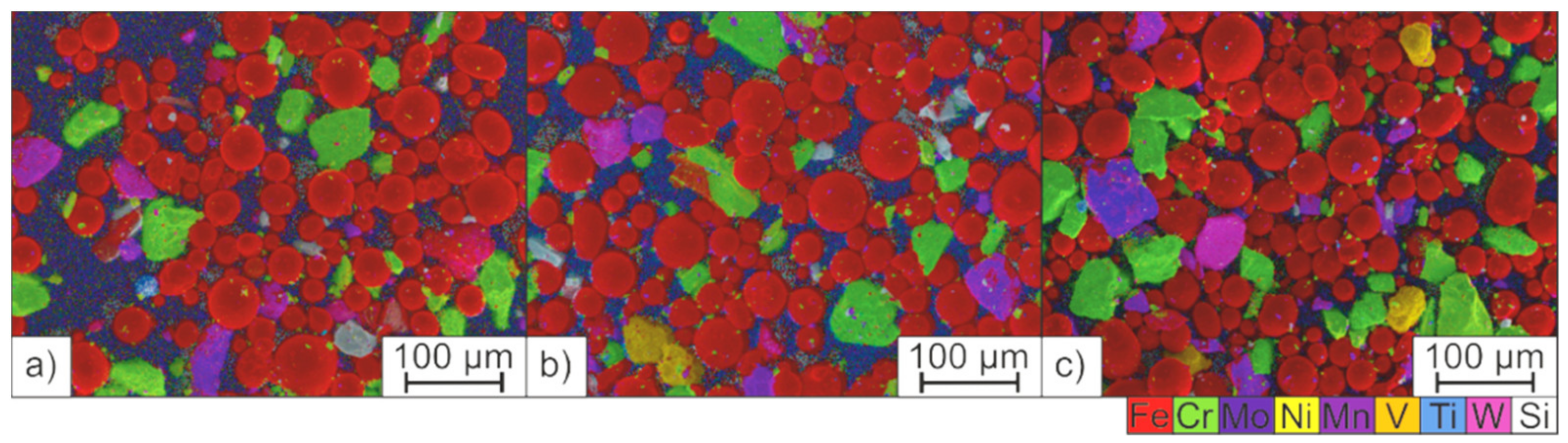
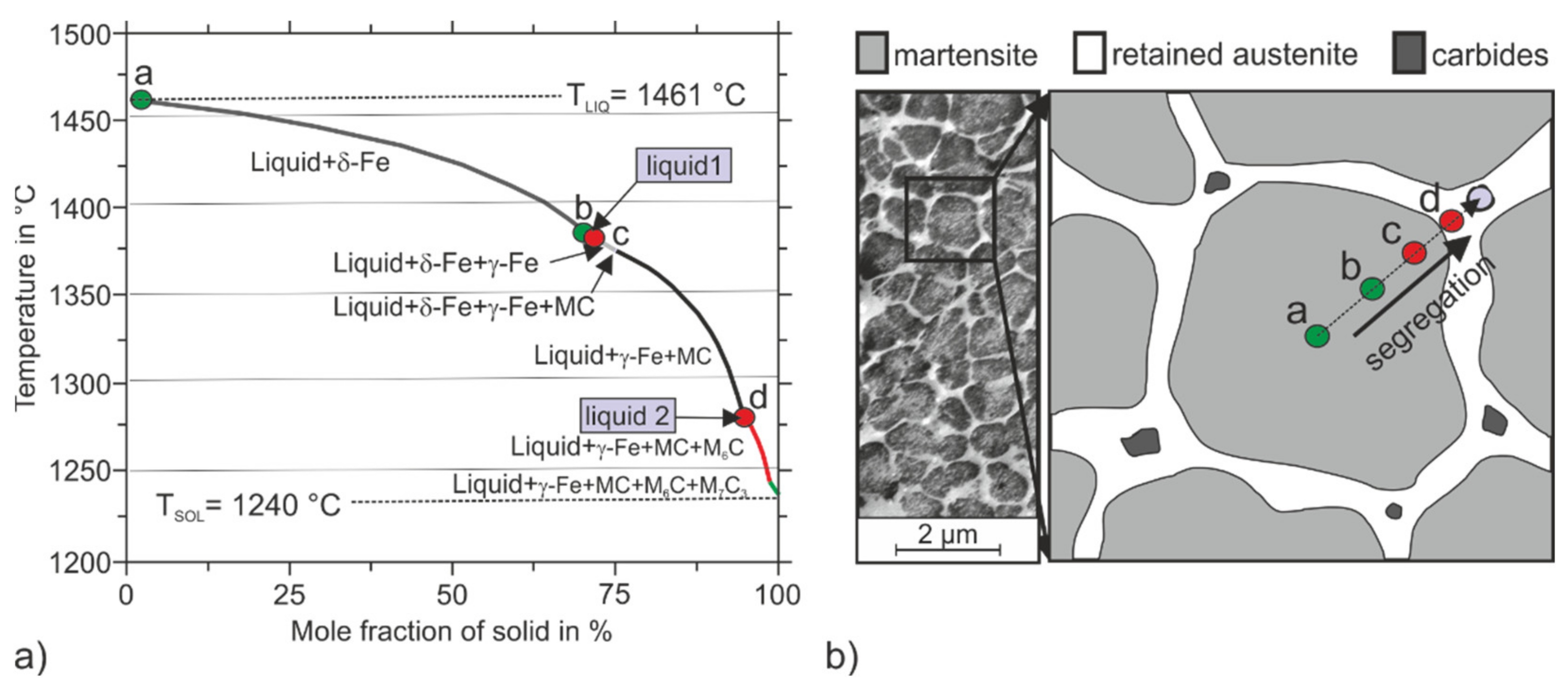
3.4. Solidification and Microstructure of the PBF-LB/M-Fabricated Tool Steel
4. Conclusions
- Admixing of gas-atomized spherical Fe powder with aspherical elemental and ferroalloy particles is suitable for the PBF-LB/M-fabrication of highly dense parts.
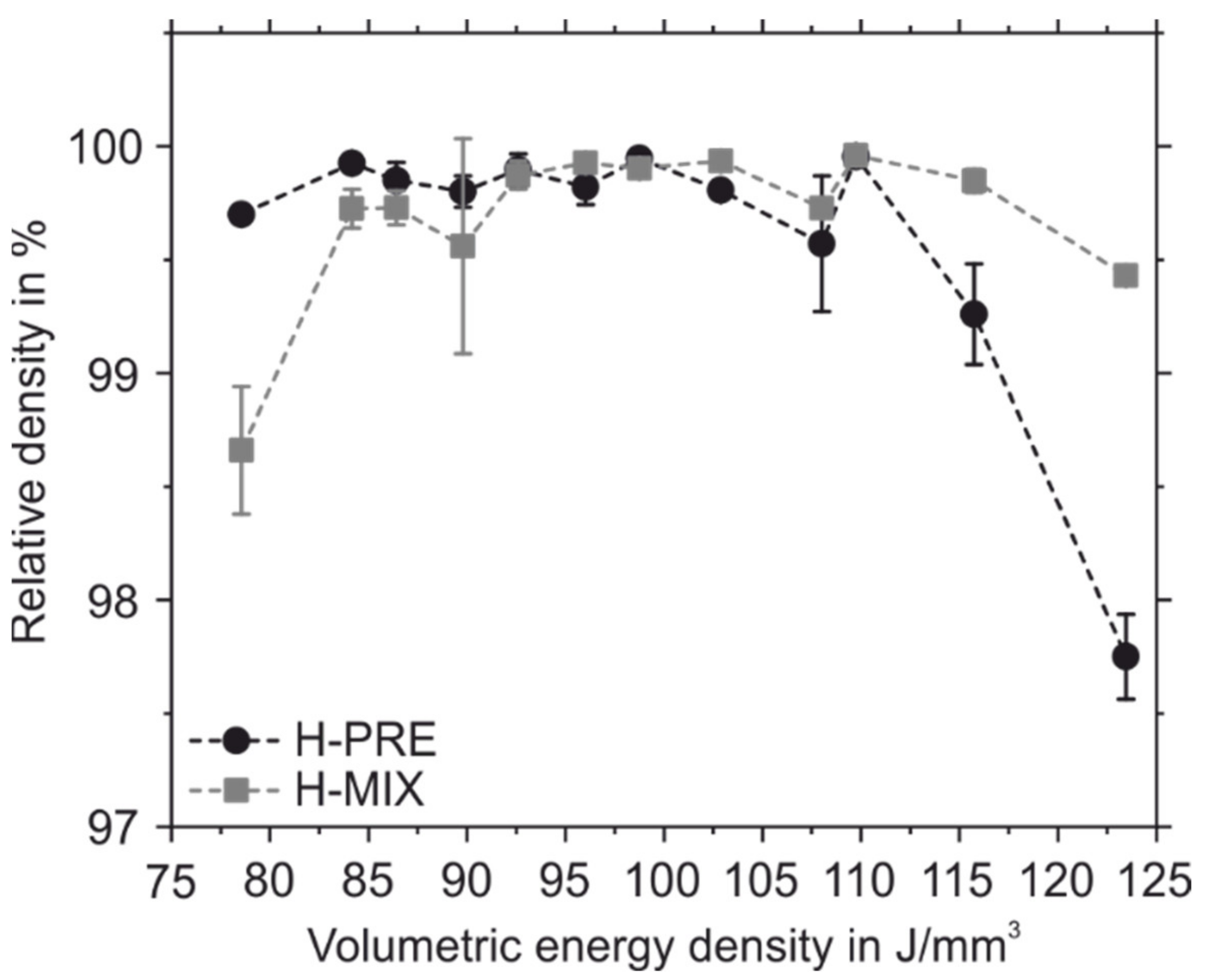
- PBF-LB/M-samples made of the powder mixture tend to have higher relative densities at higher applied volumetric energy densities than samples made of the gas-atomized pre-alloyed feedstock.
- The powder mixture has a lower CBD, poorer flow properties and lower permeability than the gas-atomized pre-alloyed reference material. Nevertheless, the fact that samples with a high relative density of 99.961% could be manufactured shows that the quality of the powder application during the PBF-LB/M-process is still sufficient.
- Mixing gas-atomized Fe powder with aspherical elemental and ferroalloy powders increases the laser reflectance of the mixture compared to the single reflectance of the components. Still, the mixed powder has a lower reflectance than the gas-atomized, pre-alloyed reference material.
- Feedstocks consisting of very fine particles show the lowest reflectance. Fine portions may remain in larger particle fractions after dry screening and thus reduce the reflectance of those feedstocks.
- Taking the LTT alloy concept into account, hot work tool steels can be achieved which can be processed by PBF-LB/M without preheating. The MS temperature should be below 200 °C so that a RA content higher than 20 vol.% is achieved.
- When using the pre-alloyed gas-atomized powder, crack-free, distortion-free, and low-stress specimens could be manufactured.
- The microstructure possesses a hierarchical built-up. A cellular substructure consisting of martensitic cell nuclei surrounded by austenitic seams can be observed.
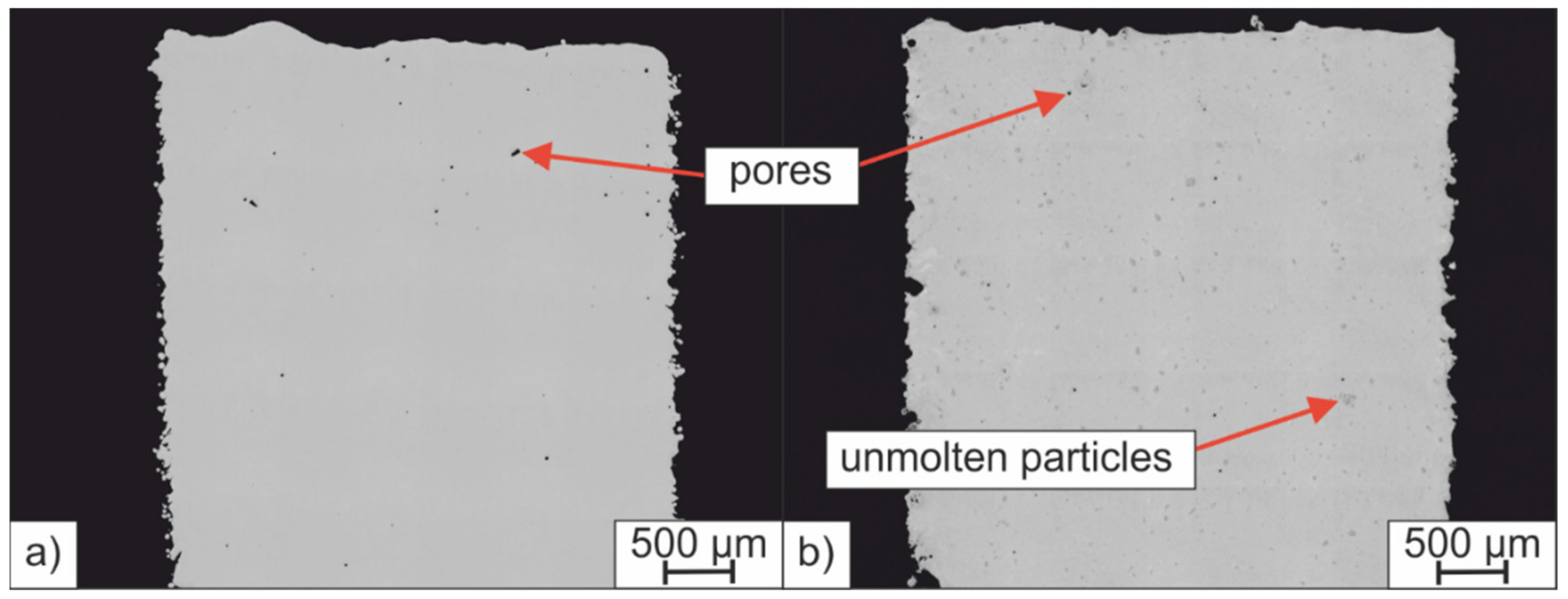
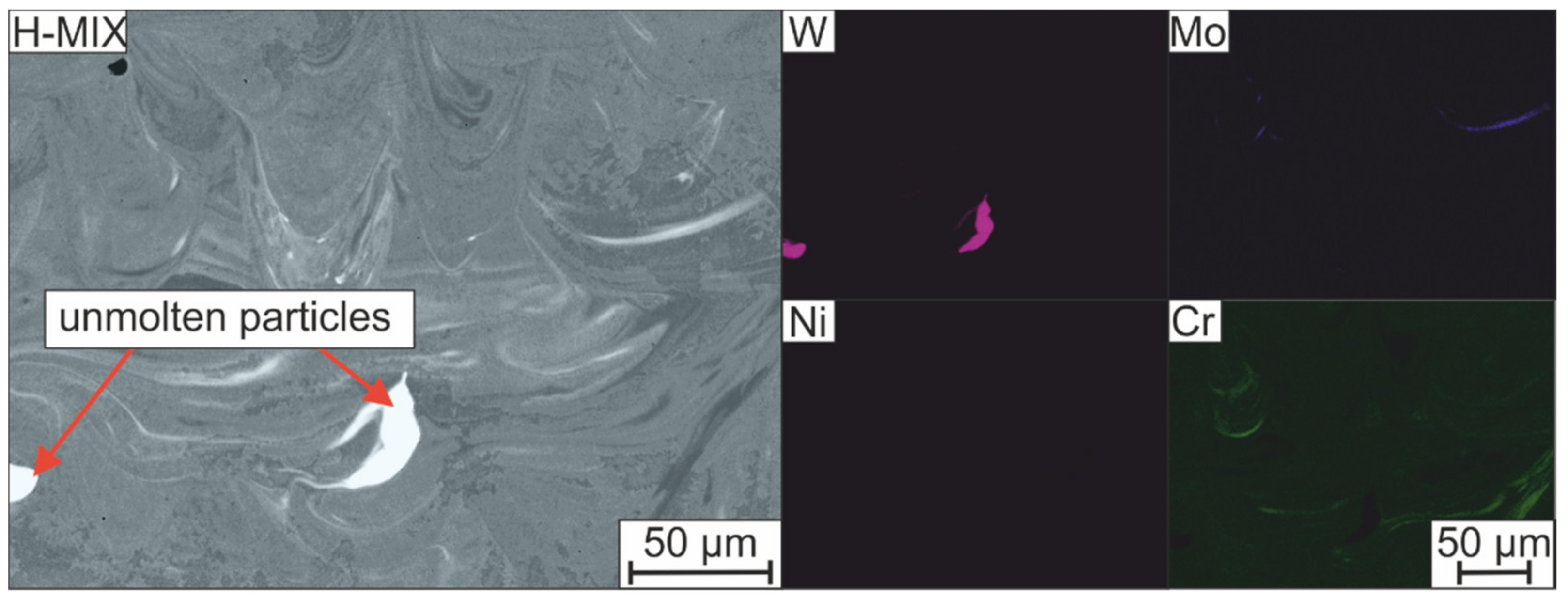
- Unmolten particles can be detected in the microstructure which was created from the powder mixture. The energy introduced into the powder bed was not sufficient to achieve a homogeneous element distribution by sufficient melt convection before solidification. The chemical inhomogeneities can counteract the LTT alloying concept and promote cold cracking.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klocke, F.; Arntz, K.; Teli, M.; Winands, K.; Wegener, M.; Oliari, S. State-of-the-art Laser Additive Manufacturing for Hot-work Tool Steels. Procedia CIRP 2017, 63, 58–63. [Google Scholar] [CrossRef]
- Atzeni, E.; Salmi, A. Economics of additive manufacturing for end-usable metal parts. Int. J. Adv. Manuf. Technol. 2012, 62, 1147–1155. [Google Scholar] [CrossRef]
- Åsberg, M.; Fredriksson, G.; Hatami, S.; Fredriksson, W.; Krakhmalev, P. Influence of post treatment on microstructure, porosity and mechanical properties of additive manufactured H13 tool steel. Mater. Sci. Eng. A 2019, 742, 584–589. [Google Scholar] [CrossRef]
- Mazur, M.; Leary, M.; McMillan, M.; Elambasseril, J.; Brandt, M. SLM additive manufacture of H13 tool steel with conformal cooling and structural lattices. Rapid Prototyp. J. 2016, 22, 504–518. [Google Scholar] [CrossRef]
- Haghdadi, N.; Laleh, M.; Moyle, M.; Primig, S. Additive manufacturing of steels: A review of achievements and challenges. J. Mater. Sci. 2021, 56, 64–107. [Google Scholar] [CrossRef]
- Deirmina, F.; Peghini, N.; AlMangour, B.; Grzesiak, D.; Pellizzari, M. Heat treatment and properties of a hot work tool steel fabricated by additive manufacturing. Mater. Sci. Eng. A 2019, 753, 109–121. [Google Scholar] [CrossRef]
- Krell, J.; Röttger, A.; Geenen, K.; Theisen, W. General investigations on processing tool steel X40CrMoV5-1 with selective laser melting. J. Mater. Processing Technol. 2018, 255, 679–688. [Google Scholar] [CrossRef]
- Mertens, R.; Dadbakhsh, S.; van Humbeeck, J.; Kruth, J.-P. Application of base plate preheating during selective laser melting. Procedia CIRP 2018, 74, 5–11. [Google Scholar] [CrossRef]
- Cheruvathur, S.; Lass, E.A.; Campbell, C.E. Additive Manufacturing of 17-4 PH Stainless Steel: Post-processing Heat Treatment to Achieve Uniform Reproducible Microstructure. JOM 2016, 68, 930–942. [Google Scholar] [CrossRef]
- Geenen, K.; Röttger, A.; Feld, F.; Theisen, W. Microstructure, mechanical, and tribological properties of M3:2 high-speed steel processed by selective laser melting, hot-isostatic pressing, and casting. Addit. Manuf. 2019, 28, 585–599. [Google Scholar] [CrossRef]
- Kučerová, L.; Zetková, I.; Jandová, A.; Bystrianský, M. Microstructural characterisation and in-situ straining of additive-manufactured X3NiCoMoTi 18-9-5 maraging steel. Mater. Sci. Eng. A 2019, 750, 70–80. [Google Scholar] [CrossRef]
- Moyer, J.M.; Ansell, G.S. The volume expansion accompanying the martensite transformation in iron-carbon alloys. Metall. Trans. A 1975, 6, 1785–1791. [Google Scholar] [CrossRef]
- Zenitani, S.; Hayakawa, N.; Yamamoto, J.; Hiraoka, K.; Morikage, Y.; Kubo, T.; Yasuda, K.; Amano, K. Development of new low transformation temperature welding consumable to prevent cold cracking in high strength steel welds. Sci. Technol. Weld. Join. 2007, 12, 516–522. [Google Scholar] [CrossRef]
- Murata, H.; Kato, N.; Tamura, H. Effect of Transformation on Residual Stress in Welding. Stress Releasement by Transformation Superplasticity (Part 5). Q. J. Jpn. Weld. Soc. 1993, 11, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Huo, L.; Zhang, Y.; Wang, D.; Jing, H. New Developed Welding Electrode for Improving the Fatigue Strength of Welded Joints. J. Mater. Sci. Technol. 2002, 18, 527–531. [Google Scholar]
- Eckerlid, J.; Nilsson, T.; Karlsson, L. Fatigue properties of longitudinal attachments welded using low transformation temperature filler. Sci. Technol. Weld. Join. 2003, 8, 353–359. [Google Scholar] [CrossRef]
- Attar, H.; Prashanth, K.G.; Zhang, L.-C.; Calin, M.; Okulov, I.V.; Scudino, S.; Yang, C.; Eckert, J. Effect of Powder Particle Shape on the Properties of In Situ Ti–TiB Composite Materials Produced by Selective Laser Melting. J. Mater. Sci. Technol. 2015, 31, 1001–1005. [Google Scholar] [CrossRef]
- Spierings, A.B.; Herres, N.; Levy, G. Influence of the particle size distribution on surface quality and mechanical properties in AM steel parts. Rapid Prototyp. J. 2011, 17, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Brika, S.E.; Letenneur, M.; Dion, C.A.; Brailovski, V. Influence of particle morphology and size distribution on the powder flowability and laser powder bed fusion manufacturability of Ti-6Al-4V alloy. Addit. Manuf. 2020, 31, 100929. [Google Scholar] [CrossRef]
- Zou, R.P.; Yu, A.B. Evaluation of the packing characteristics of mono-sized non-spherical particles. Powder Technol. 1996, 88, 71–79. [Google Scholar] [CrossRef]
- Manfredi, D.; Calignano, F.; Krishnan, M.; Canali, R.; Ambrosio, E.P.; Atzeni, E. From Powders to Dense Metal Parts: Characterization of a Commercial AlSiMg Alloy Processed through Direct Metal Laser Sintering. Materials 2013, 6, 856–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boley, C.D.; Khairallah, S.A.; Rubenchik, A.M. Calculation of laser absorption by metal powders in additive manufacturing. Appl. Opt. 2015, 54, 2477–2482. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, D.; Yang, Y.; Zhang, H.; Chen, H.; Dai, D.; Lin, K. Influence of Particle Size on Laser Absorption and Scanning Track Formation Mechanisms of Pure Tungsten Powder During Selective Laser Melting. Engineering 2019, 5, 736–745. [Google Scholar] [CrossRef]
- Gusarov, A.V.; Kruth, J.-P. Modelling of radiation transfer in metallic powders at laser treatment. Int. J. Heat Mass Transf. 2005, 48, 3423–3434. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, D.; Dai, D.; Ma, C. Laser energy absorption behavior of powder particles using ray tracing method during selective laser melting additive manufacturing of aluminum alloy. Mater. Des. 2018, 143, 12–19. [Google Scholar] [CrossRef]
- Schwendner, K.I.; Banerjee, R.; Collins, P.C.; Brice, C.A.; Fraser, H.L. Direct laser deposition of alloys from elemental powder blends. Scr. Mater. 2001, 45, 1123–1129. [Google Scholar] [CrossRef]
- Roberts, C.E.; Bourell, D.; Watt, T.; Cohen, J. A Novel Processing Approach for Additive Manufacturing of Commercial Aluminum Alloys. Phys. Procedia 2016, 83, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Taruttis, A.; Hardes, C.; Röttger, A.; Uhlenwinkel, V.; Chehreh, A.B.; Theisen, W.; Walther, F.; Zoch, H.W. Laser additive manufacturing of hot work tool steel by means of a starting powder containing partly spherical pure elements and ferroalloys. Procedia CIRP 2020, 94, 46–51. [Google Scholar] [CrossRef]
- Andrews, K.W. Empirical formulae for the calculation of some transformation temperatures. J. Iron Steel Inst. 1965, 203, 721. [Google Scholar]
- Barbier, D. Extension of the Martensite Transformation Temperature Relation to Larger Alloying Elements and Contents. Adv. Eng. Mater. 2014, 16, 122–127. [Google Scholar] [CrossRef]
- Bagheri, G.; Bonadonna, C. Aerodynamics of Volcanic Particles. In Volcanic Ash; Elsevier: Amsterdam, The Netherlands, 2016; pp. 39–52. [Google Scholar]
- Geenen, K.; Röttger, A.; Theisen, W. Corrosion behavior of 316L austenitic steel processed by selective laser melting, hot-isostatic pressing, and casting. Mater. Corros. 2017, 68, 764–775. [Google Scholar] [CrossRef]
- Parteli, E.J.; Pöschel, T. Particle-based simulation of powder application in additive manufacturing. Powder Technol. 2016, 288, 96–102. [Google Scholar] [CrossRef]
- Mussatto, A.; Groarke, R.; A-Hameed, A.; Ahad, I.U.; Vijayaraghavan, R.K.; O’Neill, A.; McNally, P.; Delaure, Y.; Brabazon, D. Evaluation via powder metallurgy of nano-reinforced iron powders developed for selective laser melting applications. Mater. Des. 2019, 182, 108046. [Google Scholar] [CrossRef]
- Leturia, M.; Benali, M.; Lagarde, S.; Ronga, I.; Saleh, K. Characterization of flow properties of cohesive powders: A comparative study of traditional and new testing methods. Powder Technol. 2014, 253, 406–423. [Google Scholar] [CrossRef]
- Liravi, F.; Vlasea, M. Powder bed binder jetting additive manufacturing of silicone structures. Addit. Manuf. 2018, 21, 112–124. [Google Scholar] [CrossRef]
- Geenen, K. Werkstofftechnische Charakterisierung Austenitischer und Martensitischer Stähle nach dem Selektiven Laserschmelzen: Bildung der Mikrostruktur und Eigenschaften. Ph.D. Thesis, Ruhr-Universität Bochum, Bochum, Germany, 2018. [Google Scholar]
- Großwendt, F.; Röttger, A.; Strauch, A.; Chehreh, A.; Uhlenwinkel, V.; Fechte-Heinen, R.; Walther, F.; Weber, S.; Theisen, W. Additive manufacturing of a carbon-martensitic hot-work tool steel using a powder mixture—Microstructure, post-processing, mechanical properties. Mater. Sci. Eng. A 2021, 827, 142038. [Google Scholar] [CrossRef]
- Chou, C.-Y.; Pettersson, N.H.; Durga, A.; Zhang, F.; Oikonomou, C.; Borgenstam, A.; Odqvist, J.; Lindwall, G. Influence of solidification structure on austenite to martensite transformation in additively manufactured hot-work tool steels. Acta Mater. 2021, 215, 117044. [Google Scholar] [CrossRef]
- Koistinen, D.P.; Marburger, R.E. A general equation prescribing the extent of the austenite-martensite transformation in pure iron-carbon alloys and plain carbon steels. Acta Metall. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Gualco, A.; Svoboda, H.G.; Surian, E.S.; Vedia, L.A.d. Effect of welding procedure on wear behaviour of a modified martensitic tool steel hardfacing deposit. Mater. Des. 2010, 31, 4165–4173. [Google Scholar] [CrossRef]
- Bleck, W.; Guo, X.; Ma, Y. The TRIP Effect and Its Application in Cold Formable Sheet Steels. Steel Res. Int. 2017, 88, 1700218. [Google Scholar] [CrossRef]
- Huber, F.; Rasch, M.; Schmidt, M. Laser Powder Bed Fusion (PBF-LB/M) Process Strategies for In-Situ Alloy Formation with High-Melting Elements. Metals 2021, 11, 336. [Google Scholar] [CrossRef]
- Aota, L.S.; Bajaj, P.; Sandim, H.R.Z.; Jägle, E.A. Laser Powder-Bed Fusion as an Alloy Development Tool: Parameter Selection for In-Situ Alloying Using Elemental Powders. Materials 2020, 13, 3922. [Google Scholar] [CrossRef] [PubMed]






| Elements | C | Cr | Si | Mn | Ni | Mo | Ti | V | W | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Nominal composition | 0.36 | 10.00 | 0.70 | 0.60 | 1.70 | 3.00 | 0.20 | 0.30 | 2.00 | bal. |
| H-PRE | 0.37 ± 0.01 | 10.41 ± 0.07 | 0.75 ± 0.01 | 0.67 ± 0.01 | 1.89 ± 0.01 | 2.97 ± 0.08 | 0.13 ± 0.00 | 0.37 ± 0.01 | 2.89 ± 0.07 | bal. |
| H-MIX | 0.29 ± 0.01 | 8.92 ± 0.06 | 0.56 ± 0.02 | 0.50 ± 0.01 | 1.86 ± 0.07 | 2.34 ± 0.03 | 0.09 ± 0.00 | 0.27 ± 0.02 | 2.14 ± 0.33 | bal. |
| Powder | Circularity | Fe | Cr | Mo | W | Ni | V | Mn | C | Al | Si | Ti | Calculated Tm or TLiq in °C | Content in Mass% | Content in vol.% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FeCrC | 0.58 | 28.1 | 61.2 | - | - | - | - | 0.3 | 7.5 | - | 2.9 | - | 1563 | 4.35 | 4.91 |
| FeMo | 0.55 | 20.3 | - | 69.7 | - | - | - | - | - | - | - | - | 1895 | 4.36 | 3.87 |
| FeW | 0.59 | 19.0 | - | - | 80.5 | - | - | - | - | - | 0.5 | - | 2719 | 2.62 | 1.95 |
| FeV | 0.51 | 17.2 | - | - | - | - | 80.5 | - | 0.2 | 1.4 | 0.7 | - | 1447 | 0.38 | 0.47 |
| FeTi | 0.61 | 29.1 | - | - | - | - | - | - | - | - | - | 70.9 | 1117 | 0.28 | 0.40 |
| FeSi | 0.51 | 24.3 | - | - | - | - | - | - | - | 0.1 | 75.5 | 0.1 | 1191 | 0.85 | 1.80 |
| Cr | 0.54 | - | 99.9 | - | - | - | - | - | - | - | - | - | 1907 | 7.13 | 7.77 |
| Ni | 0.64 | - | - | - | - | 99.9 | - | - | - | - | - | - | 1455 | 1.69 | 1.47 |
| Mn | 0.55 | - | - | - | - | - | - | 99.9 | - | - | - | - | 1414 | 0.58 | 0.61 |
| Fe | 0.85 | 99.9 | - | - | - | - | - | - | - | - | - | - | 1538 | 77.75 | 76.75 |
| H-PRE | H-MIX | Pure Fe | |
|---|---|---|---|
| d (0.1) | 31.60 | 29.10 | 25.21 |
| d (0.5) | 47.57 | 46.05 | 42.41 |
| d (0.9) | 71.28 | 72.33 | 69.49 |
| H-PRE | H-MIX | |
|---|---|---|
| CBD in g/mL | 4.67 ± 0.00 | 4.43 ± 0.02 |
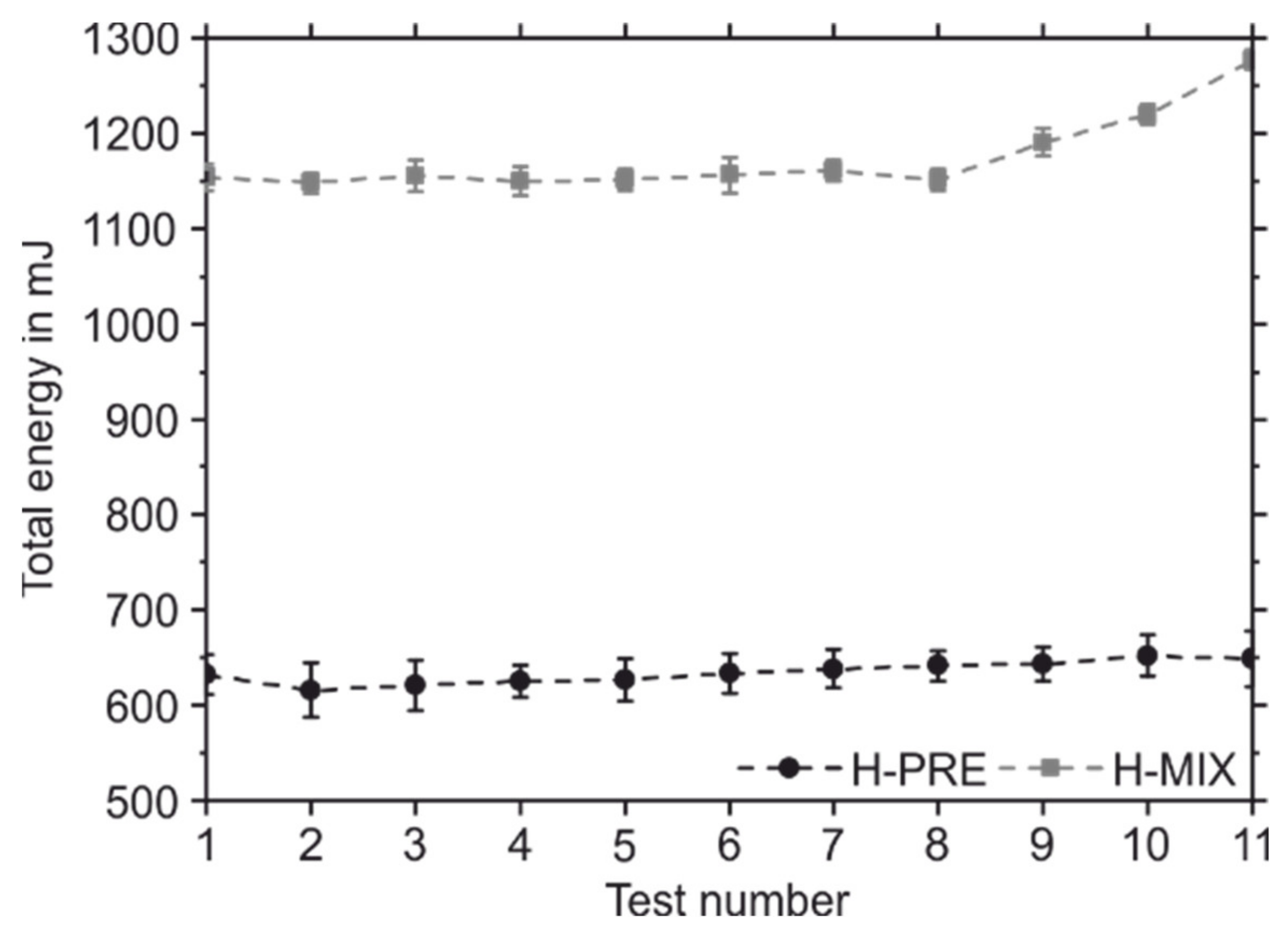
| BFE in mJ | 638.00 ± 19.98 | 1161.33 ± 11.14 |
| SE in mJ/g | 2.32 ± 0.04 | 3.61 ± 0.03 |
| FRI | 1.01 ± 0.02 | 1.11 ± 0.01 |
| PD @ 15 kPa in mbar | 4.80 ± 0.07 | 7.19 ± 0.11 |
| T in °C | C | Cr | Mo | W | Ti | V | Si | Mn | Ni | Ms in °C | RA in vol.% | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 1461 | 0.04 | 9.64 | 2.45 | 1.53 | 0.02 | 0.18 | 0.58 | 0.42 | 1.42 | 349.16 | 3.80 |
| b | 1381 | 0.09 | 9.77 | 2.77 | 1.85 | 0.03 | 0.25 | 0.82 | 0.61 | 1.91 | 309.59 | 5.60 |
| liquid 1 | 1381 | 1.03 | 10.77 | 4.13 | 2.97 | 0.26 | 0.47 | 0.79 | 0.89 | 2.26 | - | - |
| c | 1380 | 0.31 | 9.14 | 2.07 | 1.34 | 0.06 | 0.19 | 0.74 | 0.69 | 2.32 | 219.71 | 13.7 |
| d | 1274 | 0.54 | 10.72 | 3.53 | 2.46 | 0.04 | 0.27 | 1.04 | 0.96 | 2.51 | 80.78 | 55.0 |
| liquid 2 | 1274 | 1.42 | 11.81 | 5.50 | 4.05 | 0.44 | 0.64 | 0.79 | 0.97 | 2.14 | - | - |
| nominal | - | 0.36 | 10.00 | 3.00 | 2.00 | 0.20 | 0.30 | 0.80 | 0.60 | 1.80 | 193.12 | 17.90 |
| RA in vol.% | Compressive Residual Stress in MPa | |
|---|---|---|
| H-PRE | 21.74 ± 1.15 | −142.00 ± 82.52 |
| H-MIX | 27.24 ± 2.00 | −113.33 ± 57.38 |
| Cr | Si | Mn | Ni | Mo | Ti | V | W | Fe | |
|---|---|---|---|---|---|---|---|---|---|
| Unmolten FeW particles | 0.60 ± 0.20 | 0.00 ± 0.00 | 0.17 ± 0.09 | 0.99 ± 0.33 | 0.00 ± 0.00 | 0.14 ± 0.14 | 0.07 ± 0.07 | 85.30 ± 0.74 | 7.84 ± 0.73 |
| Matrix | 11.4 ± 0.36 | 0.98 ± 0.10 | 0.48 ± 0.11 | 1.97 ± 0.22 | 1.94 ± 0.39 | 0.03 ± 0.04 | 0.42 ± 0.05 | 0.61 ± 0.05 | 80.48 ± 0.19 |
| Nominal | 10.00 | 0.70 | 0.60 | 1.80 | 3.00 | 0.15 | 0.30 | 2.00 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hantke, N.; Großwendt, F.; Strauch, A.; Fechte-Heinen, R.; Röttger, A.; Theisen, W.; Weber, S.; Sehrt, J.T. Processability of a Hot Work Tool Steel Powder Mixture in Laser-Based Powder Bed Fusion. Materials 2022, 15, 2658. https://doi.org/10.3390/ma15072658
Hantke N, Großwendt F, Strauch A, Fechte-Heinen R, Röttger A, Theisen W, Weber S, Sehrt JT. Processability of a Hot Work Tool Steel Powder Mixture in Laser-Based Powder Bed Fusion. Materials. 2022; 15(7):2658. https://doi.org/10.3390/ma15072658
Chicago/Turabian StyleHantke, Nick, Felix Großwendt, Anna Strauch, Rainer Fechte-Heinen, Arne Röttger, Werner Theisen, Sebastian Weber, and Jan Torsten Sehrt. 2022. "Processability of a Hot Work Tool Steel Powder Mixture in Laser-Based Powder Bed Fusion" Materials 15, no. 7: 2658. https://doi.org/10.3390/ma15072658







