The Improvement of Durability of Reinforced Concretes for Sustainable Structures: A Review on Different Approaches
Abstract
:1. Introduction
2. Corrosion Mechanisms in Reinforced Concrete Structures
3. Corrosion Inhibitors and Surface Treatments
- (a)
- Hydrophobic treatments, based on silanes, siloxanes and silicones;
- (b)
- Treatments able to seal the capillary pores, based on sodium silicate or magnesium fluorosilicates;
- (c)
- Organic coatings forming a continuous film, with a thickness between 0.1–0.3 mm, thermoplastic (acrylic, vinyl) or thermosetting (epoxy, polyurethane);
- (d)
- Cementitious mortars containing acrylic or vinyl polymers with polymer/cement ratio in the range of 0.3–0.6 and thickness between 1 and 5 mm.
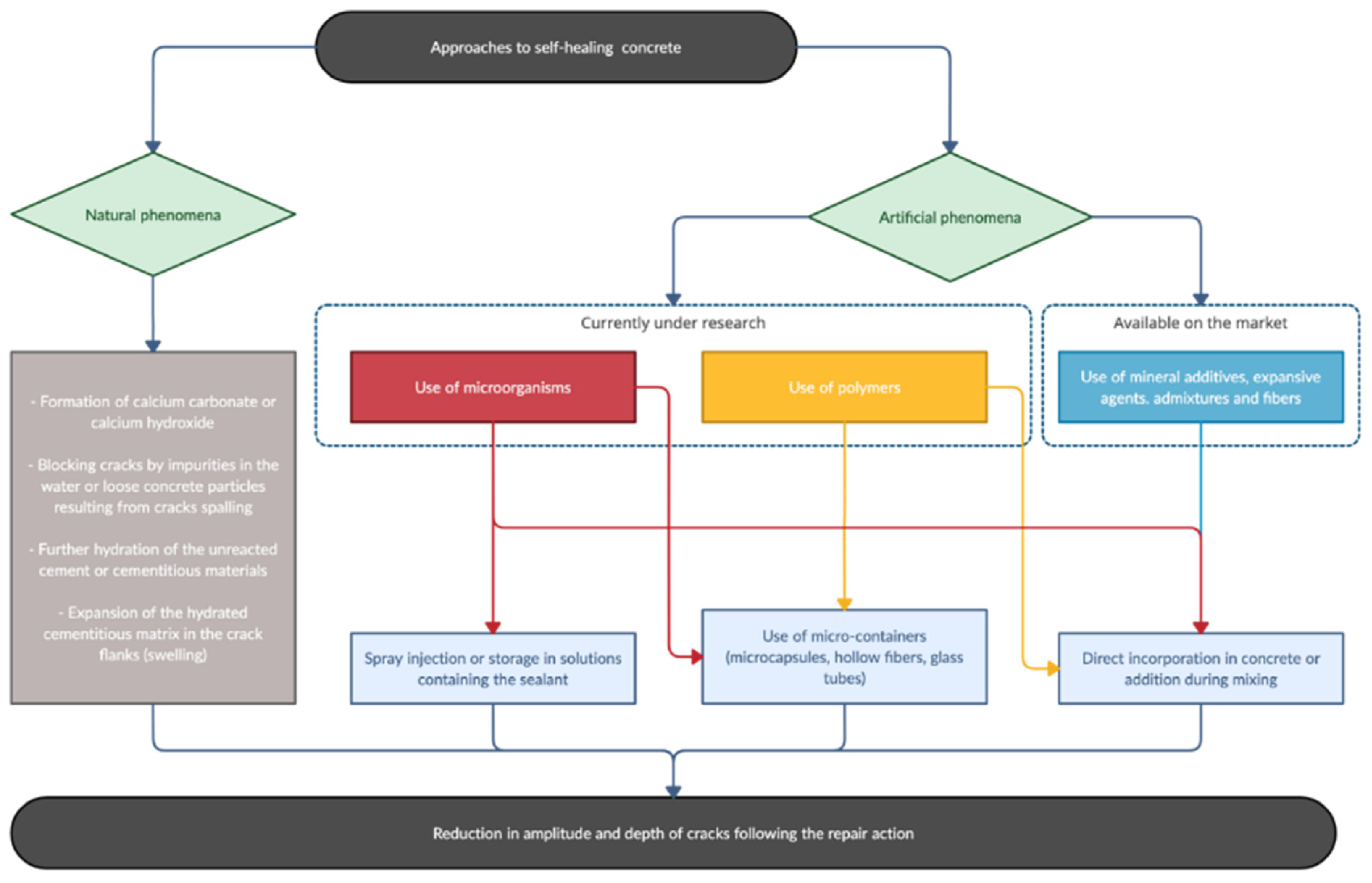
4. Self-Healing Strategies for High Durability Concrete
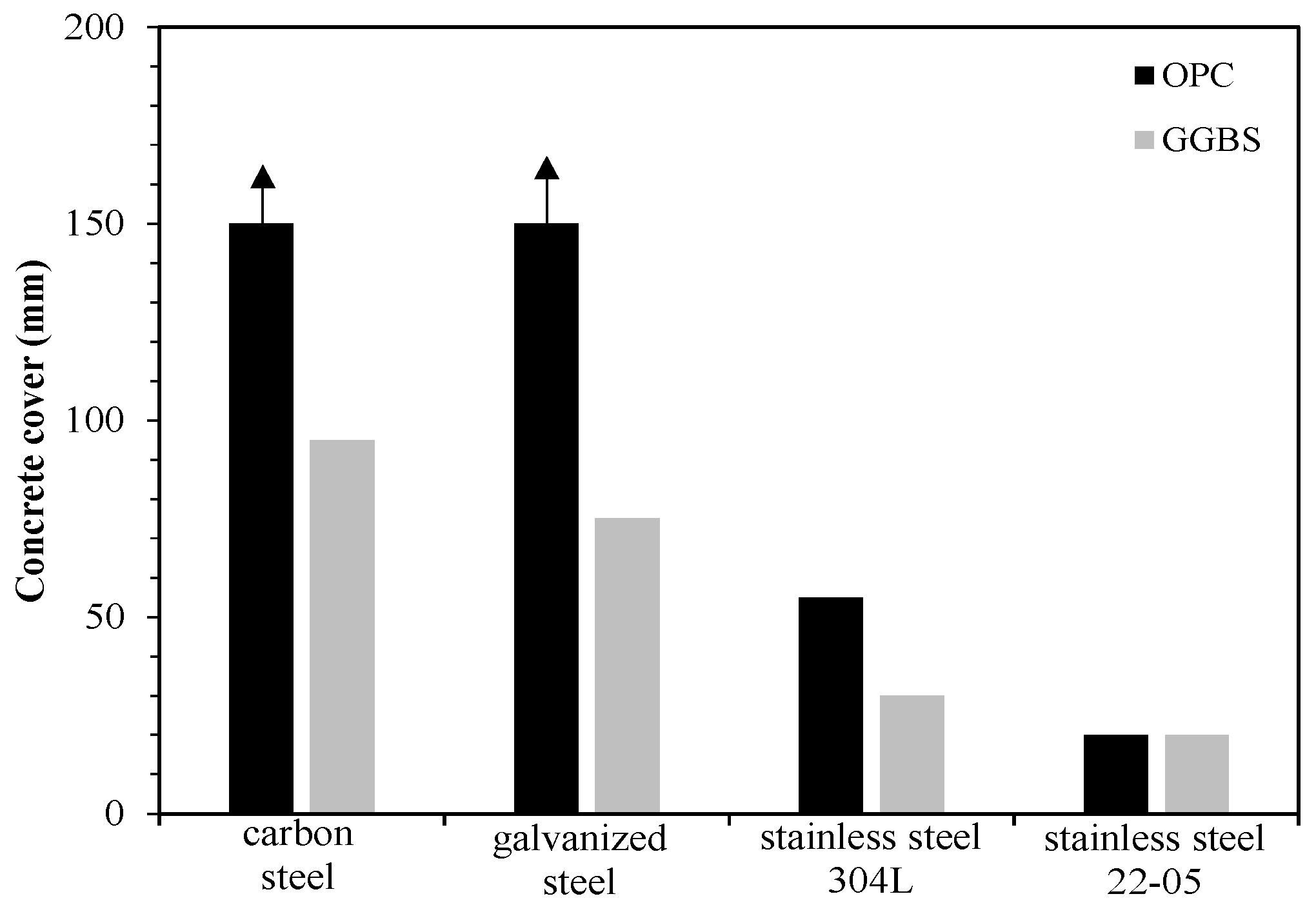
5. Corrosion Resistant Reinforcements
6. Durability Design
7. Waterproofing Recycled Aggregates
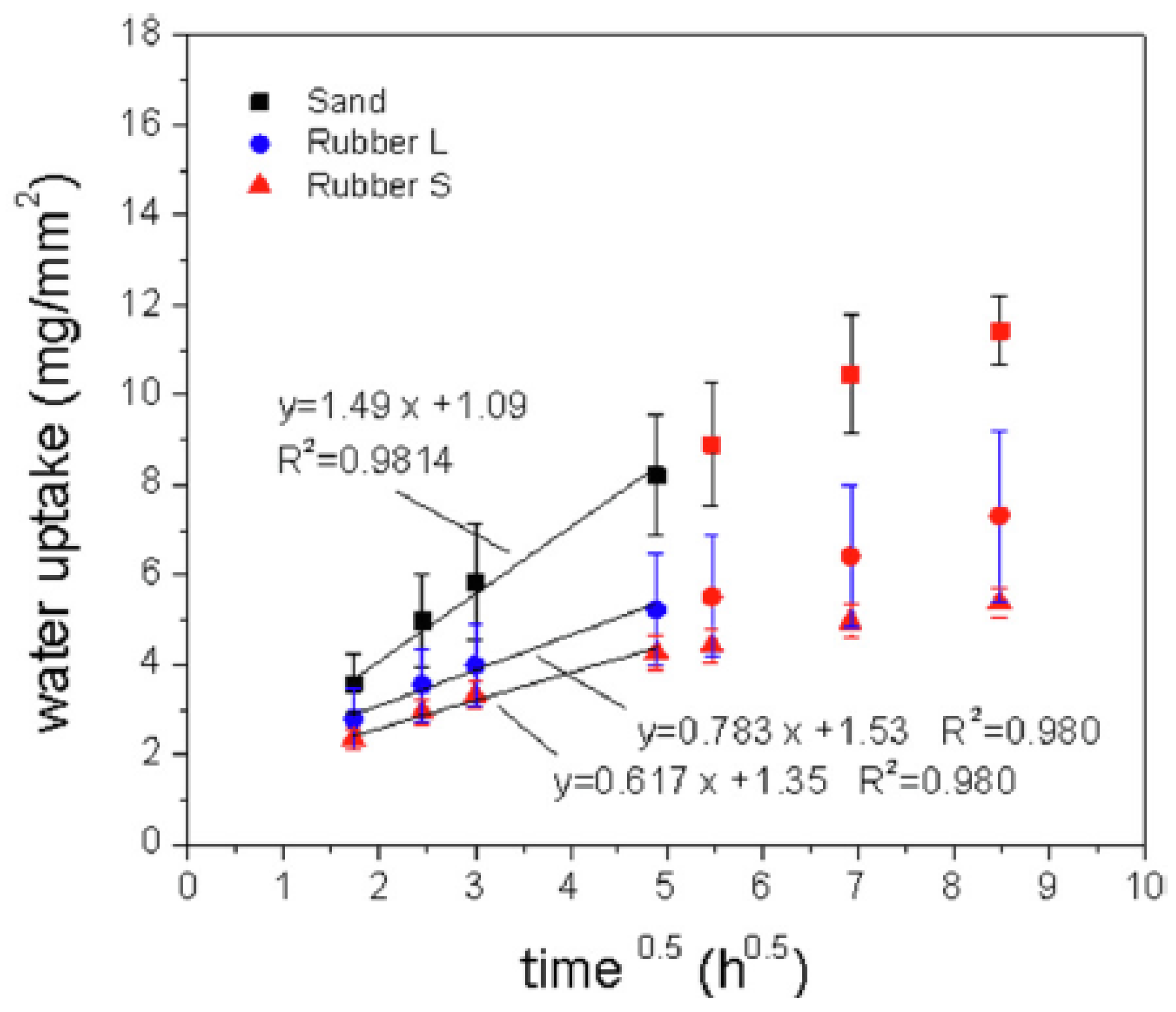
8. Durability of Special Mixtures
8.1. Fly Ash-Based Geopolymers
8.2. Alkali Activated Materials
8.3. Calcium Sulfoaluminate Cements
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damineli, B.L.; Kemeid, F.M.; Aguiar, P.S.; John, V.M. Measuring the eco-efficiency of cement use. Cem. Concr. Compos. 2010, 32, 555–562. [Google Scholar] [CrossRef]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction—Part 1. J. Appl. Biomater. Funct. Mater. 2018, 16, 186–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction–Part 2. J. Appl. Biomater. Funct. Mater. 2018, 16, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagüés, A.A.; Moreno, E.I.; Andrade, C. Evolution of pH during in-situ leaching in small concrete cavities. Cem. Concr. Res. 1997, 27, 1747–1759. [Google Scholar] [CrossRef]
- Räsänen, V.; Penttala, V. The pH measurement of concrete and smoothing mortar using a concrete powder suspension. Cem. Concr. Res. 2004, 34, 813–820. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T. Studio elettrochimico della formazione del film di passività sulle armature del calcestruzzo. In Giornate Naz; Della Corros.: Naples, Italy, 2013. [Google Scholar]
- Poursaee, A.; Hansson, C.M. Reinforcing steel passivation in mortar and pore solution. Cem. Concr. Res. 2007, 37, 1127–1133. [Google Scholar] [CrossRef]
- Andrade, C.; Keddam, M.; Nóvoa, X.R.; Pérez, M.C.; Rangel, C.M.; Takenouti, H. Electrochemical behaviour of steel rebars in concrete: Influence of environmental factors and cement chemistry. Electrochim. Acta 2001, 46, 3905–3912. [Google Scholar] [CrossRef]
- Alonso, M.C.; Sanchez, M. Analysis of the variability of chloride threshold values in the literature. Mater. Corros. 2009, 60, 631–637. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.; Vennesland, O. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Angst, U.; Vennesland, Ø. Critical chloride content in reinforced concrete. In Concrete Repair, Rehabilitation and Retrofitting II2; Taylor & Francis Group: London, UK, 2009; pp. 311–317. [Google Scholar]
- PAGE, C.L. Mechanism of corrosion protection in reinforced concrete marine structures. Nature 1975, 258, 514–515. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T. Cyclic voltammetry evaluation of inhibitors for localised corrosion in alkaline solutions. Electrochim. Acta 2014, 124, 156–164. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T. Study of localized corrosion of steel reinforcement in inhibited alkaline solutions [Studio della corrosione localizzata degli acciai per armature in soluzioni alcaline inibite]. Metall. Ital. 2013, 105, 21–31. [Google Scholar]
- Hausmann, D.A. Steel Corrosion in Concrete—How Does It Occur? Mater. Prot. 1967, 6, 19–23. [Google Scholar]
- Gouda, V.K. Corrosion and corrosion inhibition of reinforcing steel I. immersed in alkaline solutions. Br. Corros. J. 1970, 5, 198–203. [Google Scholar] [CrossRef]
- Goni, S.; Andrade, C. Synthetic concrete pore solution chemistry and rebar corrosion rate in the presence of chlorides. Cem. Concr. Res. 1990, 20, 525–539. [Google Scholar] [CrossRef]
- Diamond, S. Chloride concentrations in concrete pore solutions resulting from calcium and sodium chloride admixtures. Cem. Concr. Aggreg. 1988, 44, 489–499. [Google Scholar]
- Yonezawa, T.; Ashworth, V.; Procter, R.P.M. Pore Solution Composition and Chloride Effects on the Corrosion of Steel in Concrete. Corrosion 1988, 44, 489–499. [Google Scholar] [CrossRef]
- Glass, G.K.; Buenfeld, N.R. Theoretical basis for designing reinforced concrete cathodic protection systems. Br. Corros. J. 1997, 32, 179–184. [Google Scholar] [CrossRef]
- Arya, C.; Buenfeld, N.R.; Newman, J.B. Factors influencing chloride-binding in concrete. Cem. Concr. Res. 1990, 20, 291–300. [Google Scholar] [CrossRef]
- Luping, T.; Nilsson, L.-O. Chloride binding capacity and binding isotherms of OPC pastes and mortars. Cem. Concr. Res. 1993, 23, 247–253. [Google Scholar] [CrossRef]
- Suryavanshi, A.K.; Scantlebury, J.D.; Lyon, S.B. Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cem. Concr. Res. 1996, 26, 717–727. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Ekolu, S.O.; Thomas, M.D.A.; Hooton, R.D. Pessimum effect of externally applied chlorides on expansion due to delayed ettringite formation: Proposed mechanism. Cem. Concr. Res. 2006, 36, 688–696. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Nawa, T.; Kurumisawa, K. Electrokinetic potential of hydrated cement in relation to adsorption of chlorides. Cem. Concr. Res. 2009, 39, 340–344. [Google Scholar] [CrossRef]
- Hirao, H.; Yamada, K.; Takahashi, H.; Zibara, H. Chloride binding of cement estimated by binding isotherms of hydrates. J. Adv. Concr. Technol. 2005, 3, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Tittarelli, F. Oxygen diffusion through hydrophobic cement-based materials. Cem. Concr. Res. 2009, 39, 924–928. [Google Scholar] [CrossRef]
- De Belie, N.; Gruyaert, E.; Al-Tabbaa, A.; Antonaci, P.; Baera, C.; Bajare, D.; Darquennes, A.; Davies, R.; Ferrara, L.; Jefferson, T.; et al. A Review of Self-Healing Concrete for Damage Management of Structures. Adv. Mater. Interfaces 2018, 5, 1800074. [Google Scholar] [CrossRef]
- Berra, M.; Colombo, A.; Pastore, T. Normal and total-lightweight reinforced concretes under cyclic immersion in 3.5% chloride solution. In Concrete under Severe Conditions: Environment and Loading; E&FN Spon: London, UK, 1995; pp. 595–604. [Google Scholar]
- Berra, M.; Melchiorri, G.; Pastore, T. Durability tests on normal and total-lightweight reinforced concretes in marine environment. In Proceedings of the Fourth International Symposium on Utilization of High Strength/High Performance Concrete, Paris, France, 29–31 May 1996. [Google Scholar]
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Redaelli, E.; Polder, R.B. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair; Wiley WCH: Hoboken, NJ, USA, 2013. [Google Scholar]
- Basheer, P.A.M.; Basheer, L.; Cleland, D.J.; Long, A.E. Surface treatments for concrete: Assessment methods and reported performance. Constr. Build. Mater. 1997, 11, 413–429. [Google Scholar] [CrossRef]
- Swamy, R.N.; Tanikawa, S. An external surface coating to protect concrete and steel from aggressive environments. Mater. Struct. 1993, 26, 465–478. [Google Scholar] [CrossRef]
- Tittarelli, F.; Moriconi, G. The effect of silane-based hydrophobic admixture on corrosion of reinforcing steel in concrete. Cem. Concr. Res. 2008, 38, 1354–1357. [Google Scholar] [CrossRef]
- Coffetti, D.; Crotti, E.; Gazzaniga, G.; Gottardo, R.; Pastore, T.; Coppola, L. Protection of Concrete Structures: Performance Analysis of Different Commercial Products and Systems. Materials 2021, 14, 3719. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, M.V.; Brenna, A.; Bolzoni, F.; Berra, M.; Pastore, T.; Ormellese, M. Effect of polymer modified cementitious coatings on water and chloride permeability in concrete. Constr. Build. Mater. 2013, 49, 720–728. [Google Scholar] [CrossRef]
- Brenna, A.; Beretta, S.; Berra, M.; Diamanti, M.V.; Ormellese, M.; Pastore, T.; Pedeferri, M.P.; Bolzoni, F. Effect of polymer modified cementitious coatings on chloride-induced corrosion of steel in concrete. Struct. Concr. 2019, 21, suco.201900255. [Google Scholar] [CrossRef]
- Elsener, B. Corrosion Inhibitors for Steel in Concrete: State of the Art Report. Constr. Build. Mater. 2001, 22, 609–622. [Google Scholar]
- Pastore, T.; Cabrini, M.; Coppola, L.; Lorenzi, S.; Marcassoli, P.; Buoso, A. Evaluation of the corrosion inhibition of salts of organic acids in alkaline solutions and chloride contaminated concrete. Mater. Corros. 2011, 62, 187–195. [Google Scholar] [CrossRef]
- Bolzoni, F.; Coppola, L.; Goidanich, S.; Lazzari, L.; Ormellese, M.; Pedeferri, M.P. Corrosion inhibitors in reinforced concrete structures Part 1: Preventative technique. Corros. Eng. Sci. Technol. 2004, 39, 219–228. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Gazzaniga, G.; Pastore, T. Chloride Diffusion in Concrete Protected with a Silane-Based Corrosion Inhibitor. Materials 2020, 13, 2001. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Coppola, L.; Coffetti, D.; Pastore, T. Inhibition effect of tartrate ions on the localized corrosion of steel in pore solution at different chloride concentration. Buildings 2020, 10, 105. [Google Scholar] [CrossRef]
- Berke, N.S.; Hicks, M.C. Predicting long-term durability of steel reinforced concrete with calcium nitrite corrosion inhibitor. Cem. Concr. Compos. 2004, 26, 191–198. [Google Scholar] [CrossRef]
- Bellezze, T.; Timofeeva, D.; Giuliani, G.; Roventi, G. Effect of soluble inhibitors on the corrosion behaviour of galvanized steel in fresh concrete. Cem. Concr. Res. 2018, 107, 1–10. [Google Scholar] [CrossRef]
- Maeder, U. A New Class of Corrosion Inhibitors for Reinforced Concrete. Spec. Publ. 1996, 163, 215–232. [Google Scholar] [CrossRef]
- Cabrini, M.; Fontana, F.; Lorenzi, S.; Pastore, T.; Pellegrini, S. Effect of Organic Inhibitors on Chloride Corrosion of Steel Rebars in Alkaline Pore Solution. J. Chem. 2015, 2015, 521507. [Google Scholar] [CrossRef] [Green Version]
- Ormellese, M.; Bolzoni, F.; Goidanich, S.; Pedeferri, M.; Brenna, A. Corrosion inhibitors in reinforced concrete structures Part 3–migration of inhibitors into concrete. Corros. Eng. Sci. Technol. 2011, 46, 334–339. [Google Scholar] [CrossRef]
- Bolzoni, F.; Brenna, A.; Fumagalli, G.; Goidanich, S.; Lazzari, L.; Ormellese, M.; Pedeferri, M. Experiences on corrosion inhibitors for reinforced concrete. Int. J. Corros. Scale Inhib. 2014, 3, 254–278. [Google Scholar] [CrossRef]
- Monticelli, C.; Frignani, A.; Balbo, A.; Zucchi, F. Influence of two specific inhibitors on steel corrosion in a synthetic solution simulating a carbonated concrete with chlorides. Mater. Corros. 2011, 62, 178–186. [Google Scholar] [CrossRef]
- Djerbi, A.; Bonnet, S.; Khelidj, A.; Baroghel-bouny, V. Influence of traversing crack on chloride diffusion into concrete. Cem. Concr. Res. 2008, 38, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Marsavina, L.; Audenaert, K.; de Schutter, G.; Faur, N.; Marsavina, D. Experimental and numerical determination of the chloride penetration in cracked concrete. Constr. Build. Mater. 2009, 23, 264–274. [Google Scholar] [CrossRef]
- Kwon, S.J.; Na, U.J.; Park, S.S.; Jung, S.H. Service life prediction of concrete wharves with early-aged crack: Probabilistic approach for chloride diffusion. Struct. Saf. 2009, 31, 75–83. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Q.; Ashour, A.; Han, B. Self-healing cement concrete composites for resilient infrastructures: A review. Compos. Part B Eng. 2020, 189, 107892. [Google Scholar] [CrossRef]
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef] [Green Version]
- Coffetti, D.; Crotti, E.; Gazzaniga, G.; Carrara, M.; Pastore, T.; Coppola, L. Pathways towards sustainable concrete. Cem. Concr. Res. 2022, 154, 106718. [Google Scholar] [CrossRef]
- Hyde, G.W.; Smith, W.J. Results of experiments made to determine the permeability of cements and cement mortars. Mater. Sci. 1889, 128, 199–207. [Google Scholar] [CrossRef]
- Glanville, W. The Permeability of Portland Cement Concrete; Harrison and Sons: Ventura, CA, USA, 1931; Available online: http://www.worldcat.org/title/permeability-of-portland-cement-concrete/oclc/29871375 (accessed on 9 March 2018).
- Rooij, M.; van Tittelboom, K.; Belie, N.; Schlangen, E. Self-Healing Phenomena in Cement-Based Materials: State-of-the-Art Report of RILEM Technical Committee; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Edvardsen, C. Water permeability and autogenous healing of cracks in concrete. In Innovation in Concrete Structures: Design and Construction; ICE: London, UK, 2015; pp. 473–487. [Google Scholar] [CrossRef]
- Hearn, N. Self-sealing, autogenous healing and continued hydration: What is the difference? Mater. Struct. 1998, 31, 563–567. [Google Scholar] [CrossRef]
- Vijay, K.; Murmu, M.; Deo, S.V. Bacteria based self healing concrete—A review. Constr. Build. Mater. 2017, 152, 1008–1014. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Ghorbel, E.; Fares, H.; Cousture, A. Bacterial self-healing of concrete and durability assessment. Cem. Concr. Compos. 2019, 104, 103340. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Zuo, J.; Liu, X. Self-Healing of Concrete Cracks by Ceramsite-Loaded Microorganisms. Adv. Mater. Sci. Eng. 2018, 2018, 5153041. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, Y.; Feng, T.; Zhou, M.; Zhao, L.; Zhou, A.; Li, Z. Immobilizing bacteria in expanded perlite for the crack self-healing in concrete. Constr. Build. Mater. 2017, 148, 610–617. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Wang, J.Y.; de Belie, N.; Verstraete, W. Diatomaceous earth as a protective vehicle for bacteria applied for self-healing concrete. J. Ind. Microbiol. Biotechnol. 2012, 39, 567–577. [Google Scholar] [CrossRef]
- Xu, H.; Lian, J.; Gao, M.; Fu, D.; Yan, Y. Self-healing concrete using rubber particles to immobilize bacterial spores. Materials 2019, 12, 2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, M.; Chatrabhuti, S.; Gurdebeke, S.; Alderete, N.; van Tittelboom, K.; Raquez, J.M.; Cnudde, V.; van Vlierberghe, S.; de Belie, N.; Gruyaert, E. Poly(methyl methacrylate) capsules as an alternative to the proof-of-concept’’ glass capsules used in self-healing concrete. Cem. Concr. Compos. 2018, 89, 260–271. [Google Scholar] [CrossRef]
- Wang, J.Y.; Soens, H.; Verstraete, W.; de Belie, N. Self-healing concrete by use of microencapsulated bacterial spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Dong, B.; Fang, G.; Ding, W.; Liu, Y.; Zhang, J.; Han, N.; Xing, F. Self-healing features in cementitious material with urea-formaldehyde/epoxy microcapsules. Constr. Build. Mater. 2016, 106, 608–617. [Google Scholar] [CrossRef]
- Wang, J.Y.; Snoeck, D.; van Vlierberghe, S.; Verstraete, W.; de Belie, N. Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr. Build. Mater. 2014, 68, 110–119. [Google Scholar] [CrossRef]
- Thao, T.D.P.; Johnson, T.J.S.; Tong, Q.S.; Dai, P.S. Implementation of self-healing in concrete–Proof of concept. IES J. Part A Civ. Struct. Eng. 2009, 2, 116–125. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; de Belie, N.; van Loo, D.; Jacobs, P. Self-healing efficiency of cementitious materials containing tubular capsules filled with healing agent. Cem. Concr. Compos. 2011, 33, 497–505. [Google Scholar] [CrossRef]
- Dry, C.M. Three designs for the internal release of sealants, adhesives, and waterproofing chemicals into concrete to reduce permeability. Cem. Concr. Res. 2000, 30, 1969–1977. [Google Scholar] [CrossRef]
- Yang, Z.; Hollar, J.; He, X.; Shi, X. A self-healing cementitious composite using oil core/silica gel shell microcapsules. Cem. Concr. Compos. 2011, 33, 506–512. [Google Scholar] [CrossRef]
- Anglani, G.; Tulliani, J.M.; Antonaci, P. Behaviour of pre-cracked self-healing cementitious materials under static and cyclic loading. Materials 2020, 13, 1149. [Google Scholar] [CrossRef] [Green Version]
- Formia, A.; Terranova, S.; Antonaci, P.; Pugno, N.M.; Tulliani, J.M. Setup of extruded cementitious hollow tubes as containing/releasing devices in self-healing systems. Materials 2015, 8, 1897–1923. [Google Scholar] [CrossRef] [PubMed]
- Formia, A.; Irico, S.; Bertola, F.; Canonico, F.; Antonaci, P.; Pugno, N.M.; Tulliani, J.-M. Experimental analysis of self-healing cement-based materials incorporating extruded cementitious hollow tubes. J. Intell. Mater. Syst. Struct. 2016, 27, 2633–2652. [Google Scholar] [CrossRef]
- Li, D.; Chen, B.; Chen, X.; Fu, B.; Wei, H.; Xiang, X. Synergetic effect of superabsorbent polymer (SAP) and crystalline admixture (CA) on mortar macro-crack healing. Constr. Build. Mater. 2020, 247, 118521. [Google Scholar] [CrossRef]
- Deng, H.; Liao, G. Assessment of influence of self-healing behavior on water permeability and mechanical performance of ECC incorporating superabsorbent polymer (SAP) particles. Constr. Build. Mater. 2018, 170, 455–465. [Google Scholar] [CrossRef]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N.R. Self-sealing of cracks in concrete using superabsorbent polymers. Cem. Concr. Res. 2016, 79, 194–208. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Fang, C.; Li, D.; Han, N.; Xing, F. A self-healing cementitious composite with mineral admixtures and built-in carbonate. Cem. Concr. Compos. 2018, 92, 216–229. [Google Scholar] [CrossRef]
- Wang, X.F.; Yang, Z.H.; Fang, C.; Wang, W.; Liu, J.; Xing, F. Effect of carbonate-containing self-healing system on properties of a cementitious composite: Fresh, mechanical, and durability properties. Constr. Build. Mater. 2020, 235, 117442. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Yang, H.; Wang, L.; Tang, S.; Wu, B.; Mao, W. Pore Structural and Fractal Analysis of the Effects of MgO Reactivity and Dosage on Permeability and F–T Resistance of Concrete. Fractal Fract. 2022, 6, 113. [Google Scholar] [CrossRef]
- Qureshi, T.; Kanellopoulos, A.; Al-Tabbaa, A. Autogenous self-healing of cement with expansive minerals-I: Impact in early age crack healing. Constr. Build. Mater. 2018, 192, 768–784. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, T.; Kanellopoulos, A.; Al-Tabbaa, A. Autogenous self-healing of cement with expansive minerals-II: Impact of age and the role of optimised expansive minerals in healing performance. Constr. Build. Mater. 2019, 194, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Borg, R.P.; Cuenca, E.; Brac, E.M.G.; Ferrara, L. Crack sealing capacity in chloride-rich environments of mortars containing different cement substitutes and crystalline admixtures. J. Sustain. Cem.-Based Mater. 2018, 7, 141–159. [Google Scholar] [CrossRef]
- Ferrara, L.; Krelani, V.; Moretti, F. On the use of crystalline admixtures in cement based construction materials: From porosity reducers to promoters of self healing. Smart Mater. Struct. 2016, 25, 084002. [Google Scholar] [CrossRef]
- De Weerdt, K.; Colombo, A.; Coppola, L.; Justnes, H.; Geiker, M.R. Impact of the associated cation on chloride binding of Portland cement paste. Cem. Concr. Res. 2015, 68, 196–202. [Google Scholar] [CrossRef]
- Donnini, J.; Lancioni, G.; Bellezze, T.; Corinaldesi, V. Bond behavior of FRCM carbon yarns embedded in a cementitious matrix: Experimental and numerical results. In Key Engineering Materials; Trans Tech Publications Ltd.: Bachs, Switzerland, 2017; pp. 305–312. [Google Scholar] [CrossRef]
- Lollini, F.; Carsana, M.; Gastaldi, M.; Redaelli, E.; Bertolini, L. The challenge of the performance-based approach for the design of reinforced concrete structures in chloride bearing environment. Constr. Build. Mater. 2015, 79, 245–254. [Google Scholar] [CrossRef] [Green Version]
- ISO 14564; Epoxy-Coated Steel for the Reinforcement of Concrete. ISO: London, UK, 1999.
- Yeomans, S.R. Galvanized Steel Reinforcement in Concrete; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Roventi, G.; Bellezze, T.; Barbaresi, E.; Fratesi, R. Effect of carbonation process on the passivating products of zinc in Ca(OH)2 saturated solution. Mater. Corros. 2013, 64, 1007–1014. [Google Scholar] [CrossRef]
- Roventi, G.; Bellezze, T.; Giuliani, G.; Conti, C. Corrosion resistance of galvanized steel reinforcements in carbonated concrete: Effect of wet–dry cycles in tap water and in chloride solution on the passivating layer. Cem. Concr. Res. 2014, 65, 76–84. [Google Scholar] [CrossRef]
- Maahn, E.; Sorensen, B. Influence of Microstructure on the Corrosion Properties of Hot-Dip Galvanized Reinforcement in Concrete. Corrosion 1986, 42, 187–196. [Google Scholar] [CrossRef]
- Bellezze, T.; Malavolta, M.; Quaranta, A.; Ruffini, N.; Roventi, G. Corrosion behaviour in concrete of three differently galvanized steel bars. Cem. Concr. Compos. 2006, 28, 246–255. [Google Scholar] [CrossRef]
- Bellezze, T.; Fratesi, R.; Tittarelli, F. Corrosion behaviour of galvanized steel rebars in the presence of coating discontinuities. In Corrosion in Reinforced Concrete Structures; Raupach, M., Ed.; Woodhead Publishing: Cambridge, UK, 2007; pp. 27–37. [Google Scholar] [CrossRef]
- Schiessl, P.; Reuter, C. Coated reinforcing steels development and application in Europe. In Proceedings of the International Conference on Corrosion, Houston, TX, USA, 4–8 March 1991. [Google Scholar]
- Sagues, A.; Powers, R.G. Corrosion and corrosione control of concrete structures in Florida. What can be learned? In Proceedings of the International Conference on Repair of Concrete Structures: From Theory to Practice in a Marine Environment, Svolvear, Norway, 28–30 May 1997; pp. 49–58. [Google Scholar]
- Lollini, F.; Carsana, M.; Gastaldi, M.; Redaelli, E. Corrosion behaviour of stainless steel reinforcement in concrete. Corros. Rev. 2019, 37, 3–19. [Google Scholar] [CrossRef] [Green Version]
- The Concrete Society. Guidance on the Use of Stainless Steel Reinforcement; Technical Report No. 51; The Concrete Society: Surrey, UK, 1998. [Google Scholar]
- Bertolini, L.; Gastaldi, M. Corrosion resistance of low-nickel duplex stainless steel rebars. Mater. Corros. 2011, 62, 120–129. [Google Scholar] [CrossRef]
- Gastaldi, M.; Bertolini, L. Effect of temperature on the corrosion behaviour of low-nickel duplex stainless steel bars in concrete. Cem. Concr. Res. 2014, 56, 52–60. [Google Scholar] [CrossRef]
- Bertolini, L.; Gastaldi, M.; Pastore, T.; Pedeferri, M.; Pedeferri, P. Effects of Galvanic coupling between carbon steel and stainless steel reinforcement in concrete. In Proceedings of the International Conference on Corrosion and Rehabilitation of Reinforced Concrete Structures, Orlando, FL, USA, 7–11 December 1998. [Google Scholar]
- Trejo, D.; Gardoni, P.; Kim, J. Long-Term Performance of Glass Fiber-Reinforced Polymer Reinforcement Embedded in Concrete. ACI Mater. J. 2011, 108, 605–613. [Google Scholar]
- Tuutti, K. Corrosion of Steel in Concrete; Swedish Foundation for Concrete Research: Stockholm, Sweden, 1982. [Google Scholar]
- Page, C.L.; Treadaway, K.W.J. Aspects of the electrochemistry of steel in concrete. Nature 1982, 297, 109–115. [Google Scholar] [CrossRef]
- Arup, H. The Mechanism of the Protection of Steel in Concrete, Corrosion of Reinforcment in Concrete, Construction; Crane, A.P., Ed.; Ellis Horwood Ltd.: Chichester, UK, 1983; pp. 151–157. [Google Scholar]
- Schiessl, P. Corrosion of Steel in Concrete; Wiley: London, UK, 1988. [Google Scholar]
- Bertolini, L. Steel corrosion and service life of reinforced concrete structures. Struct. Infrastruct. Eng. 2008, 4, 123–137. [Google Scholar] [CrossRef]
- Pacheco-torgal, F.; Melchers, R.; de Belie, N.; Shi, X.; van Tittelboom, K.; Perez, A.S. Eco-Efficient Repair and Rehabilitation of Concrete Infrastructures; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- International Federation for Structural Concrete, fib. Model Code 2010; Bulletin no. 65; fib: Lausanne, Switzerland, 2010. [Google Scholar]
- EN 206:2013+A1:2016; Concrete-Specification, Performance, Production and Conformity. European Union: Maastricht, The Netherlands, 2021.
- EN 1992-1-1:2004; Eurocodice 2: Design of Concrete Structures-Part 1-1: General Rules and Rules for Buildings. European Union: Maastricht, The Netherlands, 2004.
- EN 197-1:2011; Cement-Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Union: Maastricht, The Netherlands, 2011.
- Carsana, M.; Frassoni, M.; Bertolini, L. Comparison of ground waste glass with other supplementary cementitious materials. Cem. Concr. Compos. 2014, 45, 39–45. [Google Scholar] [CrossRef]
- Lollini, F.; Redaelli, E.; Bertolini, L. Effects of portland cement replacement with limestone on the properties of hardened concrete. Cem. Concr. Compos. 2014, 46, 32–40. [Google Scholar] [CrossRef]
- FIsfahani, T.; Redaelli, E.; Lollini, F.; Li, W.; Bertolini, L. Effects of Nanosilica on Compressive Strength and Durability Properties of Concrete with Different Water to Binder Ratios. Adv. Mater. Sci. Eng. 2016, 2016, 8453567. [Google Scholar] [CrossRef] [Green Version]
- Carsana, M.; Gastaldi, M.; Lollini, F.; Redaelli, E.; Bertolini, L. Improving durability of reinforced concrete structures by recycling wet-ground MSWI bottom ash. Mater. Corros. 2016, 67, 573–582. [Google Scholar] [CrossRef]
- Lollini, F.; Redaelli, E.; Bertolini, L. A study on the applicability of the efficiency factor of supplementary cementitious materials to durability properties. Constr. Build. Mater. 2016, 120, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Lollini, F.; Redaelli, E.; Bertolini, L. Investigation on the effect of supplementary cementitious materials on the critical chloride threshold of steel in concrete. Mater. Struct. Constr. 2016, 49, 4147–4165. [Google Scholar] [CrossRef] [Green Version]
- Carsana, M.; Bertolini, L. Durability of a LWC concrete with expanded glass and silica fume. ACI Mater. J. 2017, 114, 207–213. [Google Scholar]
- International Federation for Structural Concrete, fib. Model Code for Service Life Design; Bullettin No. 34; fib: Lausanne, Switzerland, 2006. [Google Scholar]
- Lollini, F.; Gastaldi, M.; Bertolini, L. Performance parameters for the durability design of reinforced concrete structures with stainless steel reinforcement. Struct. Infrastruct. Eng. 2018, 14, 833–842. [Google Scholar] [CrossRef]
- Bertolini, L.; Lollini, F.; Redaelli, E. Durability design of reinforced concrete structures. Proc. Inst. Civ. Eng. 2011, 164, 73–82. [Google Scholar] [CrossRef]
- Lollini, F.; Redaelli, E.; Bertolini, L. Analysis of the parameters affecting probabilistic predictions of initiation time for carbonation-induced corrosion of reinforced concrete structures. Mater. Corros. 2012, 63, 1059–1068. [Google Scholar] [CrossRef]
- Bertolini, L. Materiali da Costruzione; Città Studi Edizioni: Milan, Italy, 2012; Volume II. [Google Scholar]
- Mehta, P.K. Concrete. Structure, Properties and Materials; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Bittrich, E.; Cometa, S.; de Giglio, E.; di Mundo, R.; Itaranto, N.D.; Eichhorn, K.; Keller, B.; Lednicky, F.; Mangolini, F.; Palumbo, F.; et al. Polymer Surface Characterization; GmbH&Co KG: München, Germany, 2014. [Google Scholar]
- Chalangaran, N.; Farzampour, A.; Palsar, N.; Fatemi, H. Experimental investigation of sound transmission loss in concrete containing recycled rubber crumbs. Adv. Concr. Constr. 2021, 11, 447–454. [Google Scholar] [CrossRef]
- Chalangaran, N.; Farzampour, A.; Paslar, N. Nano Silica and Metakaolin Effects on the Behavior of Concrete Containing Rubber Crumbs. CivilEng 2020, 1, 264–274. [Google Scholar] [CrossRef]
- Di Mundo, R.; Petrella, A.; Notarnicola, M. Surface and bulk hydrophobic cement composites by tyre rubber addition. Constr. Build. Mater. 2018, 172, 176–184. [Google Scholar] [CrossRef]
- Petrella, A.; di Mundo, R.; de Gisi, S.; Todaro, F.; Labianca, C.; Notarnicola, M. Environmentally Sustainable Cement Composites Based on End-of-Life Tyre Rubber and Recycled Waste Porous Glass. Materials 2019, 12, 3289. [Google Scholar] [CrossRef] [Green Version]
- Di Mundo, R.; Dilonardo, E.; Nacucchi, M.; Carbone, G.; Notarnicola, M. Water absorption in rubber-cement composites: 3D structure investigation by X-ray computed-tomography. Constr. Build. Mater. 2019, 228, 116602. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, J.; Xu, J.; Bo, M.; Li, J.; Tang, B. Research on anti-chloride ion penetration property of crumb rubber concrete at different ambient temperatures. Constr. Build. Mater. 2018, 189, 42–53. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Manzi, S.; Natali, M.E.; Rickard, W.D.A.; van Riessen, A. Room temperature alkali activation of fly ash: The effect of Na 2O/SiO2 ratio. Constr. Build. Mater. 2014, 69, 262–270. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Carabba, L.; Manzi, S.; Bignozzi, M.C. Superplasticizer addition to carbon fly ash geopolymers activated at room temperature. Materials 2016, 9, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Carabba, L.; Santandrea, M.; Carloni, C.; Manzi, S.; Bignozzi, M.C. Steel fiber reinforced geopolymer matrix (S-FRGM) composites applied to reinforced concrete structures for strengthening applications: A preliminary study. Compos. Part B Eng. 2017, 128, 83–90. [Google Scholar] [CrossRef]
- Ma, C.-K.; Awang, A.Z.; Omar, W. Structural and material performance of geopolymer concrete: A review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Dell’Aversano, R.; Gazzaniga, G. The influence of heat and steam curing on the properties of one-part fly ash/slag alkali activated materials: Preliminary results. AIP Conf. Proc. 2019, 2196, 020038. [Google Scholar] [CrossRef]
- Mobili, A.; Belli, A.; Giosuè, C.; Bellezze, T.; Tittarelli, F. Metakaolin and fly ash alkali-activated mortars compared with cementitious mortars at the same strength class. Cem. Concr. Res. 2016, 88, 198–210. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.F.; Jamshidi, M.; Ding, Y. Durability of alkali-activated binders: A clear advantage over Portland cement or an unproven issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; Karim, M.R.; Hossain, M.K.; Islam, M.N.; Zain, M.F.M. Durability of mortar and concrete containing alkali-activated binder with pozzolans: A review. Constr. Build. Mater. 2015, 93, 95–109. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S. Durability of low-calcium fly ash based geopolymer concrete culvert in a saline environment. Cem. Concr. Res. 2017, 100, 297–310. [Google Scholar] [CrossRef]
- Wardhono, A.; Gunasekara, C.; Law, D.W.; Setunge, S. Comparison of long term performance between alkali activated slag and fly ash geopolymer concretes. Constr. Build. Mater. 2017, 143, 272–279. [Google Scholar] [CrossRef]
- Ameri, F.; Shoaei, P.; Zareei, S.A.; Behforouz, B. Geopolymers vs. alkali-activated materials (AAMs): A comparative study on durability, microstructure, and resistance to elevated temperatures of lightweight mortars. Constr. Build. Mater. 2019, 222, 49–63. [Google Scholar] [CrossRef]
- Saccani, A.; Manzi, S.; Lancellotti, I.; Barbieri, L. Manufacturing and durability of alkali activated mortars containing different types of glass waste as aggregates valorization. Constr. Build. Mater. 2020, 237, 117733. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Monticelli, C.; Natali, M.E.; Balbo, A.; Chiavari, C.; Zanotto, F.; Manzi, S.; Bignozzi, M.C. Corrosion behavior of steel in alkali-activated fly ash mortars in the light of their microstructural, mechanical and chemical characterization. Cem. Concr. Res. 2016, 80, 60–68. [Google Scholar] [CrossRef]
- Monticelli, C.; Natali, M.E.; Balbo, A.; Chiavari, C.; Zanotto, F.; Manzi, S.; Bignozzi, M.C. A study on the corrosion of reinforcing bars in alkali-activated fly ash mortars under wet and dry exposures to chloride solutions. Cem. Concr. Res. 2016, 87, 53–63. [Google Scholar] [CrossRef]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z. Structural performance of reinforced geopolymer concrete members: A review. Constr. Build. Mater. 2016, 120, 251–264. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Masi, G.; Rickard, W.D.A.; Bignozzi, M.C.; van Riessen, A. The effect of organic and inorganic fibres on the mechanical and thermal properties of aluminate activated geopolymers. Compos. Part B Eng. 2015, 76, 218–228. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Qi, S.L.; Wu, B. Characterizing the bond strength of geopolymers at ambient and elevated temperatures. Cem. Concr. Compos. 2015, 58, 40–49. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Gluth, G.J.G.; Pistol, K. In-situ thermo-mechanical testing of fly ash geopolymer concretes made with quartz and expanded clay aggregates. Cem. Concr. Res. 2016, 80, 33–43. [Google Scholar] [CrossRef]
- Part, W.K.; Ramli, M.; Cheah, C.B. An overview on the influence of various factors on the properties of geopolymer concrete derived from industrial by-products. Constr. Build. Mater. 2015, 77, 370–395. [Google Scholar] [CrossRef]
- Carabba, L.; Manzi, S.; Rambaldi, E.; Ridolfi, G.; Bignozzi, M.C. High-temperature behaviour of alkali-activated composites based on fly ash and recycled refractory particles. J. Ceram. Sci. Technol. 2017, 8, 4416. [Google Scholar]
- Giosuè, C.; Mobili, A.; di Perna, C.; Tittarelli, F. Performance of lightweight cement-based and alkali-activated mortars exposed to high-temperature. Constr. Build. Mater. 2019, 220, 565–576. [Google Scholar] [CrossRef]
- Sakkas, K.; Nomikos, P.; Sofianos, A.; Panias, D. Inorganic polymeric materials for passive fire protection of underground constructions. Fire Mater. 2013, 37, 140–150. [Google Scholar] [CrossRef]
- Sotiriadis, K.; Guzii, S.; Kumpová, I.; Mácová, P.; Viani, A. The Effect of Firing Temperature on the Composition and Microstructure of a Geocement-Based Binder of Sodium Water-Glass. Solid State Phenom. 2017, 267, 58–62. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Watolla, M.B.; Gluth, G.J.G.; Sturm, P.; Rickard, W.D.A.; Krüger, S.; Schartel, B. Intumescent geopolymer-bound coatings for fire protection of steel. J. Ceram. Sci. Technol. 2017, 8, 351–364. [Google Scholar] [CrossRef]
- Carabba, L.; Moricone, R.; Scarponi, G.E.; Tugnoli, A.; Bignozzi, M.C. Alkali activated lightweight mortars for passive fire protection: A preliminary study. Constr. Build. Mater. 2019, 195, 75–84. [Google Scholar] [CrossRef]
- Pozzo, A.D.; Carabba, L.; Bignozzi, M.C.; Tugnoli, A. Life cycle assessment of a geopolymer mixture for fireproofing applications. Int. J. Life Cycle Assess. 2019, 24, 1743–1757. [Google Scholar] [CrossRef]
- Carabba, L.; Pirskawetz, S.; Krüger, S.; Gluth, G.J.G.; Bignozzi, M.C. Acoustic emission study of heat-induced cracking in fly ash-based alkali-activated pastes and lightweight mortars. Cem. Concr. Compos. 2019, 102, 145–156. [Google Scholar] [CrossRef]
- Criado, M.; Martínez-Ramirez, S.; Fajardo, S.; Gómez, P.P.; Bastidas, J.M. Corrosion rate and corrosion product characterisation using Raman spectroscopy for steel embedded in chloride polluted fly ash mortar. Mater. Corros. 2013, 64, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Law, D.W.; Adam, A.A.; Molyneaux, T.K.; Patnaikuni, I.; Wardhono, A. Long term durability properties of class F fly ash geopolymer concrete. Mater. Struct. 2015, 48, 721–731. [Google Scholar] [CrossRef]
- Tittarelli, F.; Mobili, A.; Giosuè, C.; Belli, A.; Bellezze, T. Corrosion behaviour of bare and galvanized steel in geopolymer and Ordinary Portland Cement based mortars with the same strength class exposed to chlorides. Corros. Sci. 2018, 134, 64–77. [Google Scholar] [CrossRef]
- Iorfida, A.; Verre, S.; Candamano, S.; Ombres, L. Tensile and Direct Shear Responses of Basalt-Fibre Reinforced Mortar Based Materials. In Strain-Hardening Cement-Based Composites: SHCC4; Mechtcherine, V., Slowik, V., Kabele, P., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 544–552. [Google Scholar]
- Candamano, S.; Crea, F.; Iorfida, A. Mechanical characterization of basalt fabric-reinforced alkali-activated matrix composites: A preliminary investigation. Appl. Sci. 2020, 10, 2865. [Google Scholar] [CrossRef] [Green Version]
- Coppola, B.; Palmero, P.; Montanaro, L.; Tulliani, J.M. Alkali-activation of marble sludge: Influence of curing conditions and waste glass addition. J. Eur. Ceram. Soc. 2020, 40, 3776–3787. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mechanisms of alkali-activated slag. Cem. Concr. Res. 2016, 88, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Candamano, S.; de Luca, P.; Frontera, P.; Crea, F. Production of Geopolymeric Mortars Containing Forest Biomass Ash as Partial Replacement of Metakaolin. Environments 2017, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Sgambitterra, E.; Lamuta, C.; Candamano, S.; Pagnotta, L. Brazilian disk test and digital image correlation: A methodology for the mechanical characterization of brittle materials. Mater. Struct. Constr. 2018, 51, 19. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Labrincha, J.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials-State-of-the-Art Report-Rilem-Tc 224-AAM; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Gazzaniga, G.; Pastore, T. The durability of one-part alkali activated slag-based mortars in different environments. Sustainability 2020, 12, 3561. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Sulfate attack on alkali-activated slag concrete. Cem. Concr. Res. 2002, 32, 211–216. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Ye, H.; Chen, Z.; Huang, L. Mechanism of sulfate attack on alkali-activated slag: The role of activator composition. Cem. Concr. Res. 2019, 125, 105868. [Google Scholar] [CrossRef]
- Gong, K.; White, C.E. Nanoscale Chemical Degradation Mechanisms of Sulfate Attack in Alkali-activated Slag. J. Phys. Chem. C 2018, 122, 5992–6004. [Google Scholar] [CrossRef]
- Bhutta, M.A.R.; Hussin, W.M.; Azreen, M.; Tahir, M.M. Sulphate resistance of geopolymer concrete prepared from blended waste fuel ash. J. Mater. Civ. Eng. 2014, 26, 04014080. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Hamdan, S.; van Deventer, J.S.J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. Constr. 2013, 46, 361–373. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Winnefeld, F. Synthesis and characterisation of calcium sulfoaluminate cements produced by different chemical gypsums. Adv. Cem. Res. 2019, 31, 113–123. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Tomasulo, M.; Valenti, G.L.; Dieter, H.; Montagnaro, F. Low-CO2 Cements from Fluidized Bed Process Wastes and Other Industrial By-Products. Combust. Sci. Technol. 2016, 188, 492–503. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Xu, L.; Wu, K.; Li, N.; Zhou, X.; Wang, P. Utilization of flue gas desulfurization gypsum for producing calcium sulfoaluminate cement. J. Clean. Prod. 2017, 161, 803–811. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Tomasulo, M.; Valenti, G.L.; Dieter, H.; Montagnaro, F. Calcium looping spent sorbent as a limestone replacement in the manufacture of portland and calcium sulfoaluminate cements. Environ. Sci. Technol. 2015, 49, 6865–6871. [Google Scholar] [CrossRef] [PubMed]
- Telesca, A.; Marroccoli, M.; Tomasulo, M.; Valenti, G.L. Hydration Properties and Technical Behavior of Calcium Sulfoaluminate Cements. Spec. Publ. 2015, 303, 237–254. [Google Scholar]
- Marroccoli, M.; Pace, M.L.; Telesca, A.; Valenti, G.L. Synthesis of calcium sulfoaluminate cement from Al2O3-rich by-products from aluminium manufacture. In Proceedings of the Second International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; pp. 615–623. [Google Scholar]
- Telesca, A.; Marroccoli, M.; Coffetti, D.; Coppola, L.; Candamano, S. Tartaric acid effects on hydration development and physico-mechanical properties of blended calcium sulphoaluminate cements. Cem. Concr. Compos. 2021, 124, 104275. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Su, M.; Wang, Y.; Sorrentino, F. Development in non-Portland cements. In Proceedings of the 9th International Congress on the Chemistry of Cement, New Delhi, India, 7 June 1992. [Google Scholar]
- Su, M.; Wang, Y.; Zhang, L.; Li, D. Preliminary study on the durability of sulfo/ferro-aluminate cements. In Proceedings of the 10th International Congress on the Chemistry of Cement, Gothenburg, Sweden, 2–6 June 1997; pp. 1–12. [Google Scholar]
- Zhang, L.; Su, M.; Wang, Y. Development of the use of sulfo- and ferroaluminate cements in China. Adv. Cem. Res. 1999, 11, 15–21. [Google Scholar] [CrossRef]
- Zhang, L. Microstructure and Performance of Calcium Sulfoaluminate Cements. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 2000. [Google Scholar]
- Bernardo, G.; Telesca, A.; Valenti, G.L. A porosimetric study of calcium sulfoaluminate cement pastes cured at early ages. Cem. Concr. Res. 2006, 36, 1042–1047. [Google Scholar] [CrossRef]
- Quillin, K. Performance of belite–sulfoaluminate cements. Cem. Concr. Res. 2001, 31, 1341–1349. [Google Scholar] [CrossRef]
- Gastaldi, D.; Bertola, F.; Canonico, F.; Buzzi, L.; Mutke, S.; Irico, S.; Paul, G.; Marchese, L.; Boccaleri, E. A chemical/mineralogical investigation of the behavior of sulfoaluminate binders submitted to accelerated carbonation. Cem. Concr. Res. 2018, 109, 30–41. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Dell’Aversano, R.; Gazzaniga, G.; Pastore, T. Influence of Lithium Carbonate and Sodium Carbonate on Physical and Elastic Properties and on Carbonation Resistance of Calcium Sulphoaluminate-Based Mortars. Appl. Sci. 2019, 10, 176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Glasser, F.P. Investigation of the microstructure and carbonation of CS¯A-based concretes removed from service. Cem. Concr. Res. 2005, 35, 2252–2260. [Google Scholar] [CrossRef]
- Duan, P.; Chen, W.; Ma, J.; Shui, Z. Influence of layered double hydroxides on microstructure and carbonation resistance of sulphoaluminate cement concrete. Constr. Build. Mater. 2013, 48, 601–609. [Google Scholar] [CrossRef]
- Carsana, M.; Canonico, F.; Bertolini, L. Corrosion resistance of steel embedded in sulfoaluminate-based binders. Cem. Concr. Compos. 2018, 88, 211–219. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, L.; Beretta, S.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Caputo, D.; Carsana, M.; Cioffi, R.; et al. The Improvement of Durability of Reinforced Concretes for Sustainable Structures: A Review on Different Approaches. Materials 2022, 15, 2728. https://doi.org/10.3390/ma15082728
Coppola L, Beretta S, Bignozzi MC, Bolzoni F, Brenna A, Cabrini M, Candamano S, Caputo D, Carsana M, Cioffi R, et al. The Improvement of Durability of Reinforced Concretes for Sustainable Structures: A Review on Different Approaches. Materials. 2022; 15(8):2728. https://doi.org/10.3390/ma15082728
Chicago/Turabian StyleCoppola, Luigi, Silvia Beretta, Maria Chiara Bignozzi, Fabio Bolzoni, Andrea Brenna, Marina Cabrini, Sebastiano Candamano, Domenico Caputo, Maddalena Carsana, Raffaele Cioffi, and et al. 2022. "The Improvement of Durability of Reinforced Concretes for Sustainable Structures: A Review on Different Approaches" Materials 15, no. 8: 2728. https://doi.org/10.3390/ma15082728
APA StyleCoppola, L., Beretta, S., Bignozzi, M. C., Bolzoni, F., Brenna, A., Cabrini, M., Candamano, S., Caputo, D., Carsana, M., Cioffi, R., Coffetti, D., Colangelo, F., Crea, F., De Gisi, S., Diamanti, M. V., Ferone, C., Frontera, P., Gastaldi, M. M., Labianca, C., ... Todaro, F. (2022). The Improvement of Durability of Reinforced Concretes for Sustainable Structures: A Review on Different Approaches. Materials, 15(8), 2728. https://doi.org/10.3390/ma15082728

























