Valorization of β-Chitin Extraction Byproduct from Cuttlefish Bone and Its Application in Food Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Wastewater and Cuttlefish Bone
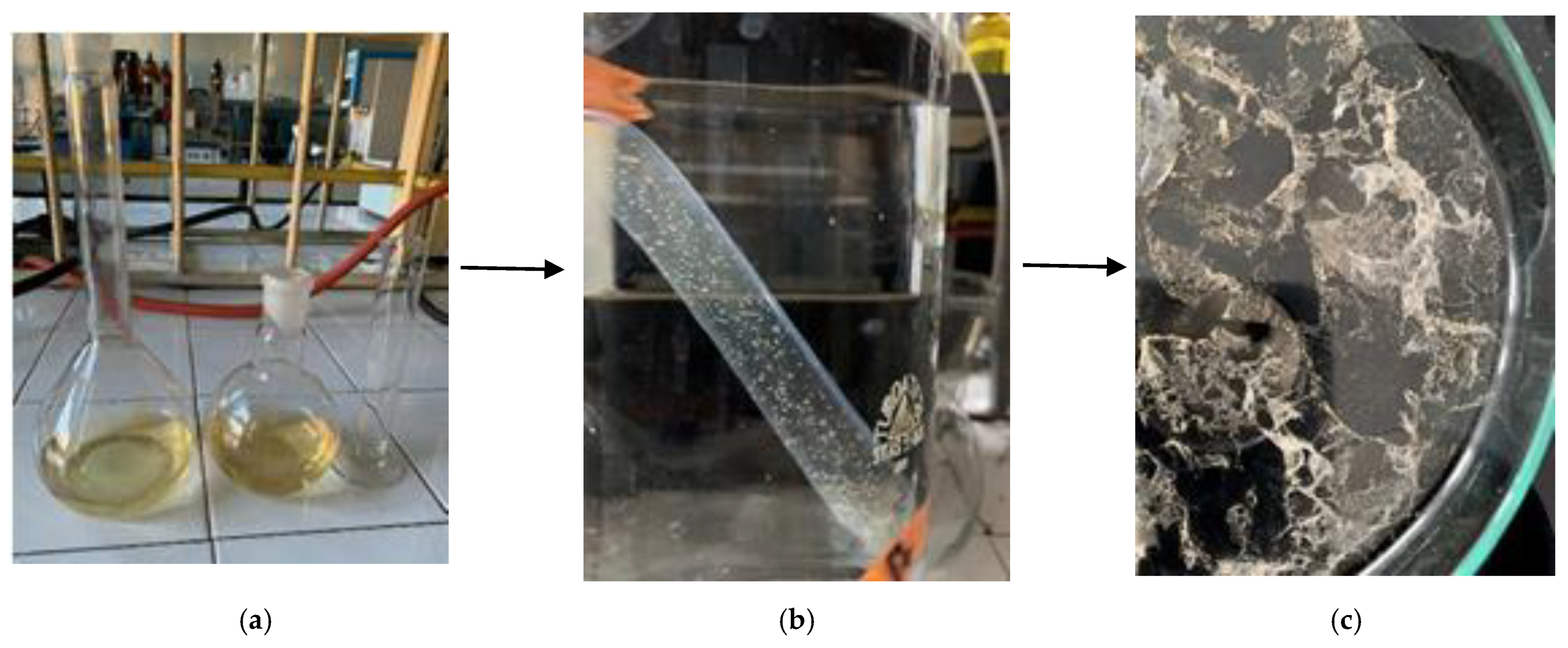
2.2. Preparation of the Deproteinization Liquid Extract
2.3. Separation and Purification of Chitin Forms from the Deproteinization Liquid Extract
2.4. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy (SEM/EDX)
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. X-ray Diffraction (XRD)
2.7. Ultraviolet/Visible Spectroscopy (UV-Vis)
2.8. OPA Fluorescent Assay for Protein Quantification
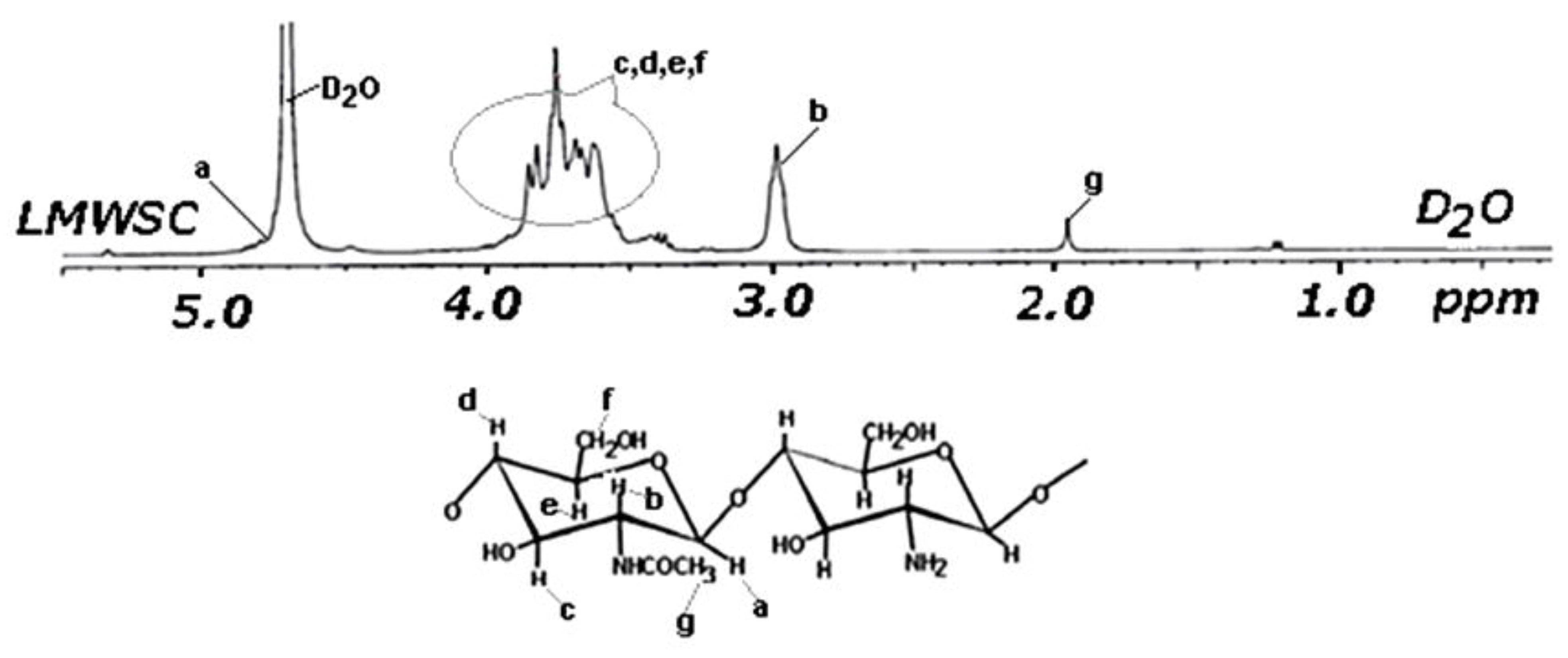
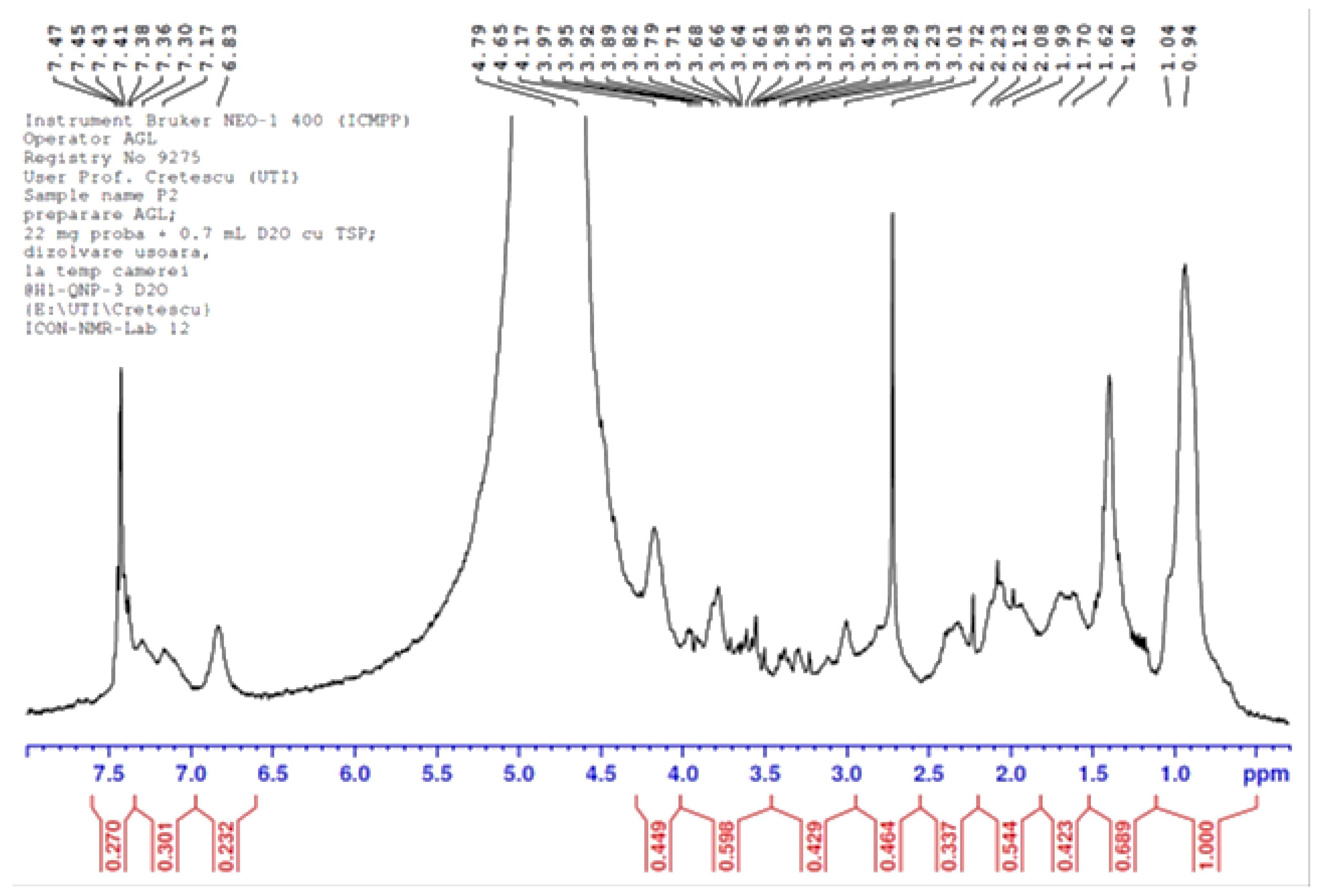
2.9. Proton Nuclear Magnetic Resonance (1H-NMR)
2.10. Zeta Potential Measurement
2.11. JarTest
2.12. Analytical Methods
3. Results and Discussion
3.1. Characterization of Raw Cuttlefish Bone and Deproteinization Liquid Extract
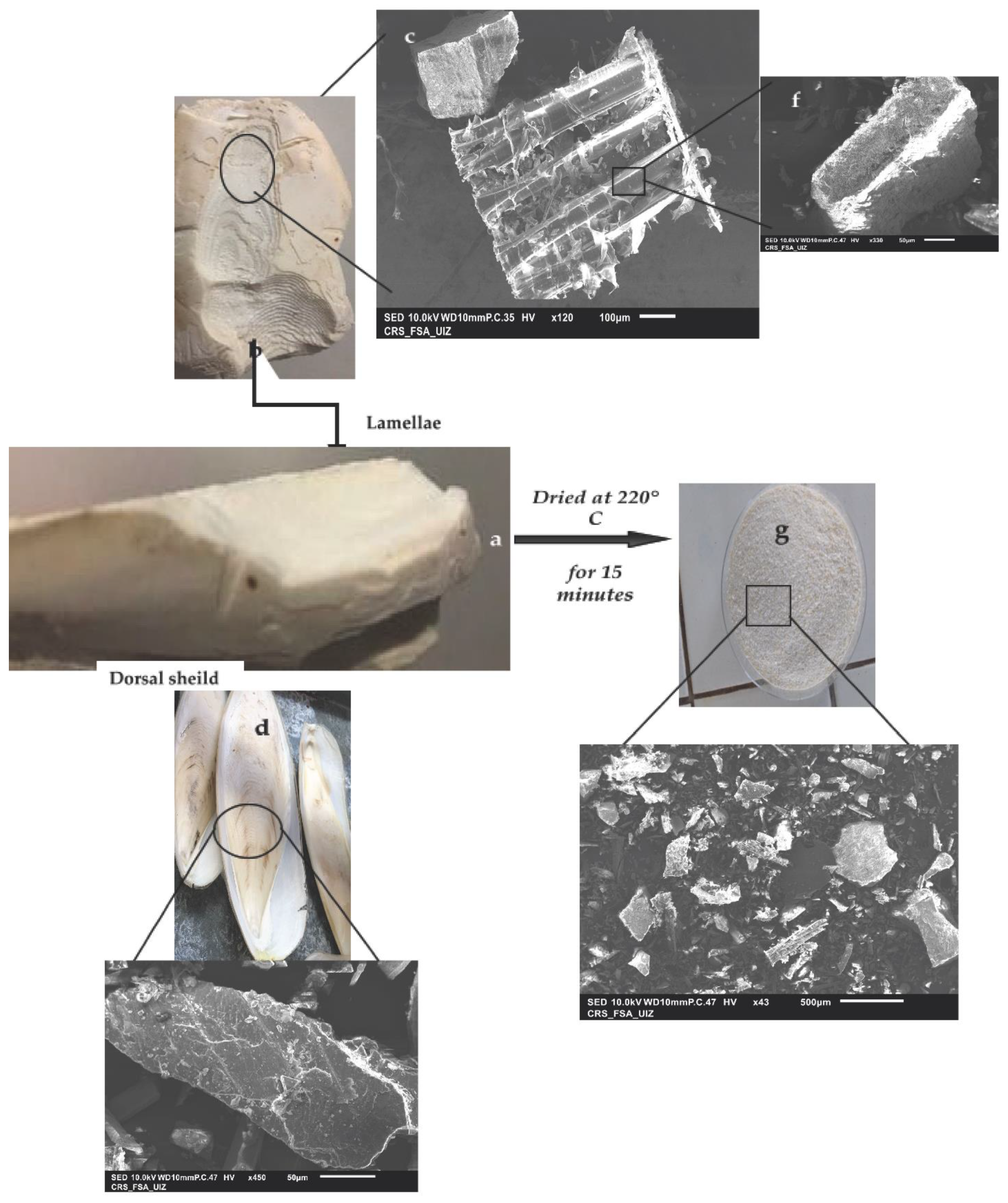
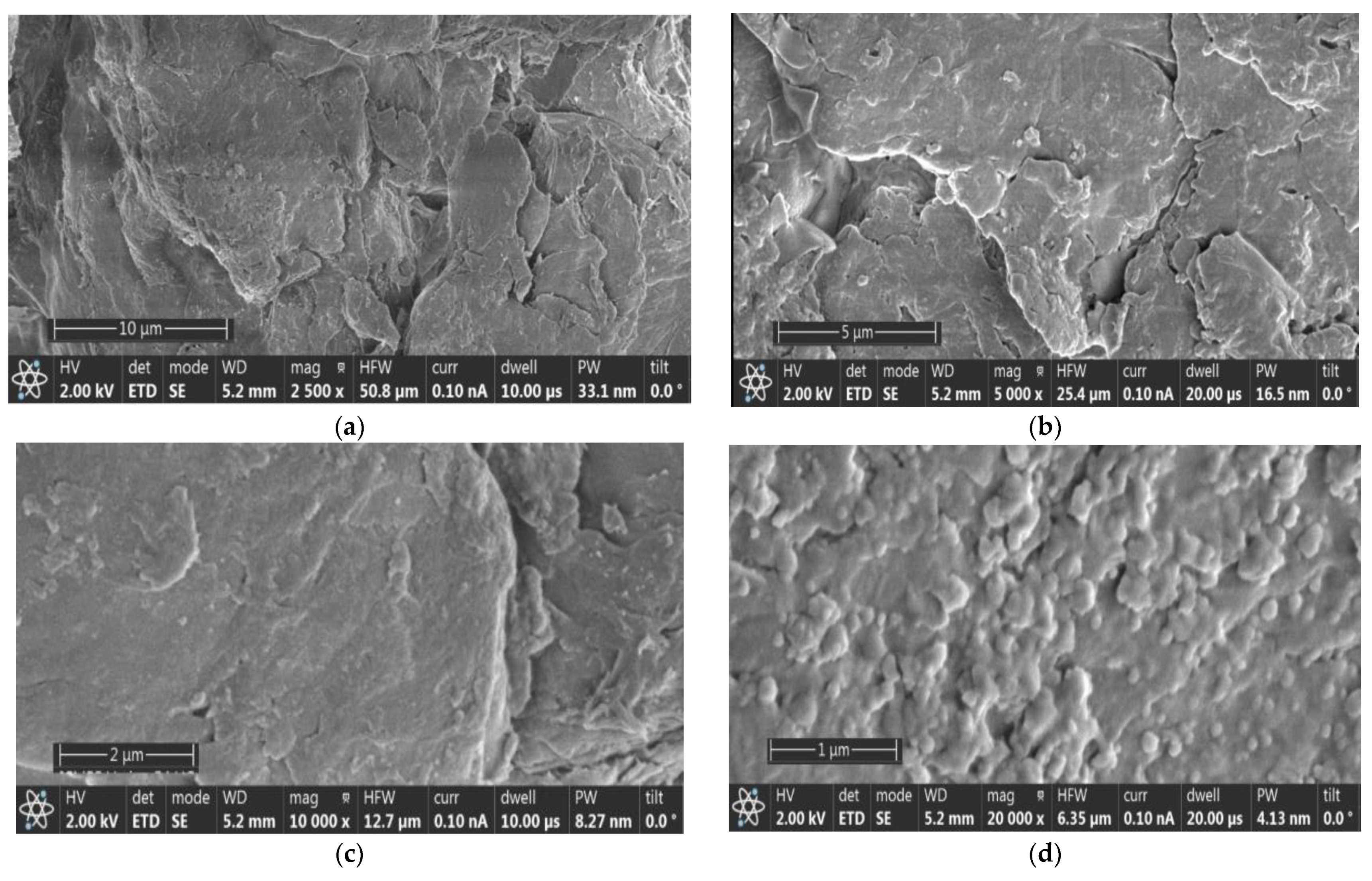
3.1.1. Analysis by SEM of Cuttlefish Bone and Chitin Fractions from the LC Extract
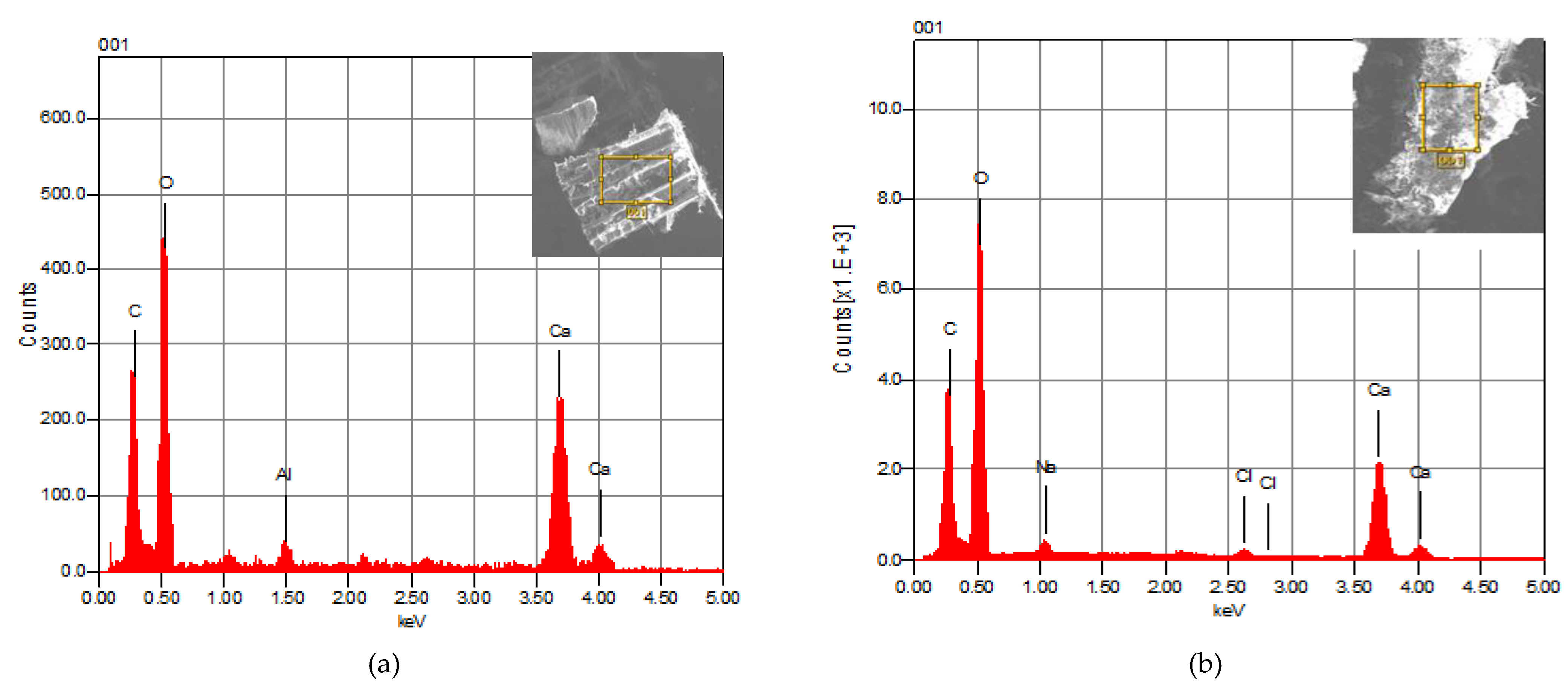
3.1.2. Analysis by Energy-Dispersive X-ray Spectroscopy (SEM/EDX)
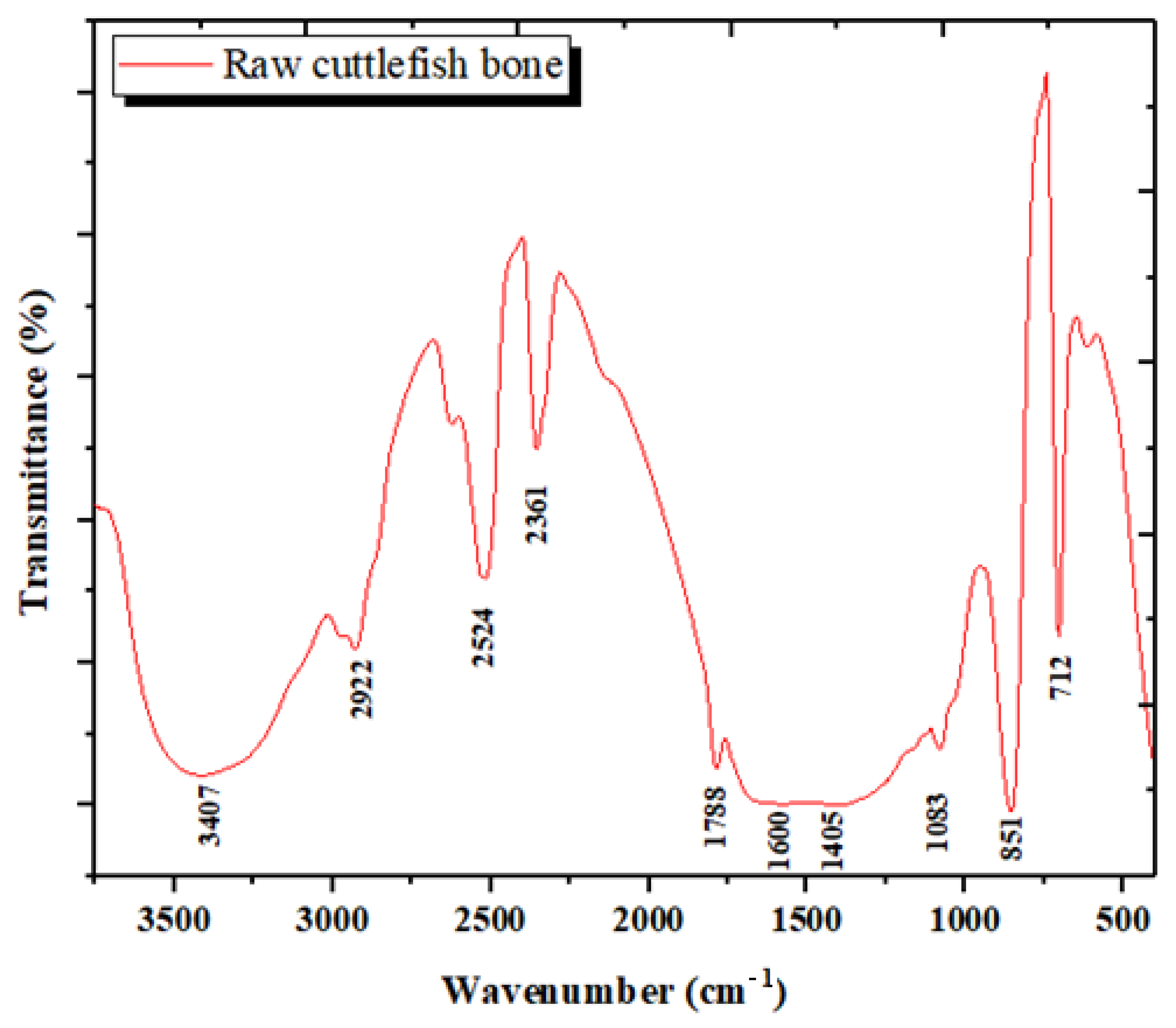
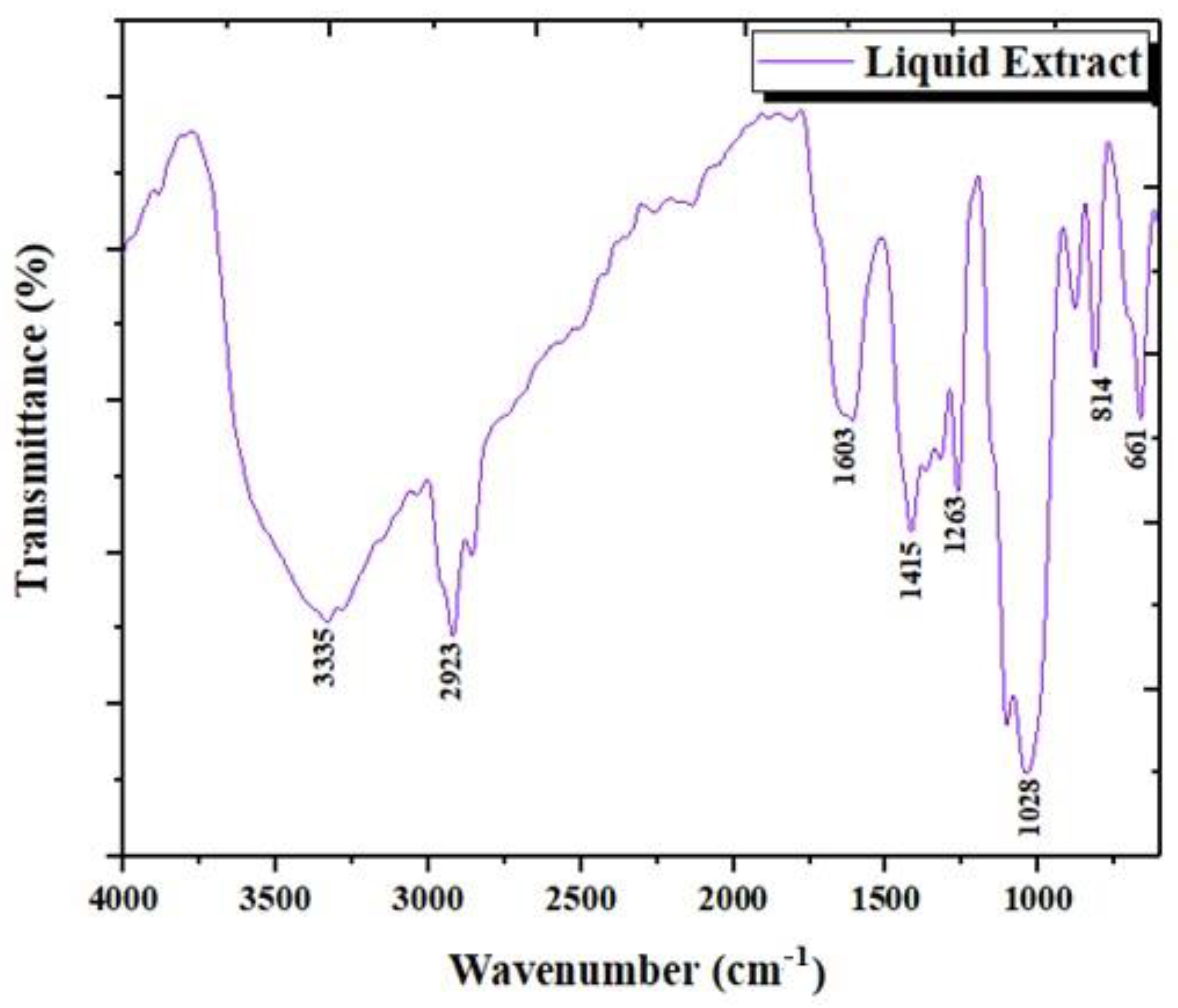
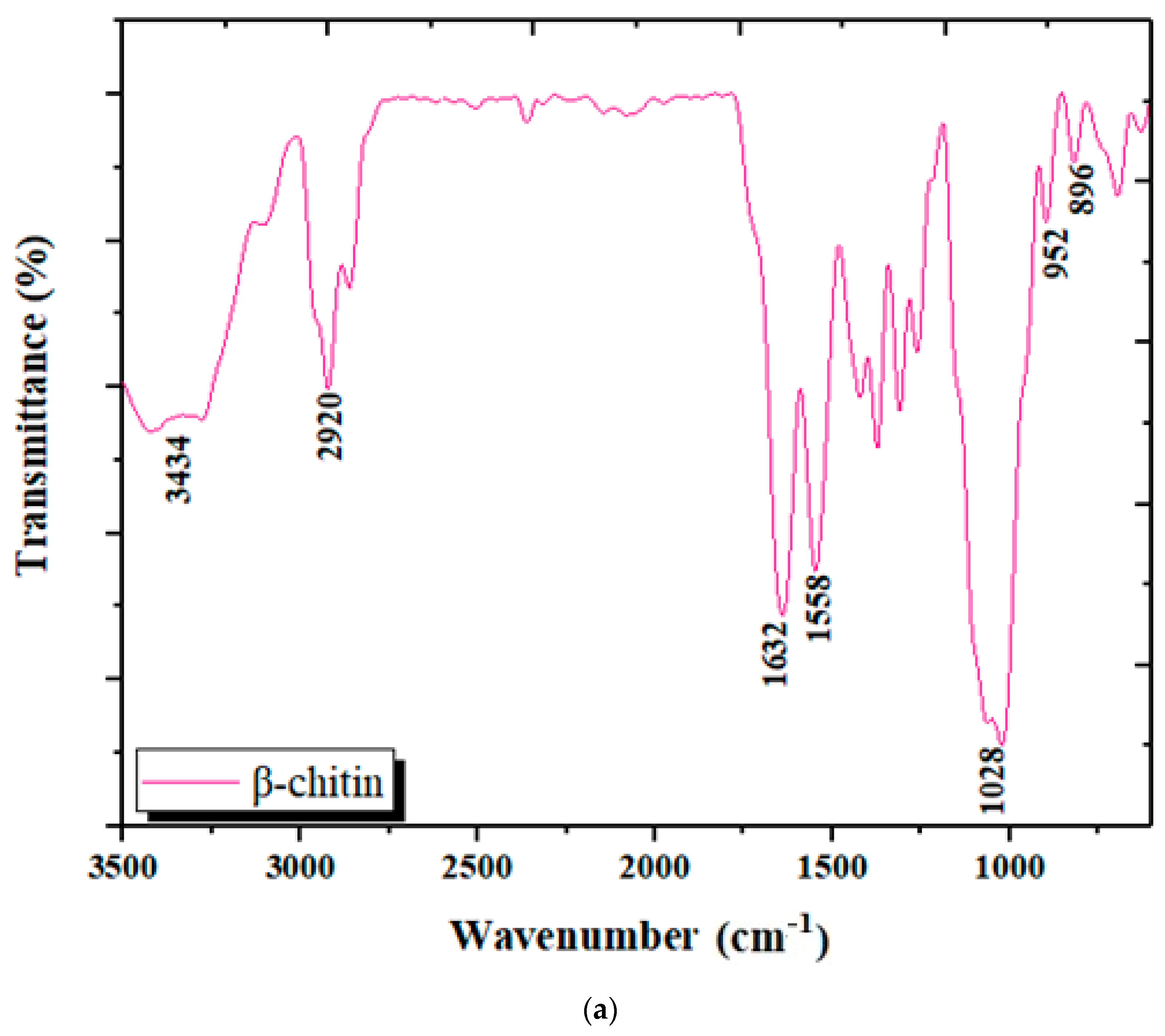
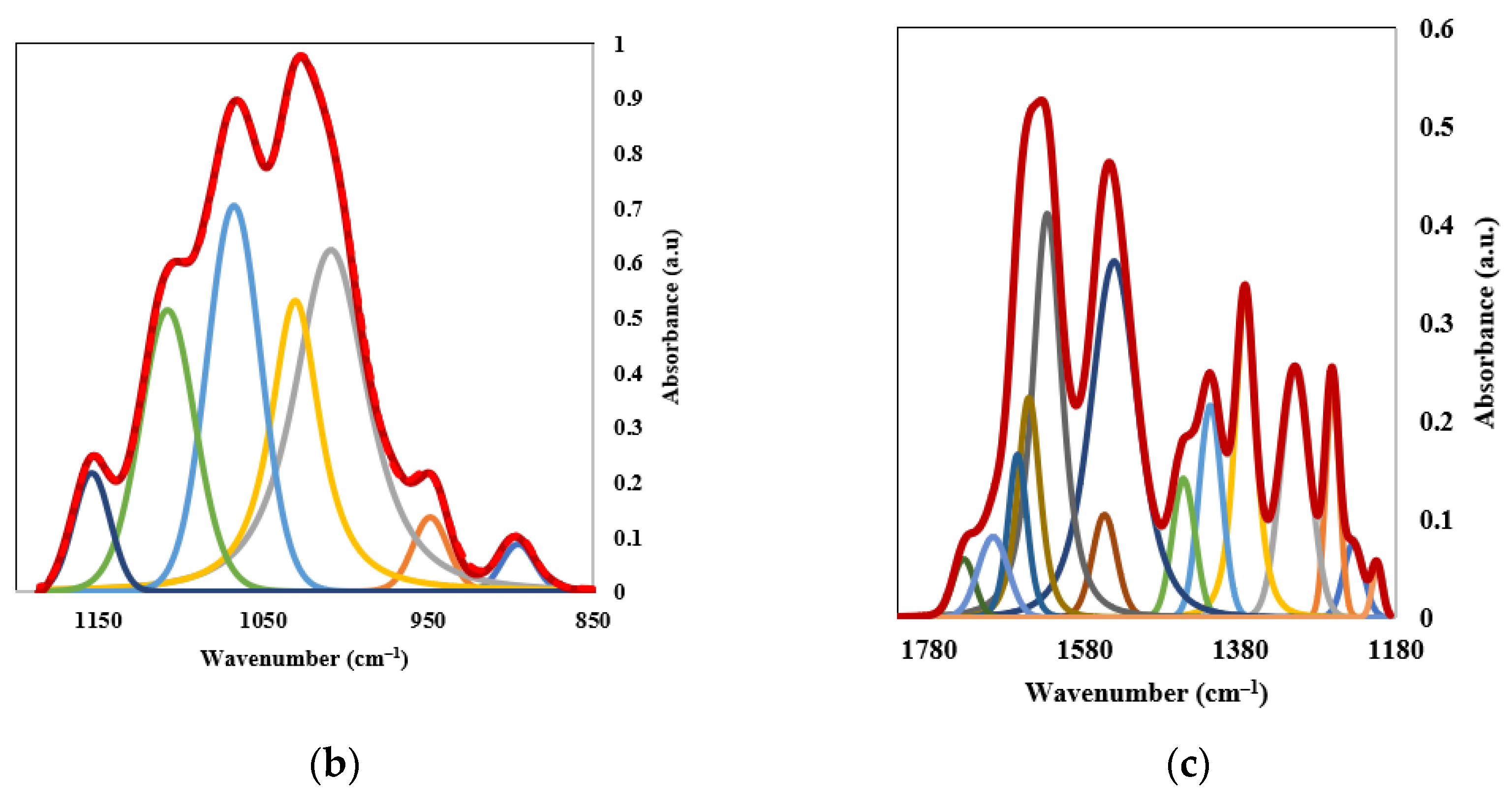
3.1.3. Analysis by Infrared Spectroscopy (FTIR)
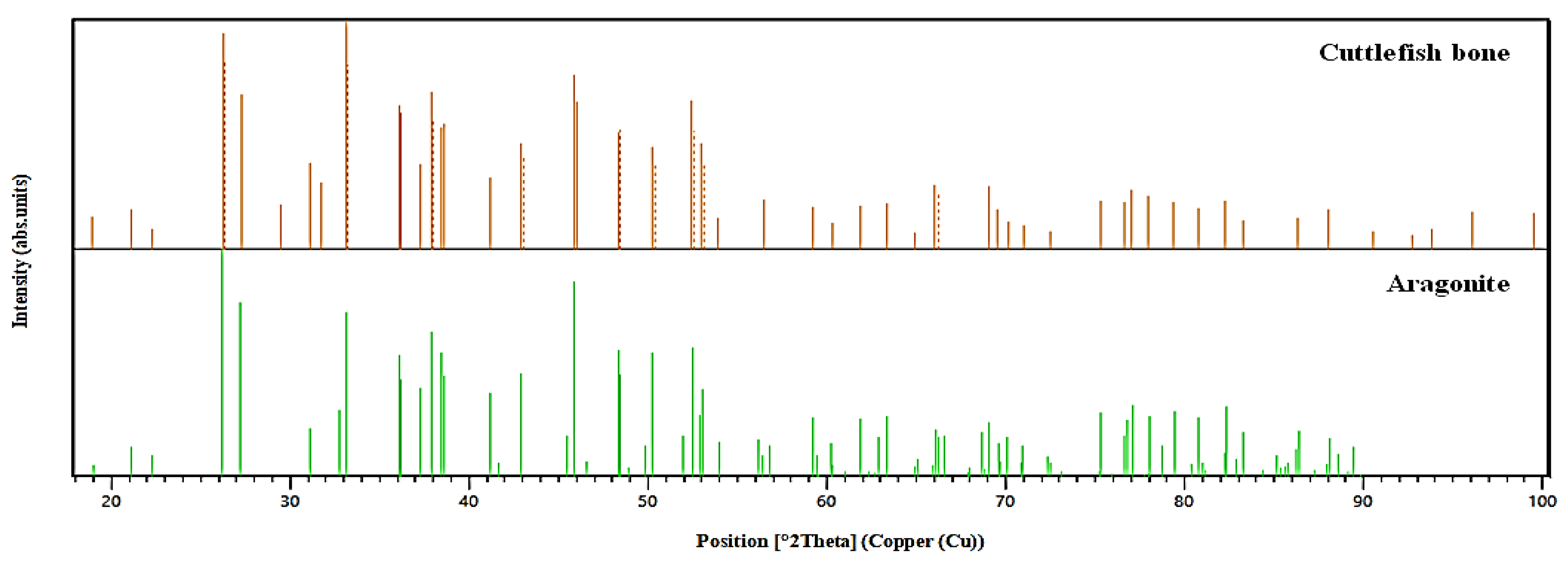
3.1.4. Analysis by XRD Spectroscopy
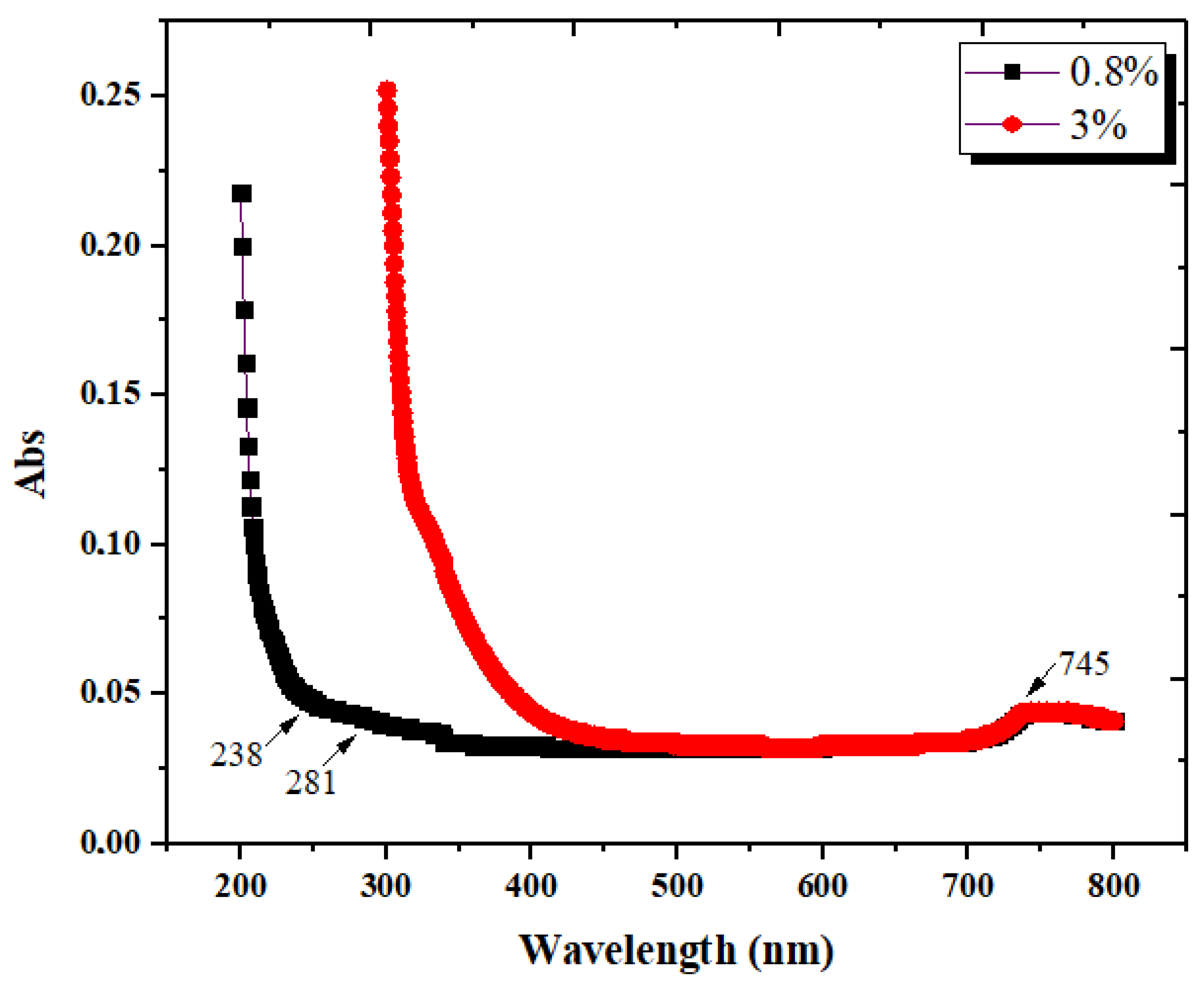
3.1.5. Ultraviolet/Visible Spectroscopy (UV-Vis)
3.1.6. Determination of Protein Content of the LC Extract
3.1.7. NMR Analysis of Chitin Oligosaccharides Separated from the LC Extract
3.1.8. Zeta Potential Measurements
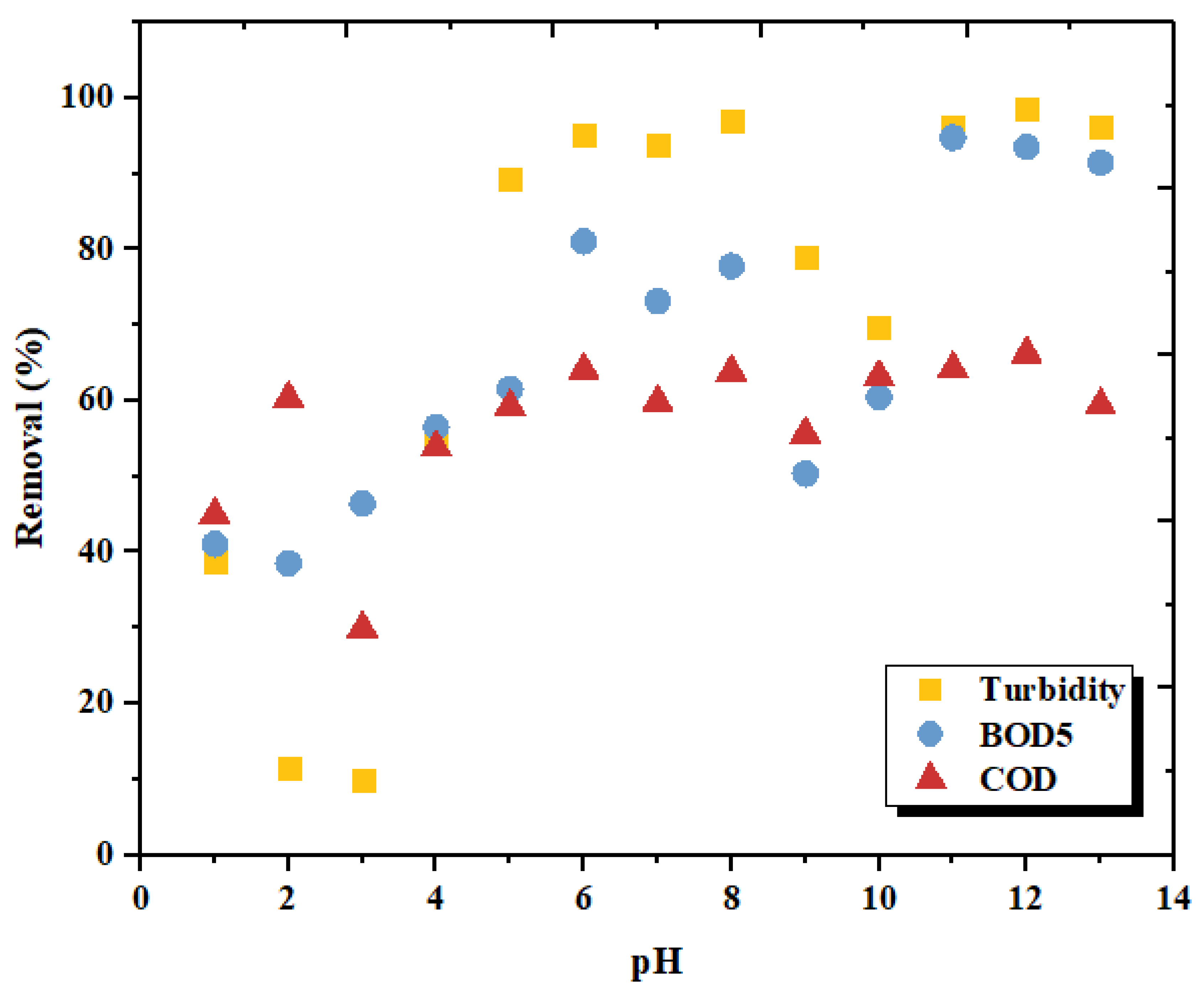
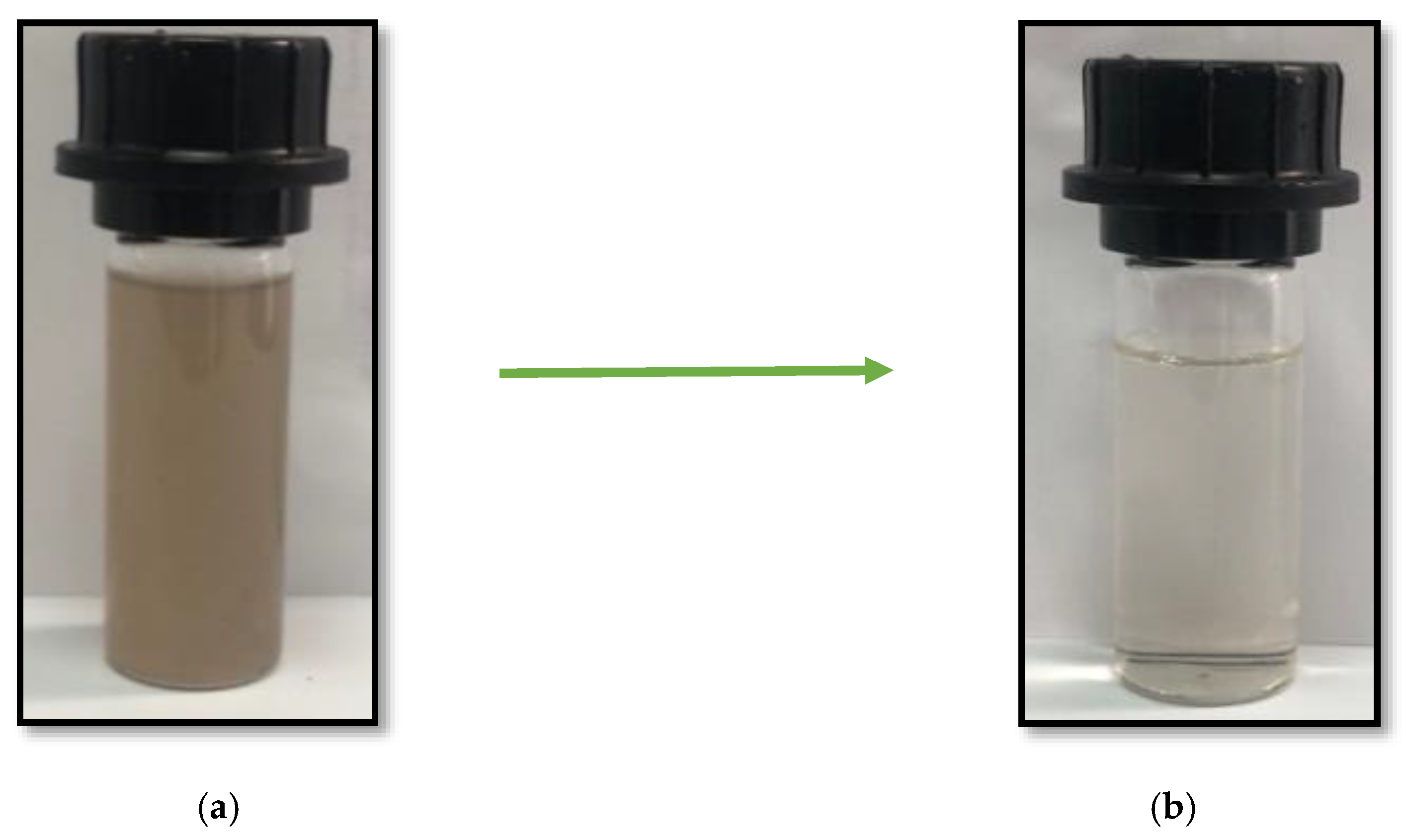
3.2. Effect of pH on the Coagulation Treatment Efficiency
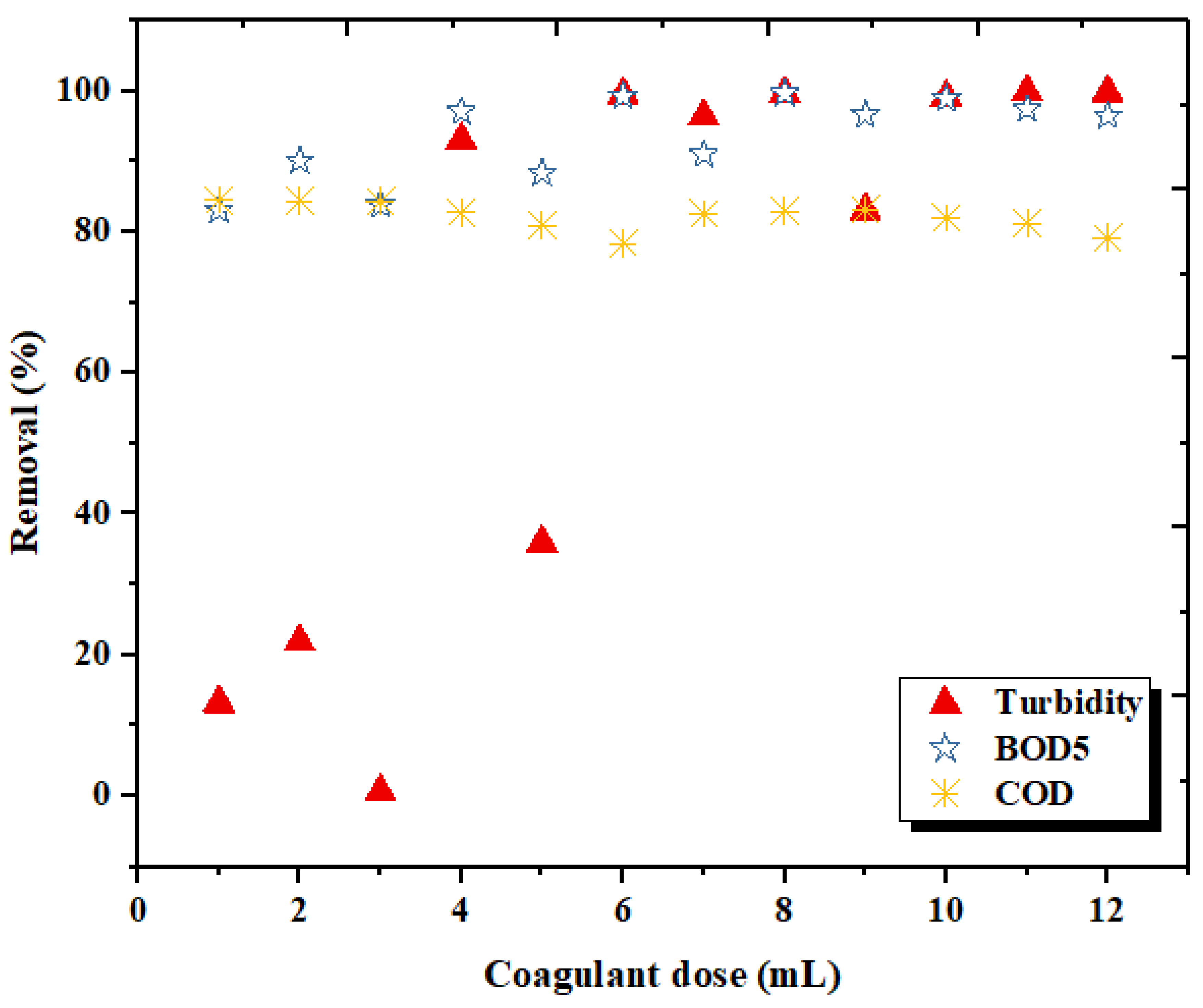
3.3. Effect of Coagulant Dosage on the Coagulation Treatment Efficiency
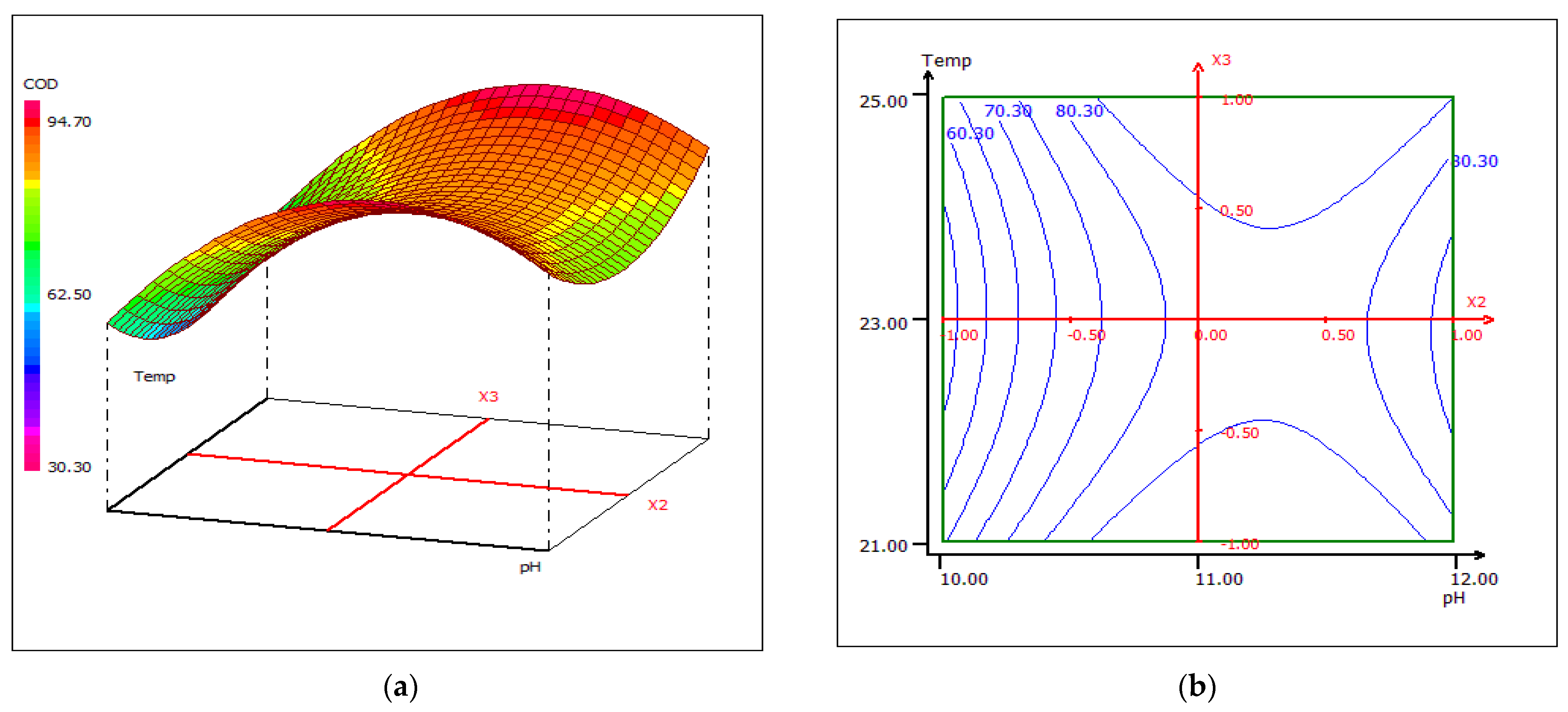
3.4. Optimization Using the Box–Behnken Experimental Design
3.4.1. Statistical Analysis
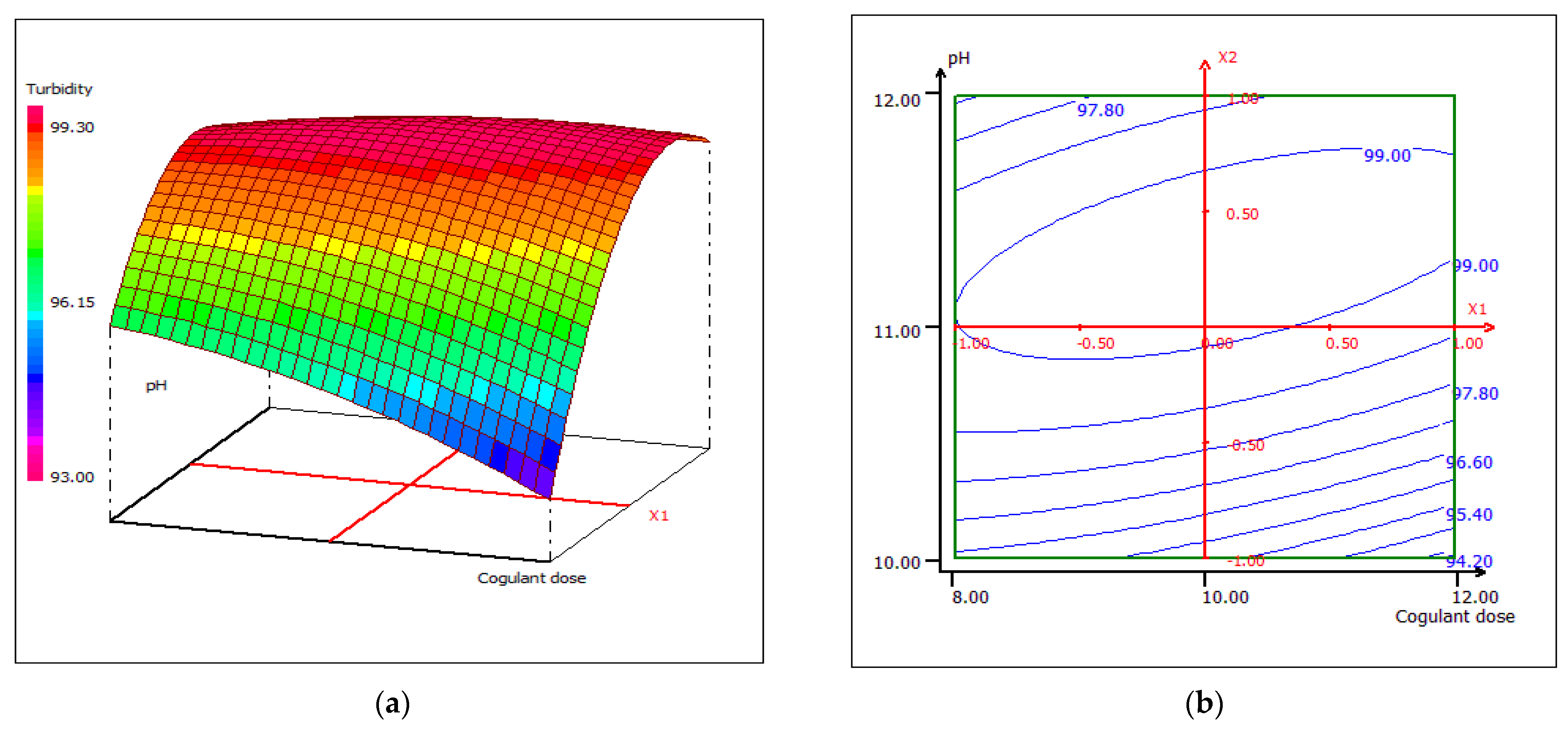
3.4.2. Response Surface Plotting and Optimization of Turbidity Removal
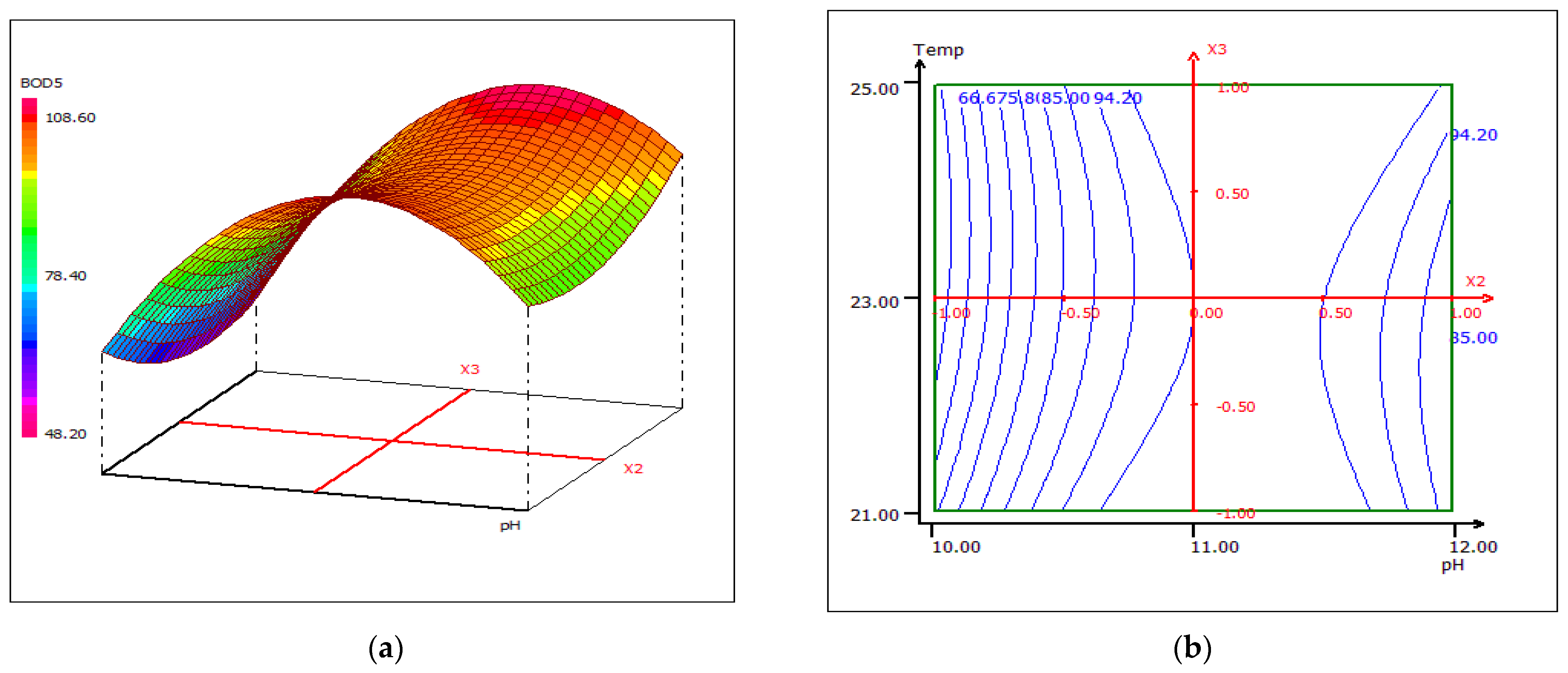
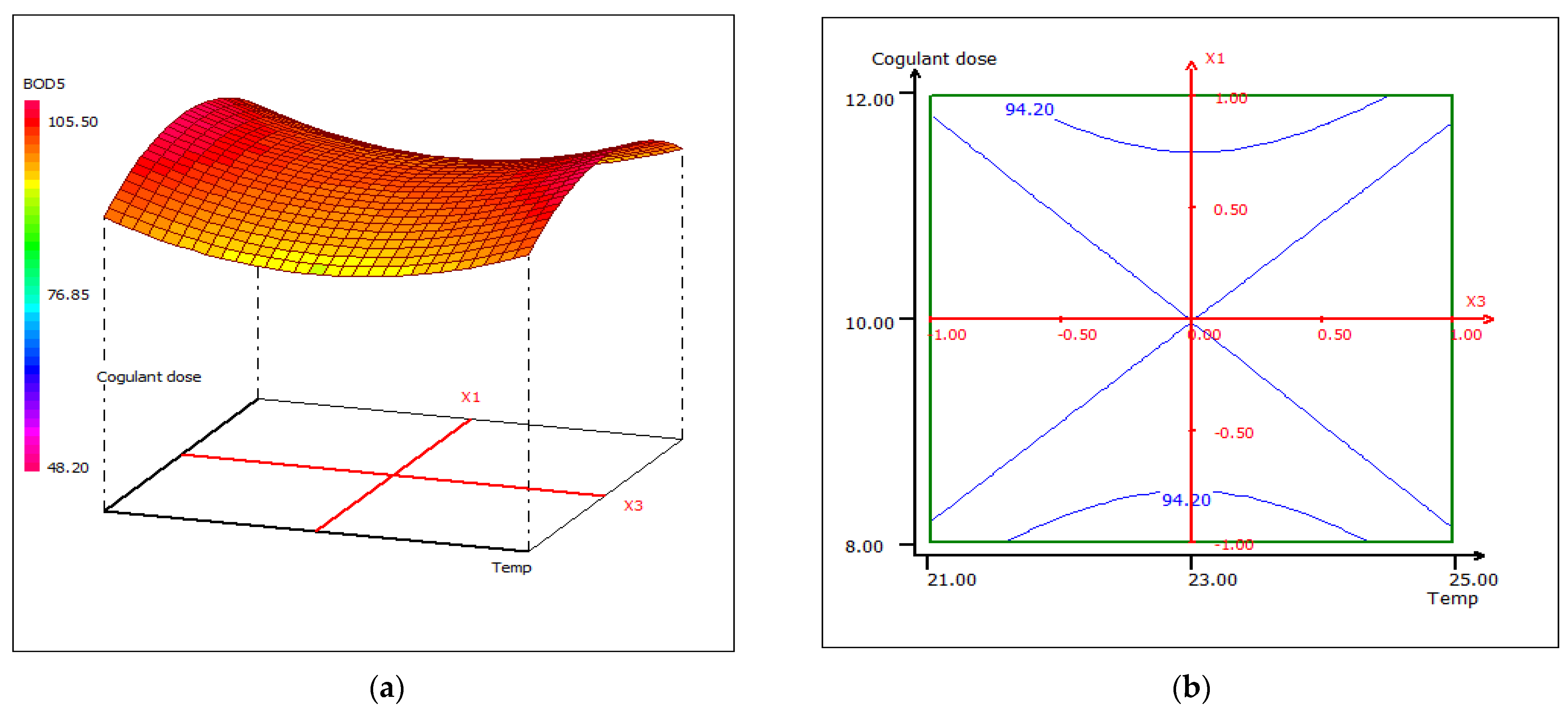
3.4.3. Response Surface Plotting and Optimization of BOD5 Removal
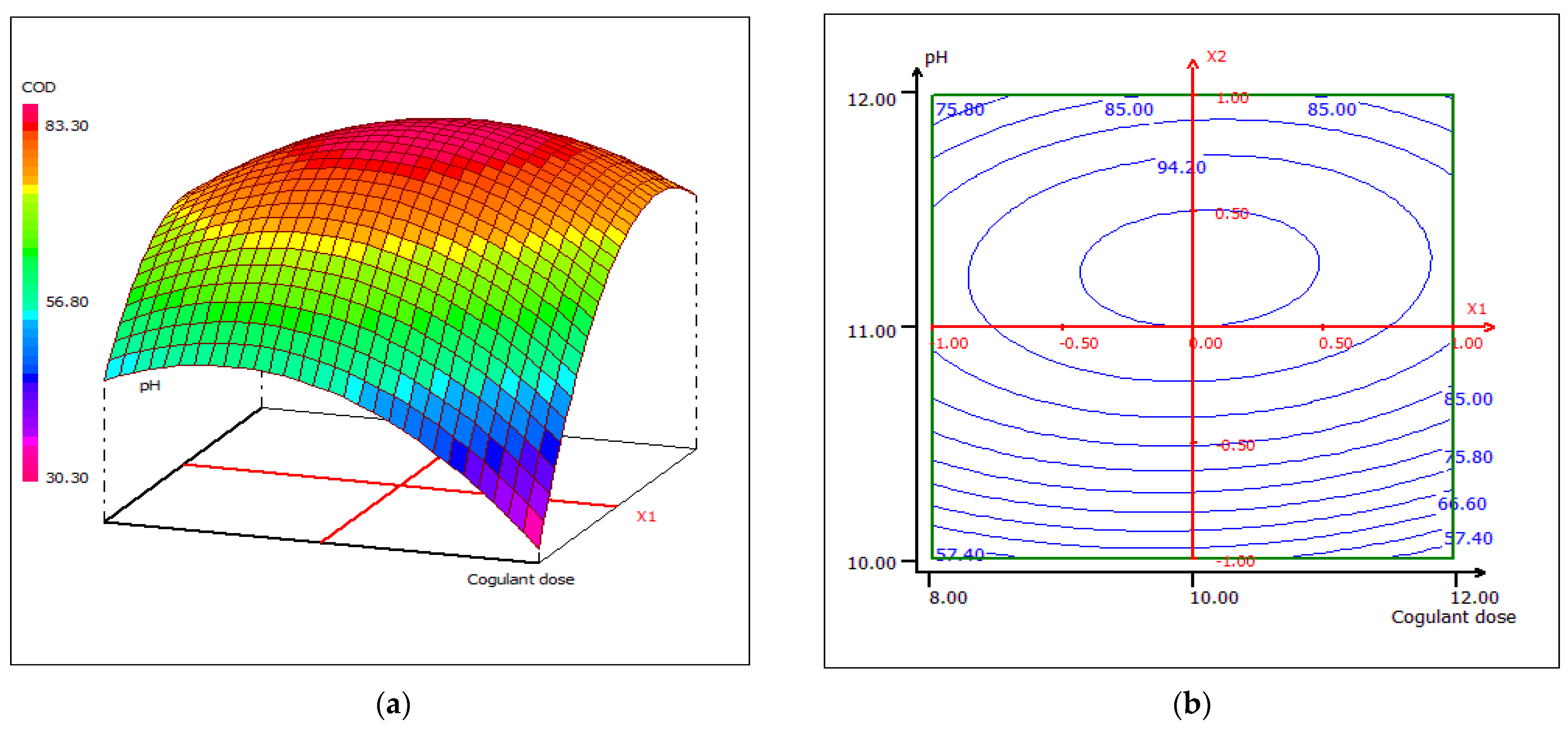
3.4.4. Response Surface Plotting and Optimization of COD Removal
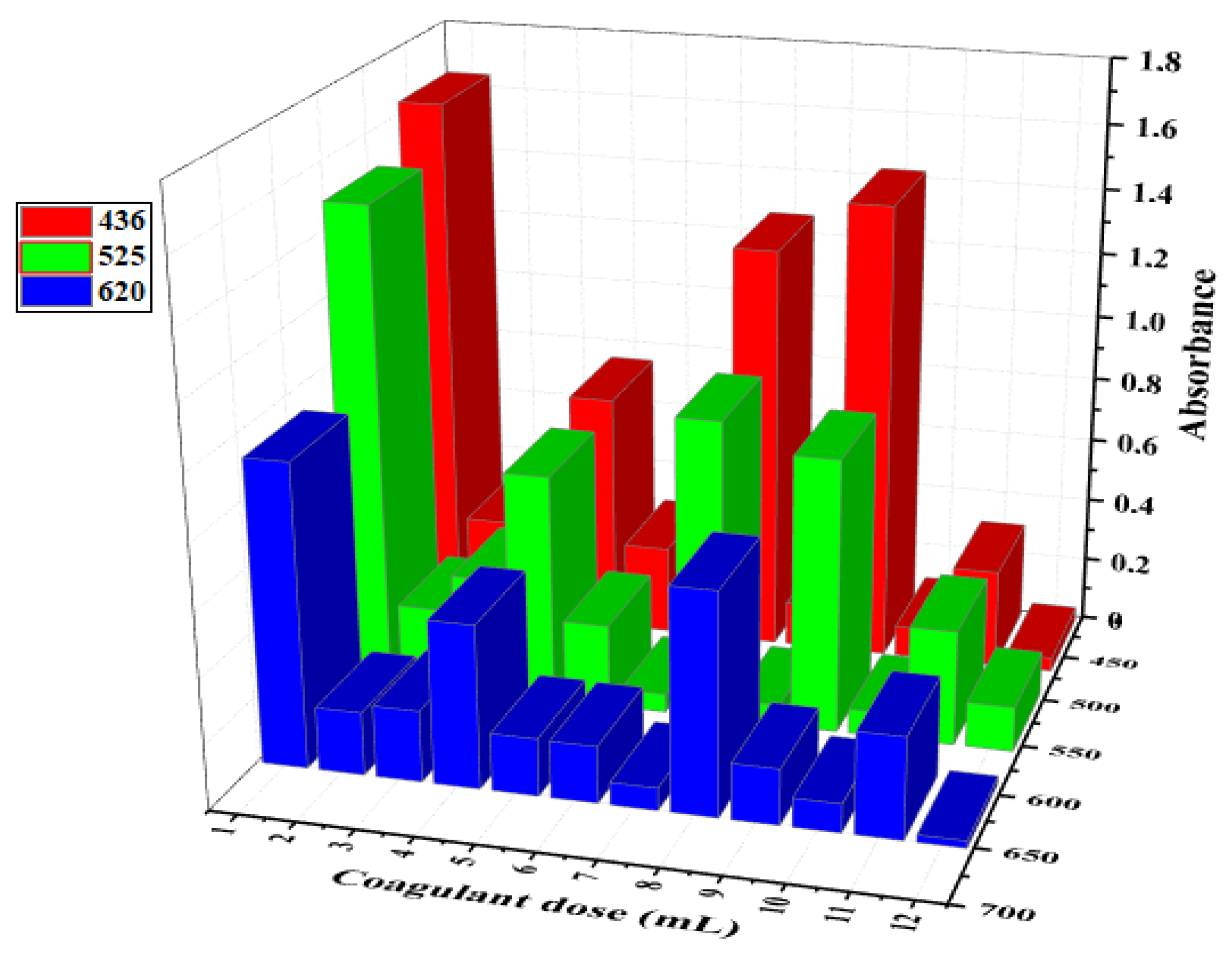
3.5. Optical Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mavrov, V.; Bélières, E. Reduction of water consumption and wastewater quantities in the fish industry by water recycling using membrane processes. Desalination 2000, 131, 75–86. [Google Scholar] [CrossRef]
- Teresita, D.; López, R.J. Contamination from fishing resources processing in Cuba and its environmental impact. Hydraul. Environ. Eng. 2013, 2, 17–26. [Google Scholar]
- García, M.A.; Montelongo, I.; Rivero, A.; Paz, N.; Fernández, M.; Núñez, V.M. Treatment of Wastewater from Fish Processing Industry using Chitosan Acid Salts. Int. J. Water Wastewater Treat. 2016, 2, 2381–5299. [Google Scholar] [CrossRef]
- Kim, H.W.; Batra, A.; Priya, S.; Uchino, K.; Markley, D.; Newnham, R.E.; Hofmann, H.F. Energy harvesting using a piezoelectric “cymbal” transducer in dynamic environment. Jpn. J. Appl. Phys. 2004, 43, 6178–6183. [Google Scholar] [CrossRef]
- Zemaitaitiene, R.J.; Zliobaite, E.; Klimaviciute, R.; Zemaitaitis, A. The role of anionic substances in removal of textile dyes from solutions using cationic flocculant. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 37–47. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Zhang, Z.G.; Yu, W.; Lockwood, F.E.; Grulke, E.A. Anomalous thermal conductivity enhancement in nanotube suspensions. Appl. Phys. Lett. 2001, 79, 2252–2254. [Google Scholar] [CrossRef]
- Divakaran, R.; Pillai, V.S. Flocculation of kaolinite suspensions in water by chitosan. Water Res. 2001, 35, 3904–3908. [Google Scholar] [CrossRef]
- Özacar, M.; Şengil, I.A. Adsorption of reactive dyes on calcined alunite from aqueous solutions. J. Hazard. Mater. 2003, 98, 211–224. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Imron, M.F.; Chik, C.E.N.C.E.; Owodunni, A.A.; Ahmad, A.; Alnawajha, M.M.; Rahim, N.F.M.; Said, N.S.M.; Abdullah, S.R.S.; Kasan, N.A.; et al. What compound inside biocoagulants/bioflocculants is contributing the most to the coagulation and flocculation processes? Sci. Total Environ. 2021, 806, 1–30. [Google Scholar] [CrossRef]
- Roussy, J.; Chastellan, P.; Van Vooren, M.; Guibal, E. Treatment of ink-containing wastewater by coagulation/flocculation using biopolymers. Water SA 2005, 31, 369–376. [Google Scholar] [CrossRef]
- Özacar, M.; Şengil, I.A. Effectiveness of tannins obtained from valonia as a coagulant aid for dewatering of sludge. Water Res. 2000, 34, 1407–1412. [Google Scholar] [CrossRef]
- Andrade, P.V.; Palanca, C.F.; de Oliveira, M.A.C.; Ito, C.Y.K.; dos Reis, A.G. Use of Moringa oleifera seed as a natural coagulant in domestic wastewater tertiary treatment: Physicochemical, cytotoxicity and bacterial load evaluation. J. Water Process Eng. 2021, 40, 1–11. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Aliu, Y.D. Evaluation of plantain peelings ash extract as coagulant aid in the coagulation of colloidal particles in low pH aqua system. Water Qual. Res. J. Can. 2008, 43, 231–238. [Google Scholar] [CrossRef]
- Vijayaraghavan, G.; Sivakumar, T.; Kumar, A.V. Application of plant based coagulants for waste water treatment. Int. J. Adv. Eng. Res. Stud. 2011, 5, 1–6. [Google Scholar]
- Okolo, B.I.; Nnaji, P.C.; Oke, E.O.; Adekunle, K.F.; Ume, C.S.; Onukwuli, O.D. Optimizing bio-coagulants for brewery wastewater treatment using response surface methodology. Niger. J. Technol. 2018, 36, 1104–1113. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kaur, N.; Kaur, R.; Kaur, R. A review on valorization of chitinous waste. J. Polym. Res. 2021, 28, 406. [Google Scholar] [CrossRef]
- Shushizadeh, M.-R.; Pour, E.M.; Zare, A.; Lashkari, Z. Persian gulf β-chitin extraction from sepia pharaonis sp. cuttlebone and preparation of its derivatives. Bioact. Carbohydr. Diet. Fibre 2015, 6, 133–142. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Saito, Y.; Okano, T.; Gaill, F.; Chanzy, H.; Putaux, J.-L. Structural data on the intra-crystalline swelling of β-chitin. Int. J. Biol. Macromol. 2000, 28, 81–88. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, M.H.; Park, W.H. Preparation and structural investigation of novel β-chitin nanocrystals from cuttlefish bone. ACS Biomater. Sci. Eng. 2019, 5, 1744–1752. [Google Scholar] [CrossRef]
- Hafza, N.I.; Zia, K.M.; Rehman, S.; Rahila, I.; Sultana, S. Utilization of Shellfish Industrial Waste for Isolation, Purification, and Characterizations of Chitin from Crustacean’s Sources in Pakistan. J. Polym. Environ. 2021, 29, 2337–2348. [Google Scholar] [CrossRef]
- Ben Nasr, A.; Walha, K.; Charcosset, C.; Ben Amar, R. Removal of fluoride ions using cuttlefish bones. J. Fluor. Chem. 2011, 132, 57–62. [Google Scholar] [CrossRef]
- Taghi Ghaneian, M.; Momtaz, M.; Dehvari, M. An Investigation of the Efficacy of Cuttlefish Bone Powder in the Removal of Reactive Blue 19 Dye from Aqueous Solutions: Equilibrium and Isotherm Studies. J. Community Health Res. 2012, 1, 88–92. [Google Scholar]
- Sandesh, K.; Suresh Kumar, R.; JagadeeshBabu, P.E. Rapid removal of cobalt (II) from aqueous solution using cuttlefish bones; equilibrium, kinetics, and thermodynamic study. Asia-Pac. J. Chem. Eng. 2013, 8, 144–153. [Google Scholar] [CrossRef]
- Nouj, N.; Hafid, N.; El Alem, N.; Cretescu, I. Novel Liquid Chitosan-Based Biocoagulant for Treatment Optimization of Fish Processing Wastewater from a Moroccan Plant. Materials 2021, 14, 7133. [Google Scholar] [CrossRef] [PubMed]
- Baird, R.; Eaton, A.D.; Rice, E.W.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017; Volume 1. [Google Scholar]
- Okey-Onyesolu, C.; Onukwuli, O.; Okoye, C. Deturbidization of Vegetable Oil Refinery Wastewater with Extracted Fish Scale Biomass via Coagulation Process; Non-linear Kinetics Studies. J. Eng. Res. Rep. 2018, 2, 1–14. [Google Scholar] [CrossRef]
- Yi, Z.; Jie, C.; Li-Na, Z. A Review of Chitin Solvents and Their Dissolution Mechanisms. Chin. J. Polym. Sci. 2020, 38, 1047. [Google Scholar] [CrossRef]
- Noble, J.E.; Bailey, M.J.A. Quantitation of protein, Chapter 8. In Methods in Enzymology, Guide to Protein Purification, 2nd ed.; Burgess, R.R., Deutscher, M.P., Eds.; Elsevier Inc.: San Diego, CA, USA, 2009; Volume 463, pp. 73–95. [Google Scholar]
- Benyounis, K.Y.; Olabi, A.G.; Hashmi, M.S.J. Optimizing the laser-welded butt joints of medium carbon steel using RSM. J. Mater. Process Technol. 2005, 164, 986–989. [Google Scholar] [CrossRef] [Green Version]
- Amran, A.H.; Zaidi, N.S.; Syafiuddin, A.; Zhan, L.Z.; Bahrodin, M.B.; Mehmood, M.A.; Boopathy, R. Potential of Carica papaya Seed-Derived Bio-Coagulant to Remove Turbidity from Polluted Water Assessed through Experimental and Modeling-Based Study. Appl. Sci. 2021, 11, 5715. [Google Scholar] [CrossRef]
- Ivankovic, H.; Tkalcec, E.; Orlic, S.; Gallego Ferrer, G.; Schauperl, Z. Hydroxyapatite formation from cuttlefish bones: Kinetics. J. Mater. Sci. Mater. Med. 2010, 21, 2711–2722. [Google Scholar] [CrossRef] [Green Version]
- Sarin, P.; Lee, S.J.; Apostolov, Z.D.; Kriven, W.M. Porous biphasic calcium phosphate scaffolds from cuttlefish bone. J. Am. Ceram. Soc. 2011, 94, 2362–2370. [Google Scholar] [CrossRef]
- Florek, M.; Fornal, E.; Gómez-Romero, P.; Zieba, E.; Paszkowicz, W.; Lekki, J.; Nowak, J.; Kuczumow, A. Complementary microstructural and chemical analyses of Sepia officinalis endoskeleton. Mater. Sci. Eng. C 2009, 29, 1220–1226. [Google Scholar] [CrossRef]
- Hasmath, F.M.; Meenakshi, S. Decolorization and detoxification of Acid blue 158 dye using cuttlefish bone powder as co-adsorbent via photocatalytic method. J. Water Process. Eng. 2014, 2, 22–30. [Google Scholar] [CrossRef]
- Shimojoh, M.; Fukushima, K.; Kurita, K. Low-molecular-weight chitosans derived from β-chitin: Preparation, molecular characteristics and aggregation activity. Carbohydr. Polym. 1998, 35, 223–231. [Google Scholar] [CrossRef]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan Amphiphilic Derivatives. Chemistry and Applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Alagui, A.; Vottero, P. Investigation of different natural sources of chitin: Influence of the source and deacetylation process on the physicochemical characteristics of chitosan. Polym. Int. 2000, 49, 337–344. [Google Scholar] [CrossRef]
- Furuhashi, T.; Schwarzinger, C.; Miksik, I.; Smrz, M.; Beran, A. Molluscan shell evolution with review of shell calcification hypothesis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 154, 351–371. [Google Scholar] [CrossRef]
- Raabe, D.; Sachs, C.; Romano, P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005, 53, 4281–4292. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S.; Fischer, F.D.; Kolednik, O. Hindered crack propagation in materials with periodically varying young’s modulus—Lessons from biological materials. Adv. Mater. 2007, 19, 2657–2661. [Google Scholar] [CrossRef]
- Focher, B.; Naggi, A.; Torri, G.; Cosani, A.; Terbojevich, M. Structural differences between chitin polymorphs and their precipitates from solutions-evidence from CP-MAS 13C-NMR, FT-IR and FT-Raman spectroscopy. Carbohydr. Polym. 1992, 17, 97–102. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polym. Guildf 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Dehvari, M.; Ehrampoush, M.H.; Ghaneian, M.T.; Jamshidi, B.; Tabatabaee, M. Adsorption kinetics and equilibrium studies of reactive red 198 dye by cuttlefish bone powder. Iran. J. Chem. Chem. Eng. 2017, 36, 143–151. [Google Scholar]
- Curti, F.; Drăgușin, D.M.; Serafim, A.; Sorescu, A.; Stancu, I.C.; Iovu, H.; Marinescu, R. Cuttlefish bone-based ink for 3D printing of scaffolds for orthopedic applications. U.P.B. Sci. Bull. 2021, 83, 1–14. [Google Scholar]
- Jang, M.K.; Kong, B.G.; Jeong, Y.I.; Lee, C.H.; Nah, J.W. Physicochemical Characterization of α-Chitin, β-Chitin, and Ɣ-Chitin Separated from Natural Resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Arjama, M.; Mehnath, S.; Rajan, M.; Jeyaraj, M. Injectable cuttlefish HAP and macromolecular fibroin protein hydrogel for natural bone mimicking matrix for enhancement of osteoinduction progression. React. Funct. Polym. 2021, 160, 1381–5148. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods – A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Liu, T.G.; Li, B.; Huang, W.; Lv, B.; Chen, J.; Zhang, J.X.; Zhu, L.P. Effects and kinetics of a novel temperature cycling treatment on the N-deacetylation of chitin in alkaline solution. Carbohydr. Polym. 2009, 77, 110–117. [Google Scholar] [CrossRef]
- Yi-Cheng, H.; Hao-Wen, C. Using hydroxyapatite from fish scales to prepare chitosan/gelatin/hydroxyapatite membrane: Exploring potential for bone tissue engineering. J. Mar. Sci. Technol. 2013, 6, 716–722. [Google Scholar]
- Yang, T.; Jia, Z.; Chen, H.; Deng, Z.; Liu, W.; Chen, L.; Li, L. Mechanical design of the highly porous cuttlebone: A bioceramic hard buoyancy tank for cuttlefish. Proc. Natl. Acad. Sci. USA 2020, 117, 23450–23459. [Google Scholar] [CrossRef]
- Ianiro, A.; Di Giosia, M.; Fermani, S.; Samori, C.; Barbalinardo, M.; Valle, F.; Pellegrini, G.; Biscarini, F.; Zerbetto, F.; Calvaresi, M.; et al. Customizing Properties of β-Chitin in Squid Pen (Gladius) by Chemical Treatments. Mar. Drugs. 2014, 12, 5979–5992. [Google Scholar] [CrossRef]
- Garrido, T.; Etxabide, A.; de la Caba, K.; Guerrero, P. Versatile soy protein films and hydrogels by the incorporation of β-chitin from squid pens (Loligo sp.). Green Chem. 2017, 19, 5923–5931. [Google Scholar] [CrossRef]
- Zhangquan, P.; Guo, L.; Zhang, Z.; Tesche, B.; Wilke, T.; Ogermann, D.; Hu, S.; Kleinermanns, K. Micelle-Assisted One-Pot Synthesis of Water-Soluble Polyaniline-Gold Composite Particles. Langmuir 2006, 22, 10915–10918. [Google Scholar] [CrossRef]
- Shimahara, K.; Takiguchi, Y. Preparation of Crustacean Chitin, Chapter 47. In Methods in Enzymology, Biomass; Part B: Lignin, Pectin, and Chitin; Wood, W.A., Lellogg, S.T., Eds.; Academic Press: San Diego, CA, USA, 1988; Volume 161, pp. 417–423. [Google Scholar]
- Feng, M.; Lu, X.; Hou, D.; Zhang, S. Solubility, chain characterization, and derivatives of chitin, Chapter 4. In Handbook of Chitin and Chitosan, Preparation and Properties; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 101–129. [Google Scholar]
- Jeong, Y.-I.; Kim, D.-G.; Jang, M.-K.; Nah, J.-W. Preparation and spectroscopic characterization of methoxy poly (ethylene glycol)-grafted water-soluble chitosan. Carbohydr. Res. 2008, 343, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Dahmane El, M.; Taourirte, M.; Eladlani, N.; Rhazi, M. Extraction and Characterization of Chitin and Chitosan from Parapenaeus longirostris from Moroccan Local Sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment, 3rd ed.; IWA Publishing: London, UK, 2016; pp. 357–419. [Google Scholar]
- Lu, G.W.; Gao, P. Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems: Science and Technology; Kulkarni, V.S., Ed.; Elsevier: Oxford, UK, 2010; pp. 59–94. [Google Scholar]
- Thompson, D.W.; Pownall, P.G. Surface electrical properties of calcite. J. Colloid. Interface Sci. 1989, 131, 74–82. [Google Scholar] [CrossRef]
- Tolaimate, A.; Desbrieres, J.; Rhazi, M.; Alagui, A. Contribution to the preparation of chitins and chitosans with controlled physico-chemical properties. Polym. Guildf 2003, 44, 7939–7952. [Google Scholar] [CrossRef]
- Sagheer, F.A.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Badot, P.M.; Crini, G.; Guibal, E. Chitine et Chitosane: Du Biopolymère à l’application. Psychoanal. Rev. 2014, 101, 129–139. [Google Scholar] [CrossRef]
- Geetha, D.M.; Shinoon Al-Hashmi, Z.S.; Chandra, S.G. Treatment of vegetable oil mill effluent using crab shell chitosan as adsorbent. Int. J. Environ. Sci. Technol. 2012, 9, 713–718. [Google Scholar] [CrossRef]
- Abou Oualid, H.; Abdellaoui, Y.; Laabd, M.; El Ouardi, M.; Brahmi, Y.; Iazza, M.; Abou Oualid, J. Eco-efficient green seaweed codium decorticatum biosorbent for textile dyes: Characterization, mechanism, recyclability, and RSM optimization. ACS Omega 2020, 5, 22192–22207. [Google Scholar] [CrossRef] [PubMed]
- Okolo, B.I.; Nnaji, P.C.; Menkiti, M.C.; Onukwuli, O.D. Process Optimization for Turbidity Removal from Paint Wastewater Using Bio-Coagulant. J. Eng. Appl. Sci. 2018, 12, 1–10. [Google Scholar]
- Rodrigues, A.C.; Boroski, M.; Shimada, N.S.; Garcia, J.C.; Nozaki, J.; Hioka, N. Treatment of paper pulp and paper mill wastewater by coagulation-flocculation followed by heterogeneous photocatalysis. J. Photochem. Photobiol. A Chem. 2008, 194, 1–10. [Google Scholar] [CrossRef]
- He, W.; Gao, W.; Fatehi, P. Oxidation of Kraft Lignin with Hydrogen Peroxide and its Application as a Dispersant for Kaolin Suspensions. ACS Sustain. Chem. Eng. 2017, 5, 10597–10605. [Google Scholar] [CrossRef]
- Khouni, I.; Marrot, B.; Ben Amar, R. Decolourization of the reconstituted dye bath effluent by commercial laccase treatment: Optimization through response surface methodology. Chem. Eng. J. 2010, 156, 121–133. [Google Scholar] [CrossRef]






| No Exp | Experimental Parameters | Responses | ||||
|---|---|---|---|---|---|---|
| X1 Coagulant Dose (mL) | X2 Initial pH | X3 Temperature (°C) | Y1 Turbidity Removal (%) | Y2 BOD5 Removal (%) | Y3 COD Removal (%) | |
| 1 | 8.00 | 10.00 | 23.00 | 96.00 | 52.64 | 50.35 |
| 2 | 12.00 | 10.00 | 23.00 | 93.00 | 48.17 | 30.25 |
| 3 | 8.00 | 12.00 | 23.00 | 98.00 | 75.75 | 53.50 |
| 4 | 12.00 | 12.00 | 23.00 | 99.07 | 80.32 | 68.96 |
| 5 | 8.00 | 11.00 | 21.00 | 99.12 | 97.89 | 80.91 |
| 6 | 12.00 | 11.00 | 21.00 | 98.90 | 97.58 | 79.90 |
| 7 | 8.00 | 11.00 | 25.00 | 99.68 | 97.76 | 82.92 |
| 8 | 12.00 | 11.00 | 25.00 | 99.85 | 96.46 | 79.09 |
| 9 | 10.00 | 10.00 | 21.00 | 96.44 | 70.63 | 65.34 |
| 10 | 10.00 | 12.00 | 21.00 | 98.32 | 86.42 | 82.18 |
| 11 | 10.00 | 10.00 | 25.00 | 97.43 | 61.30 | 62.75 |
| 12 | 10.00 | 12.00 | 25.00 | 98.00 | 97.11 | 84.73 |
| 13 | 10.00 | 11.00 | 23.00 | 99.13 | 98.80 | 81.94 |
| 14 | 10.00 | 11.00 | 23.00 | 99.13 | 98.80 | 81.94 |
| 15 | 10.00 | 11.00 | 23.00 | 99.13 | 98.80 | 81.94 |
| Coded Variables | Factor | Coded Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| X1 | Coagulant dosage (mL/0.5 L) | 8 | 10 | 12 |
| X2 | Initial pH | 10 | 11 | 12 |
| X3 | Temperature (°C) | 21 | 23 | 25 |
| Parameter | Value | Moroccan Standard Limit of Direct Discharge | ||
|---|---|---|---|---|
| Min | Mean | Max | ||
| Temperature | 12 | 18.5 | 25 | 30 °C |
| pH | 6.62 | 7.06 | 7.50 | 5.5–9.5 |
| Conductivity | 9000 | 15,500 | 22,000 | 2700 µS/cm |
| Turbidity | 850 | 925 | >1000 | - |
| BOD5 | 1700 | 2345.5 | 2990.9 | 100 mg O2/L |
| COD | 2380 | 3735 | 5090 | 500 mg O2/L |
| pH of colloidal suspension | 7.0 | 8.0 | 9.0 | 10.0 | 11.0 |
| Measured zeta potential (mV) | +10.4 | +2.07 | −3.97 | −7.12 | −10.3 |
| Parameter | Standard Deviation | R2 | R2 Adjusted | R2 Predicted |
|---|---|---|---|---|
| Turbidity | 0.946 | 0.901 | 0.722 | N.D |
| BOD5 | 0.760 | 0.999 | 0.998 | 0.990 |
| COD | 0.520 | 1.00 | 0.999 | 0.994 |
| Coefficient | b0 | b1 | b2 | b3 | b1–1 | b2–2 | b3–3 | b1–2 | b1–3 | b2–3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | Turbidity | 99.130 | −248 | 1.315 | 0.273 | −0.386 | −2.226 | 0.644 | 1.017 | 0.097 | −0.328 |
| BOD5 removal | 98.800 | −0.189 | 13.357 | 0.014 | −8.011 | −26.569 | 6.634 | 2.248 | −0.248 | 5.005 | |
| COD removal | 81.940 | −1.185 | 10.085 | 0.145 | −12.110 | −19.065 | 10.875 | 8.890 | −0.705 | 1.285 | |
| F. inflation | Turbidity | - | 1.00 | 1.00 | 1.01 | 1.01 | 1.01 | 1.00 | 1.00 | 1.00 | 1.00 |
| BOD5 removal | - | 1.00 | 1.00 | 1.00 | 1.01 | 1.01 | 1.01 | 1.00 | 1.00 | 1.00 | |
| COD removal | - | 1.00 | 1.00 | 1.00 | 1.01 | 1.01 | 1.01 | 1.00 | 1.00 | 1.00 | |
| Standard deviation | Turbidity | 0.546 | 0.335 | 0.335 | 0.335 | 0.493 | 0.493 | 0.493 | 0.473 | 0.473 | 0.473 |
| BOD5 removal | 0.439 | 0.269 | 0.269 | 0.269 | 0.396 | 0.396 | 0.396 | 0.380 | 0.380 | 0.380 | |
| COD removal | 0.300 | 0.184 | 0.184 | 0.184 | 0.271 | 0.271 | 0.271 | 0.260 | 0.260 | 0.260 | |
| t exp | Turbidity | 181.43 | −0.74 | 3.93 | 0.81 | −0.78 | −4.52 | 1.31 | 2.15 | 0.21 | −0.69 |
| BOD5 removal | 225.03 | −0.70 | 49.68 | 0.05 | −20.24 | −67.13 | 16.76 | 5.94 | −0.65 | 13.16 | |
| COD removal | 272.89 | −6.44 | 54.85 | 0.79 | −44.74 | −70.44 | 40.18 | 34.19 | −2.71 | 4.94 | |
| Significance (%) | Turbidity | <0.01 *** | 49.3 | 1.11 * | 45.2 | 46.8 | 0.628 ** | 24.8 | 8.4 | 84.5 | 52.0 |
| BOD5 removal | <0.01 *** | 51.4 | <0.01 *** | 96.1 | <0.01 *** | <0.01 *** | <0.01 *** | 0.193 ** | 54.4 | <0.01 *** | |
| COD removal | <0.01 *** | 0.134 ** | <0.01 *** | 46.6 | <0.01 *** | <0.01 *** | <0.01 *** | <0.01 *** | 4.22 * | 0.432 ** | |
| Sources of Variation | Regression | Residual | Total | ||||||||
| Sum of squares | Turbidity | 40.572 | 4.478 | 45.050 | |||||||
| BOD5 removal | 4.59662 × 103 | 2.891 | 4.59951 × 103 | ||||||||
| COD removal | 3.55346 × 103 | 1.352 | 3.55481 × 103 | ||||||||
| Degree of freedom | Turbidity | 9 | 5 | 14 | |||||||
| BOD5 removal | 9 | 5 | 14 | ||||||||
| COD removal | 9 | 5 | 14 | ||||||||
| Mean square | Turbidity | 4.5080 | 0.8956 | - | |||||||
| BOD5 removal | 5.10736 × 102 | 5.78295 × 102 | - | ||||||||
| COD removal | 3.94829 × 102 | 2.70480 × 10−1 | - | ||||||||
| Ratio | Turbidity | 5.033 | - | - | |||||||
| BOD5 removal | 883.174 | - | - | ||||||||
| COD removal | 1459.734 | - | - | ||||||||
| Significance (%) | Turbidity | 4.49 * | - | - | |||||||
| BOD5 removal | <0.01 *** | - | - | ||||||||
| COD removal | <0.01 *** | - | - | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouj, N.; Hafid, N.; El Alem, N.; Buciscanu, I.I.; Maier, S.S.; Samoila, P.; Soreanu, G.; Cretescu, I.; Stan, C.D. Valorization of β-Chitin Extraction Byproduct from Cuttlefish Bone and Its Application in Food Wastewater Treatment. Materials 2022, 15, 2803. https://doi.org/10.3390/ma15082803
Nouj N, Hafid N, El Alem N, Buciscanu II, Maier SS, Samoila P, Soreanu G, Cretescu I, Stan CD. Valorization of β-Chitin Extraction Byproduct from Cuttlefish Bone and Its Application in Food Wastewater Treatment. Materials. 2022; 15(8):2803. https://doi.org/10.3390/ma15082803
Chicago/Turabian StyleNouj, Nisrine, Naima Hafid, Noureddine El Alem, Ingrid Ioana Buciscanu, Stelian Sergiu Maier, Petrisor Samoila, Gabriela Soreanu, Igor Cretescu, and Catalina Daniela Stan. 2022. "Valorization of β-Chitin Extraction Byproduct from Cuttlefish Bone and Its Application in Food Wastewater Treatment" Materials 15, no. 8: 2803. https://doi.org/10.3390/ma15082803