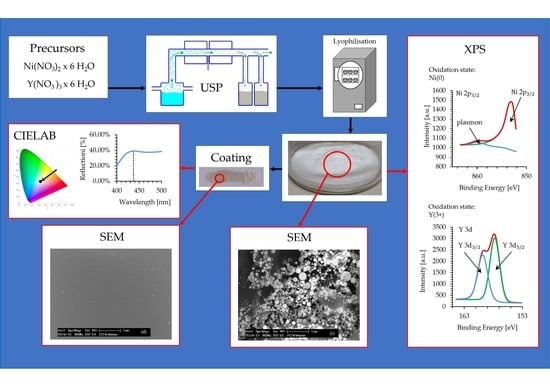
Synthesis of Ni/Y2O3 Nanocomposite through USP and Lyophilisation for Possible Use as Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Process
2.2.1. USP Synthesis
2.2.2. Lyophilisation
2.3. Ink Preparation from the Ni/Y2O3 Nanocomposite Powder
2.4. Characterisation
2.4.1. Thermo Gravimetric Analysis (TGA) and Differential Thermal Analysis (DTA)
2.4.2. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.4.3. Scanning Electron Microscopy with an Energy-Dispersive X-ray Spectroscope (SEM-EDX)
2.4.4. X-ray Photoelectron Spectroscopy (XPS)
2.4.5. Colour Measurement
2.4.6. Statistics
3. Results
3.1. TGA/DTA
3.2. ICP-MS
3.3. SEM-EDX
3.4. XPS Analysis
3.5. Colour of the Ni/Y2O3 Nanocomposite Coating
4. Discussion
5. Conclusions
- -
- An Ni/Y nitrate-based aqueous solution as a precursor allows USP synthesis of Ni/Y2O3 nanocomposite particles, which are collected in an aqueous PVP suspension.
- -
- Lyophilisation proved to be a suitable process for water removal and for obtaining nanocomposite Ni/Y2O3 powder.
- -
- ICP-MS and SEM/EDX analyses of the Ni/Y2O3 nanocomposite powder showed the impact of precursor concentrations on the final particle formation and their composition.
- -
- XPS research confirmed that the Ni/Y2O3 nanocomposite particles are composed of elemental Ni0 and Y2O3.
- -
- The mechanism of Ni/Y2O3 nanocomposite formation was set up with the initial formation of pure Y2O3 and Ni doping on its surface.
- -
- The prepared Ni/Y2O3 nanocomposite ink allowed the preparation of a coating that has a light grey-silver colour.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Veleva, L.; Diaz-Ballote, L.; Wipf, D.O. An In Situ Electrochemical Study of Electrodeposited Nickel and Nickel-Yttrium Oxide Composite Using Scanning Electrochemical Microscopy. J. Electrochem. Soc. 2003, 150, C1. [Google Scholar] [CrossRef]
- Adekoya, J.A.; Ogunniran, K.O.; Siyanbola, T.O.; Dare, E.O.; Revaprasadu, N. Band Structure, Morphology, Functionality, and Size- Dependent Properties of Metal Nanoparticles. In Noble and Precious Metals-Properties, Nanoscale Effects and Applications; Seehra, M.S., Bristow, A.D., Eds.; IntechOpen: London, UK, 2018; pp. 15–42. [Google Scholar] [CrossRef] [Green Version]
- Taherian, Z.; Khataee, A.; Orooji, Y. Facile synthesis of yttria-promoted nickel catalysts supported on MgO-MCM-41 for syngas production from greenhouse gases. Renew. Sustain. Energy Rev. 2020, 134, 110130. [Google Scholar] [CrossRef]
- Abdullah, B.; Abd Ghani, N.A.; Vo, D.V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Park, J.H.; Yeo, S.; Chang, T.S. Effect of supports on the performance of Co-based catalysts in methane dry reforming. J. CO2 Util. 2018, 26, 465–475. [Google Scholar] [CrossRef]
- Sun, G.B.; Hidajat, K.; Wu, X.S.; Kawi, S. A crucial role of surface oxygen mobility on nanocrystalline Y2O3 support for oxidative steam reforming of ethanol to hydrogen over Ni/Y2O3 catalysts. Appl. Catal. B Environ. 2008, 81, 303–312. [Google Scholar] [CrossRef]
- Li, Y.; Men, Y.; Liu, S.; Wang, J.; Wang, K.; Tang, Y.; An, W.; Pan, X.; Li, L. Remarkably efficient and stable Ni/Y2O3 catalysts for CO2 methanation: Effect of citric acid addition. Appl. Catal. B Environ. 2021, 293, 120206. [Google Scholar] [CrossRef]
- Hasan, M.; Asakoshi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. CO2 methanation mechanism over Ni/Y2O3: An in situ diffuse reflectance infrared Fourier transform spectroscopic study. Phys. Chem. Chem. Phys. 2021, 23, 5551–5558. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. Size-Dependent Catalytic Activity of Monodispersed Nickel Nanoparticles for the Hydrolytic Dehydrogenation of Ammonia Borane. ACS Appl. Mater. Interfaces 2018, 10, 517–525. [Google Scholar] [CrossRef]
- Varničić, M.; Pavlović, M.M.; Pantović, S.E.; Mihailović, M.; Pantović Pavlović, M.R.; Stopić, S.; Friedrich, B. Spray-pyrolytic tunable structures of mn oxides-based composites for electrocatalytic activity improvement in oxygen reduction. Metals 2022, 12, 22. [Google Scholar] [CrossRef]
- Stopic, S.; Wenz, F.; Husovic, T.V.; Friedrich, B. Synthesis of silica particles using ultrasonic spray pyrolysis method. Metals 2021, 11, 463. [Google Scholar] [CrossRef]
- Stopić, S.; Ilić, I.; Uskoković, D. Structural and morphological transformations during NiO and Ni particles generation from chloride precursor by ultrasonic spray pyrolysis. Mater. Lett. 1995, 24, 369–376. [Google Scholar] [CrossRef]
- Özcelik, D.Y.; Ebin, B.; Stopic, S.; Gürmen, S.; Friedrich, B. Mixed oxides NiO/ZnO/Al2O3 synthesized in a single step via ultrasonic spray pyrolysis (USP) method. Metals 2022, 12, 73. [Google Scholar] [CrossRef]
- Rahemi Ardekani, S.; Sabour Rouh Aghdam, A.; Nazari, M.; Bayat, A.; Yazdani, E.; Saievar-Iranizad, E. A comprehensive review on ultrasonic spray pyrolysis technique: {Mechanism}, main parameters and applications in condensed matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.-H.; Yan, G.; Li, X.; Guo, H.; Liu, Y.; Zhang, Q.; Guo, Z. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019, 48, 3015–3072. [Google Scholar] [CrossRef]
- Majerič, P.; Jenko, D.; Friedrich, B.; Rudolf, R. Formation of bimetallic Fe/Au submicron particles with ultrasonic spray pyrolysis. Metals 2018, 8, 278. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Beirowski, J.; Inghelbrecht, S.; Arien, A.; Gieseler, H. Freeze-Drying of Nanosuspensions, 1: Freezing Rate versus Formulation Design as Critical Factors to Preserve the Original Particle Size Distribution. J. Pharm. Sci. 2011, 100, 1958–1968. [Google Scholar] [CrossRef]
- Beirowski, J.; Inghelbrecht, S.; Arien, A.; Gieseler, H. Freeze drying of nanosuspensions, 2: The role of the critical formulation temperature on stability of drug nanosuspensions and its practical implication on process design. J. Pharm. Sci. 2011, 100, 4471–4481. [Google Scholar] [CrossRef]
- Beirowski, J.; Inghelbrecht, S.; Arien, A.; Gieseler, H. Freeze-Drying of Nanosuspensions, Part 3: Investigation of Factors Compromising Storage Stability of Highly Concentrated Drug Nanosuspensions. J. Pharm. Sci. 2012, 101, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Bhargava, A.; Gupta, R.; Jain, N.; Panwar, J. Synthesis and Applications of Noble Metal Nanoparticles: A Review. Adv. Sci. Eng. Med. 2017, 9, 527–544. [Google Scholar] [CrossRef]
- Shariq, M.; Majerič, P.; Friedrich, B.; Budic, B.; Jenko, D.; Dixit, A.R.; Rudolf, R. Application of Gold(III) Acetate as a New Precursor for the Synthesis of Gold Nanoparticles in PEG Through Ultrasonic Spray Pyrolysis. J. Clust. Sci. 2017, 28, 1647–1665. [Google Scholar] [CrossRef]
- Jelen, Ž.; Majerič, P.; Zadravec, M.; Anžel, I.; Rakuša, M.; Rudolf, R. Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests. Nanotechnol. Rev. 2021, 10, 1978–1992. [Google Scholar] [CrossRef]
- Amis, T.M.; Renukuntla, J.; Bolla, P.K.; Clark, B.A. Selection of cryoprotectant in lyophilization of progesterone-loaded stearic acid solid lipid nanoparticles. Pharmaceutics 2020, 12, 892. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalt. Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wang, W. Role of Freeze Drying in Nanotechnology. Dry. Technol. 2007, 25, 29–35. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Friess, W. Freeze-drying of nanoparticles: How to overcome colloidal instability by formulation and process optimization. Eur. J. Pharm. Biopharm. 2021, 165, 345–360. [Google Scholar] [CrossRef]
- Ravnik, J.; Golobič, I.; Sitar, A.; Avanzo, M.; Irman, Š.; Kočevar, K.; Cegnar, M.; Zadravec, M.; Ramšak, M.; Hriberšek, M. Lyophilization model of mannitol water solution in a laboratory scale lyophilizer. J. Drug Deliv. Sci. Technol. 2018, 45, 28–38. [Google Scholar] [CrossRef]
- Ravnik, J.; Ramšak, M.; Zadravec, M.; Kamenik, B.; Hriberšek, M. Experimental and stochastic analysis of lyophilisation. Eur. J. Pharm. Biopharm. 2021, 159, 108–122. [Google Scholar] [CrossRef]
- ISO/CIE 11664-4:2019; Colorimetry—Part 4: CIE 1976 L*a*b* Colour Space. International Organization for Standardization: Geneva, Switzerland, 2019; Volume 8.
- ISO 13322-1; Particle Size Analysis—Image Analysis Methods. Part 1: Static Image Analysis Methods. International Organization for Standardization: Geneva, Switzerland, 2014.
- Melnikov, P.; Nascimento, V.A.; Consolo, L.Z.Z.; Silva, A.F. Mechanism of thermal decomposition of yttrium nitrate hexahydrate, Y(NO3)3·6H2O and modeling of intermediate oxynitrates. J. Therm. Anal. Calorim. 2013, 111, 115–119. [Google Scholar] [CrossRef]
- Brockner, W.; Ehrhardt, C.; Gjikaj, M. Thermal decomposition of nickel nitrate hexahydrate, Ni(NO3)2·6H2O, in comparison to Co(NO3)2·6H2O and Ca(NO3)2·4H2O. Thermochim. Acta 2007, 456, 64–68. [Google Scholar] [CrossRef]
- Marzun, G.; Wagener, P.; Barcikowski, S. Colloidal Nanoparticles for Heterogeneous Catalysis. Iraqi J. Appl. Phys. 2015, 11, 20. [Google Scholar] [CrossRef]
- Johnson, B.F.G. Nanoparticles in Catalysis; Springer: Berlin, Germany, 2003; Volume 24, ISBN 9783030566296. [Google Scholar] [CrossRef]
- Astruc, D. (Ed.) Nanoparticles and Catalysis; Wiley-VCH: Weinheim, Germany, 2008; Volume 1, ISBN 978-3-527-31572-7. [Google Scholar]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Electron Spectroscopy; Perkin-Elmer Corp: Waltham, MA, USA, 1979; Volume 192. [Google Scholar]
- Thermo Scientific XPS Nickel, Transition Metal. Available online: https://www.jp.xpssimplified.com/elements/nickel.php (accessed on 17 February 2022).
- Robie, R.A.; Hemingway, B.S. Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 bar 105, 3rd ed.; U.S. Goverment Printing Office: Washington, DC, USA, 1984.
- National Institute of Standards and Technology Ni2O3. Available online: https://webbook.nist.gov/cgi/formula?ID=B1001815&Mask=800 (accessed on 17 February 2022).
- National Institute of Standards and Technology Nickel Monoxide. Available online: https://webbook.nist.gov/cgi/formula?ID=C1313991&Mask=20 (accessed on 17 February 2022).
- National Institute of Standards and Technology Nickel. Available online: https://webbook.nist.gov/cgi/inchi?ID=C7440020&Mask=1 (accessed on 17 February 2022).
- He, Y.; Vardhanabhuti, B. Improved Heat Stability of Whey Protein Isolate by Glycation with Inulin. Dairy 2021, 2, 135–147. [Google Scholar] [CrossRef]














| Concentration [mol/L] | Ni/Y Nitrate | ||
|---|---|---|---|
| Ni(NO3)2 × 6 H2O | Y(NO3)3 × 6 H2O | ||
| Ni/Y2O3-0.100/0.200 | 0.100 | 0.200 | 0.50 |
| Ni/Y2O3-0.050/0.200 | 0.050 | 0.200 | 0.25 |
| Ni/Y2O3-0.025/0.100 | 0.025 | 0.100 | 0.25 |
| Suspension Ni/Y2O3 | Ni [μg/mL] | Y [μg/mL] |
|---|---|---|
| Ni/Y2O3-0.100/0.200 | 87.3 | 263.0 |
| Ni/Y2O3-0.050/0.200 | 100.3 | 568.8 |
| Ni/Y2O3-0.025/0.100 | 29.3 | 185.8 |
| L* | a* | b* | C* | h |
|---|---|---|---|---|
| 77.134 | 1.398 | 1.846 | 2.322 | 52.434 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Švarc, T.; Stopić, S.; Jelen, Ž.; Zadravec, M.; Friedrich, B.; Rudolf, R. Synthesis of Ni/Y2O3 Nanocomposite through USP and Lyophilisation for Possible Use as Coating. Materials 2022, 15, 2856. https://doi.org/10.3390/ma15082856
Švarc T, Stopić S, Jelen Ž, Zadravec M, Friedrich B, Rudolf R. Synthesis of Ni/Y2O3 Nanocomposite through USP and Lyophilisation for Possible Use as Coating. Materials. 2022; 15(8):2856. https://doi.org/10.3390/ma15082856
Chicago/Turabian StyleŠvarc, Tilen, Srećko Stopić, Žiga Jelen, Matej Zadravec, Bernd Friedrich, and Rebeka Rudolf. 2022. "Synthesis of Ni/Y2O3 Nanocomposite through USP and Lyophilisation for Possible Use as Coating" Materials 15, no. 8: 2856. https://doi.org/10.3390/ma15082856
APA StyleŠvarc, T., Stopić, S., Jelen, Ž., Zadravec, M., Friedrich, B., & Rudolf, R. (2022). Synthesis of Ni/Y2O3 Nanocomposite through USP and Lyophilisation for Possible Use as Coating. Materials, 15(8), 2856. https://doi.org/10.3390/ma15082856