Effects of Stepped Heating on the Initial Growth of Oxide Scales on NiCrAlHf Bond Coat Alloy under Air and Water Vapor Atmospheres
Abstract
:1. Introduction
2. Experimental
3. Results
3.1. Microstructures of NiCrAlHf Bond Coat Alloy
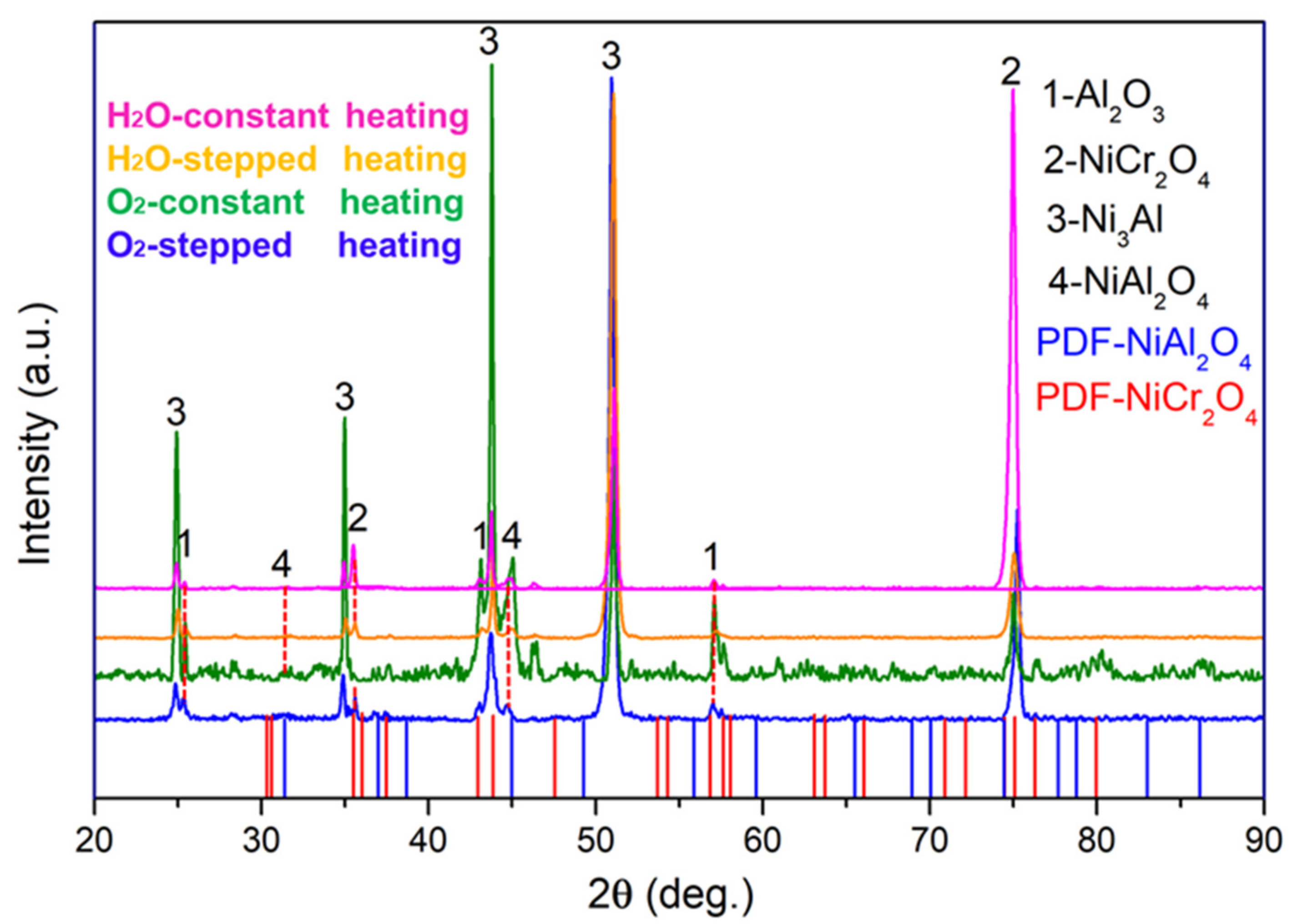
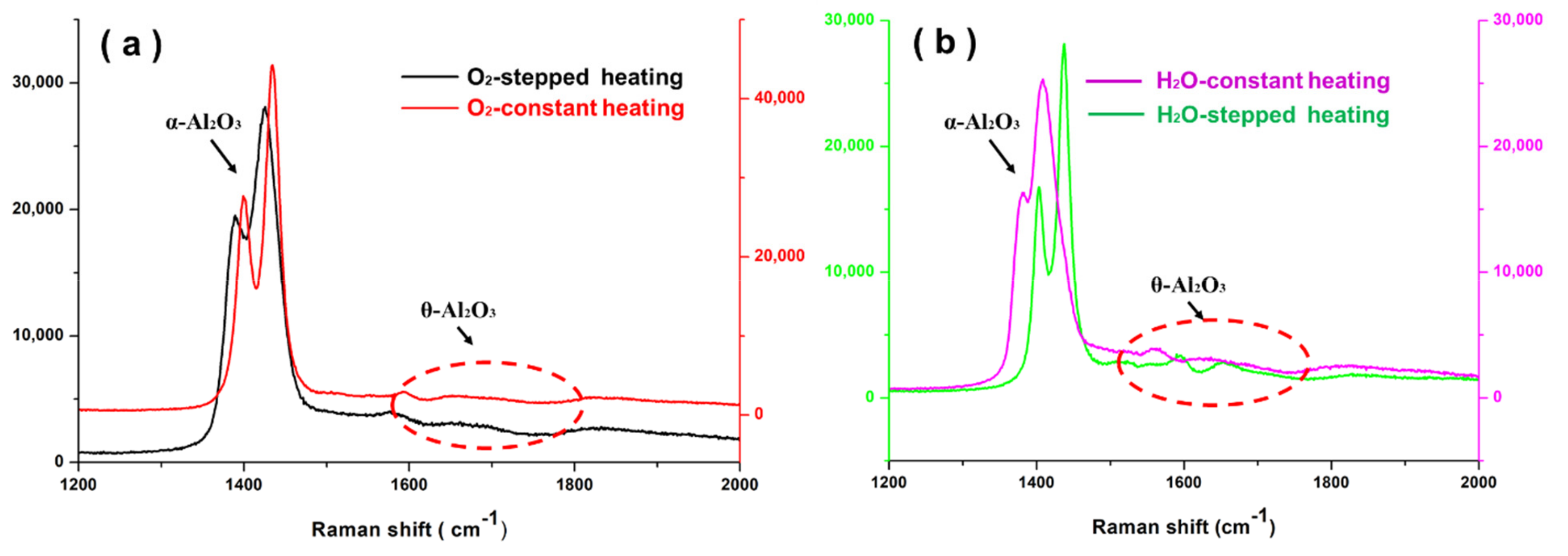
3.2. Phase Composition and Stress Distribution of NiCrAlHf Bond Coat Alloy
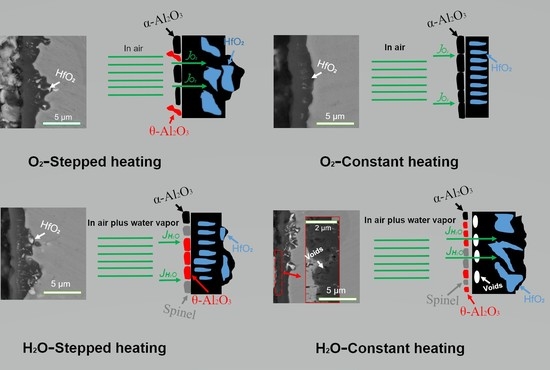
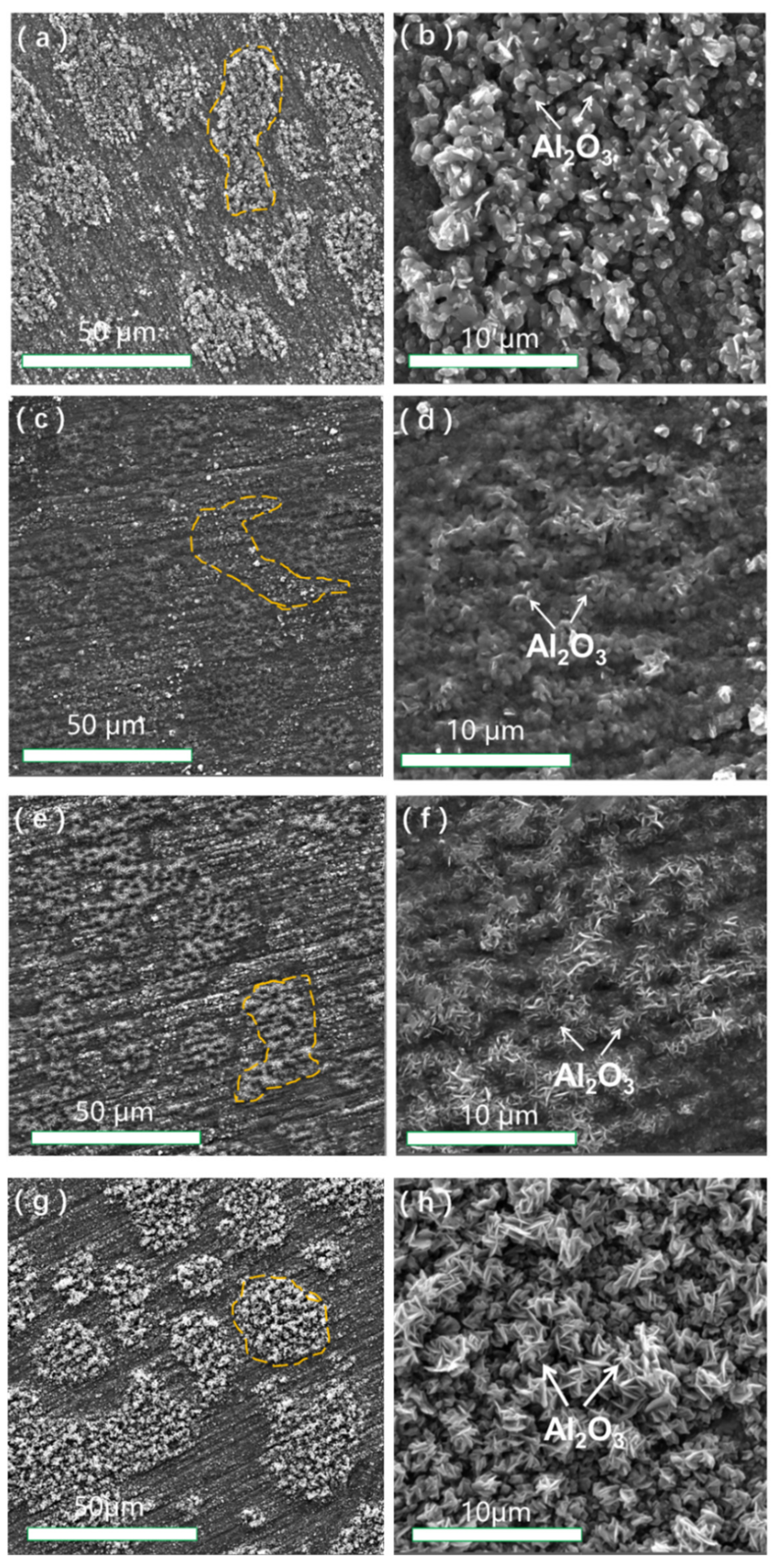
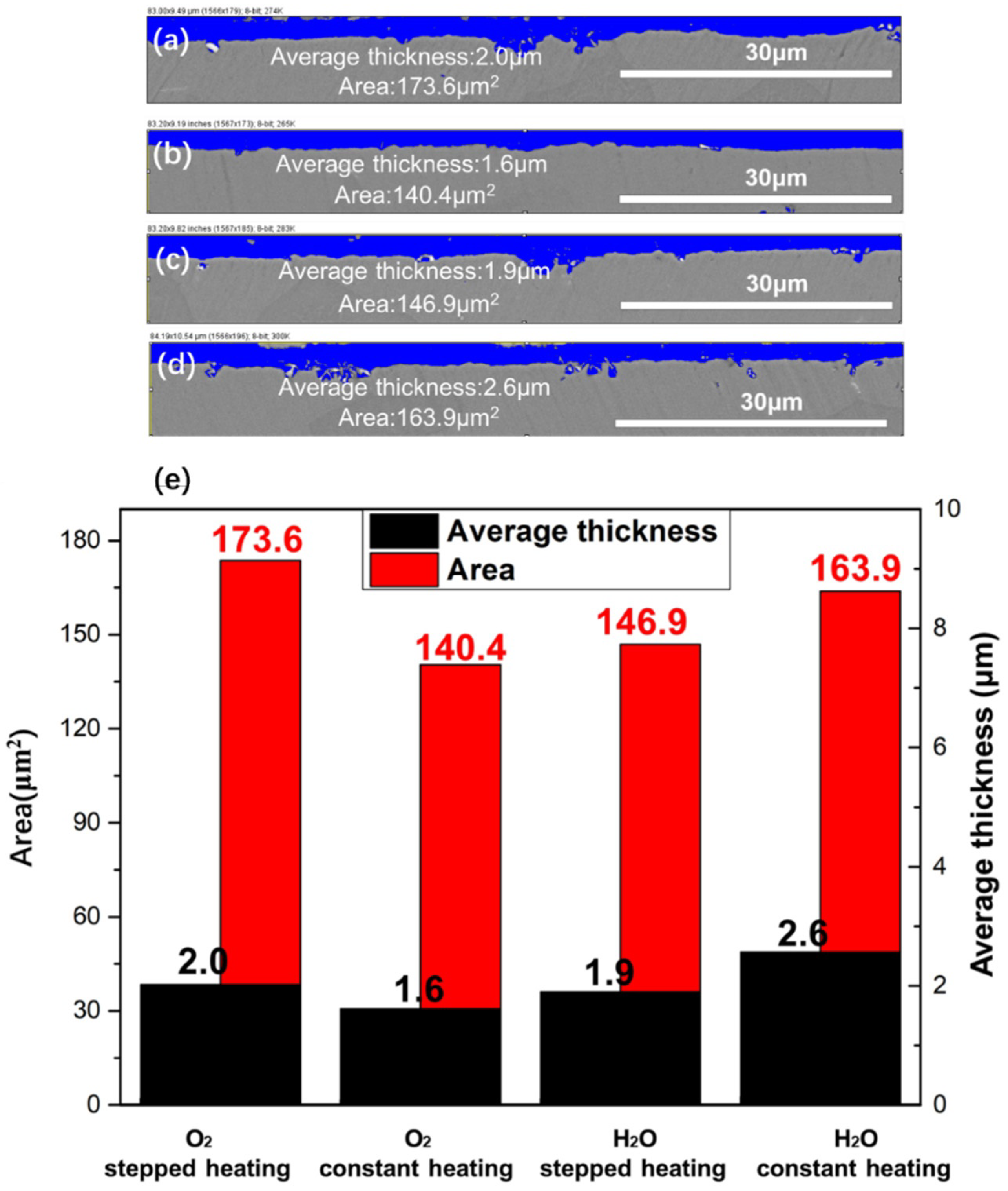
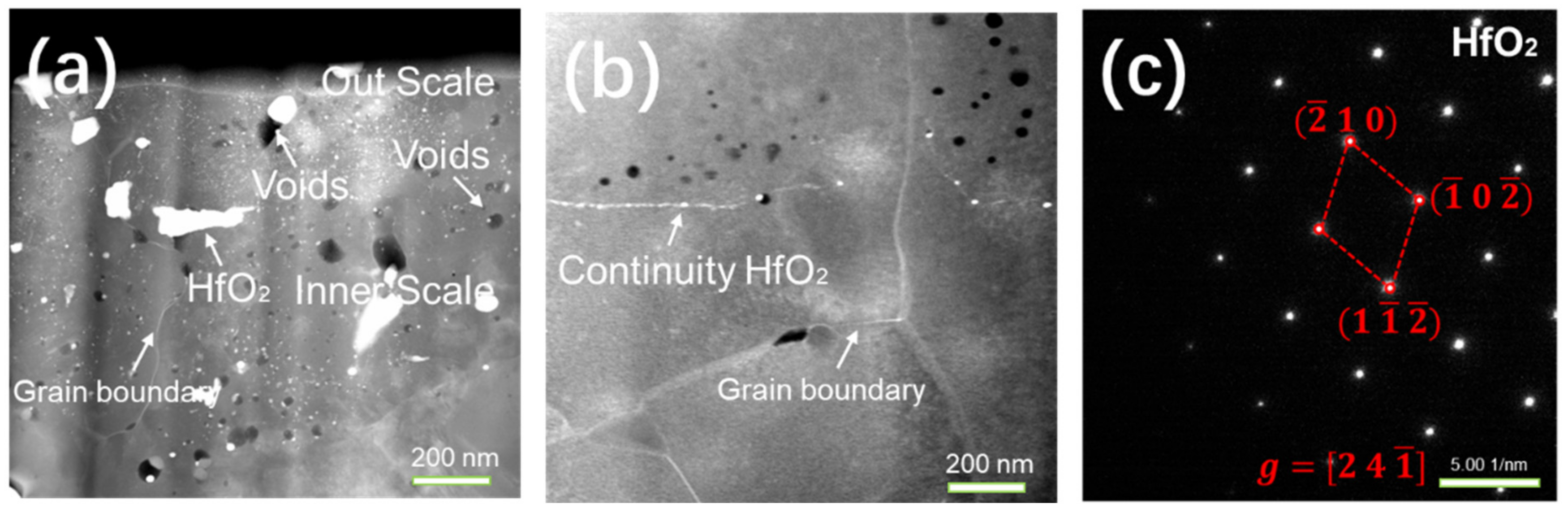
3.3. Cross-Sectional Morphologies of NiCrAlHf Bond Coat Alloy
4. Discussion
4.1. Effects of Temperature and Atmosphere on the Transformation of Al2O3
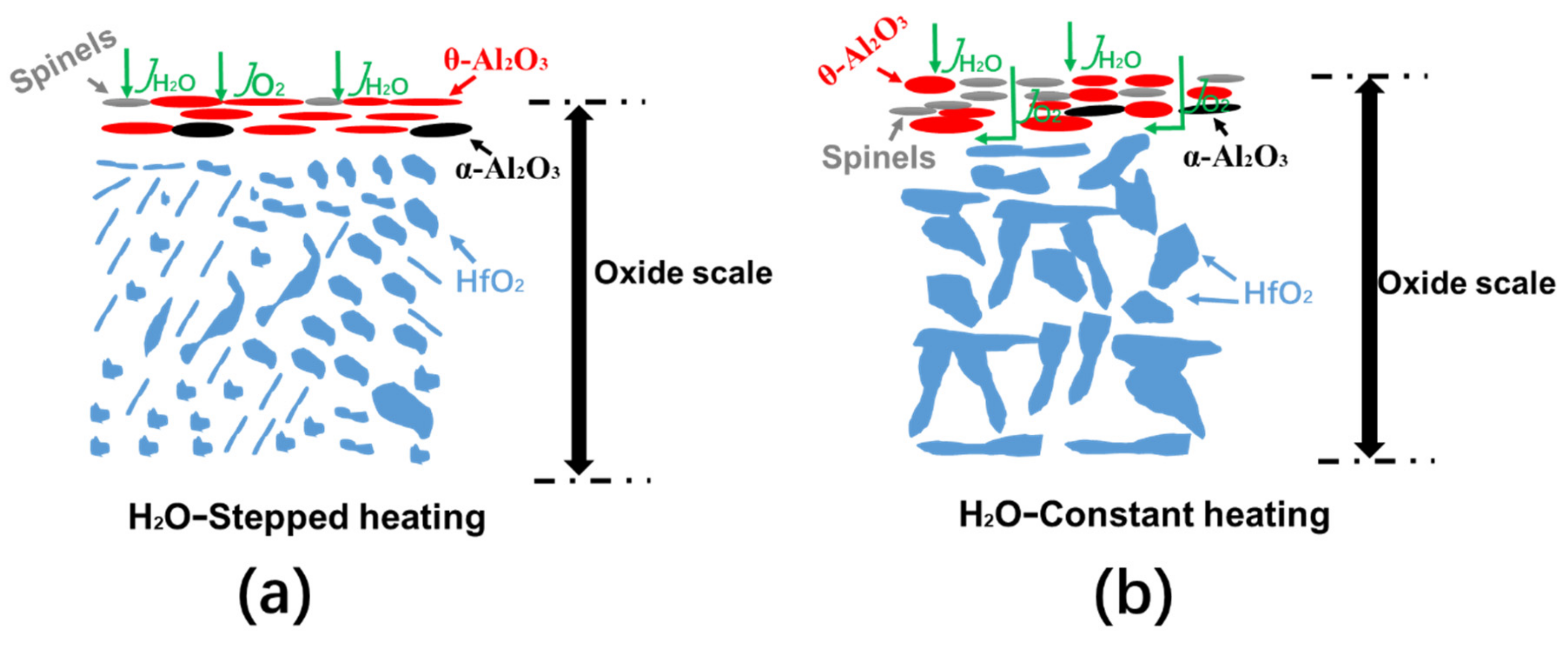
4.2. Effects of Heating Method and Atmosphere on the Morphologies of NiCrAlHf Bond Coat Alloy Oxide Scale
5. Conclusions
- (1)
- In an air atmosphere, stepped heating slows down the transformation of alumina, reduces the oxygen resistance ability of the alloy surface layer, and accelerates the thickening of the alloy oxide layer. In addition, Hf is rapidly oxidized and large HfO2 particles are formed in the oxide scale;
- (2)
- In a water vapor atmosphere, constant heating is favorable for the formation of spinel over stepped heating and the formation of spinel can destabilize the alloy and drive oxygen into the alloy, thereby accelerating the thickening of the alloy oxide scale;
- (3)
- In a water vapor atmosphere during constant heating, large particles of HfO2 can be observed in the oxide scale, which further explains the poor oxygen resistance property produced by constant heating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saboohi, Z.; Ommi, F.; Fakhrtabatabaei, A. Development of an Augmented Conceptual Design Tool for Aircraft Gas Turbine Combustors. Int. J. Multiphys. 2016, 10, 53–74. [Google Scholar] [CrossRef] [Green Version]
- Bester, N.; Yates, A. Assessment of the Operational Performance of Fischer-Tropsch Synthetic-Paraffinic Kerosene in a T63 Gas Turbine Compared to Conventional Jet A-1 Fuel. In Proceedings of the ASME Turbo Expo: Power for Land, Sea, Air, Orlando, FL, USA, 8–12 June 2009; pp. 1063–1077. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, L.; Jodoin, B. Cold spray for production of in-situ monocrystalline MCrAlY coatings—Part II: Isothermal oxidation performance. Surf. Coat. Technol. 2021, 409, 126828. [Google Scholar]
- Quadakkers, W.J.; Shemet, V.; Sebold, D.; Anton, R.; Wessel, E.; Singheiser, L. Oxidation characteristics of a platinized MCrAlY bond coat for TBC systems during cyclic oxidation at 1000 °C. Surf. Coat. Technol. 2005, 199, 77–82. [Google Scholar] [CrossRef]
- Han, D.; Liu, D.; Niu, Y.; Qi, Z.; Pan, Y.; Xu, H.; Zheng, X.; Chen, G. Interface stability of NiCrAlY coating without and with a Cr or Mo diffusion barrier on Ti-42Al-5Mn alloy. Corros. Sci. 2021, 188, 109538. [Google Scholar] [CrossRef]
- Zschau, H.E.; Neve, S.; Masset, P.J.; Schuetze, M.; Baumann, H.; Bethge, K. Characterization of the long time oxidation protection of fluorine implanted technical TiAl-alloys using ion beam methods. Nucl. Instrum. Methods Phys. Res. B 2008, 266, 2441–2445. [Google Scholar] [CrossRef]
- Guo, X.L.; Liu, Z.; Li, L.; Cheng, J.C.; Su, H.Z.; Zhang, L.F. Revealing the long-term oxidation and carburization mechanism of 310S SS and Alloy 800H exposed to supercritical carbon dioxide. Mater. Charact. 2022, 183, 111603. [Google Scholar] [CrossRef]
- Swadzba, L.; Moskal, G.; Hetmanczyk, M.; Mendala, B.; Jarczyk, G. Long-term cyclic oxidation of Al–Si diffusion coatings deposited by Arc-PVD on TiAlCrNb alloy. Surf. Coat. Technol. 2004, 184, 93–101. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, Z.; Chen, Y.Z.; Shi, J.C.; Wang, Z.Y.; Wang, S.; Liu, F. Effect of Al content on high temperature oxidation resistance of AlxCoCrCuFeNi high entropy alloys (x = 0, 0.5, 1, 1.5, 2). Vacuum 2019, 169, 108837. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Kwakernaak, C.; Sloof, W.G. The effects of alloy microstructure refinement on the short-term thermal oxidation of NiCoCrAlY alloys. Metall. Mater. Trans. A 2006, 37, 683–693. [Google Scholar] [CrossRef]
- Chen, L.; Luo, H.; Li, Z.; Sha, A. Effect of Al doping on the early-stage oxidation of Ni-Al alloys: A ReaxFF molecular dynamics study. Appl. Surf. Sci. 2021, 563, 150097. [Google Scholar] [CrossRef]
- Lance, M.J.; Unocic, K.A.; Haynes, J.A.; Pint, B.A. Effect of water vapor on thermally-grown alumina scales on Pt-modified and simple aluminide bond coatings. Surf. Coat. Technol. 2013, 237, 2–7. [Google Scholar] [CrossRef]
- Zhu, D.D.; Wang, X.L.; Zhao, J.; Lu, J.; Zhou, Y.C.; Cai, C.Y.; Huang, J.Y.; Zhou, G.W. Effect of water vapor on high-temperature oxidation of NiAl alloy. Corros. Sci. 2020, 177, 108963. [Google Scholar] [CrossRef]
- Wollgarten, K.; Galiullin, T.; Nowak, W.; Quadakkers, W.; Naumenko, D. Effect of alloying additions and presence of water vapour on short-term air oxidation behaviour of cast Ni-base superalloys. Corros. Sci. 2020, 173, 108774. [Google Scholar] [CrossRef]
- Duan, W.; Song, P.; Li, C.; Huang, T.; Ge, Z.; Feng, J.; Lu, J. Effect of water vapor on the failure behavior of thermal barrier coating with Hf-doped NiCoCrAlY bond coating. J. Mater. Res. 2019, 34, 2653–2663. [Google Scholar] [CrossRef]
- Unocic, K.A.; Leonard, D.N.; Pint, B.A. Effect of boron on the oxidation behavior of NiCrAlYHfTi in H2O and CO2 environments. Surf. Coat. Technol. 2014, 260, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Pettit, F.S.; Randklev, E.H.; Felten, E.J. Formation of NiAl2O4 by solid state reaction. J. Am. Ceram. Soc. 2010, 49, 199–203. [Google Scholar] [CrossRef]
- Pint, B.A.; Treska, M.; Hobbs, L.H. The effect of various oxide dispersions on the phase composition and morphology of A12O3 scales grown on β-NiA1. Oxid. Met. 1997, 47, 1–20. [Google Scholar] [CrossRef]
- Yang, J.C.; Schumann, E.; Levin, I.; Rühle, M. Transient oxidation of NiAl. Acta Meter. 1998, 46, 2195–2201. [Google Scholar] [CrossRef]
- Tolpygo, V.K.; Clarke, D.R. Microstructural study of the theta-alpha transformation in alumina scales formed on Nickel-aluminides. High Temp. Technol. 2000, 17, 59–70. [Google Scholar] [CrossRef]
- Takayuki, T.; Hideo, S.; Atsuo, Y.; Okada, K. Crystallinity of boehmite and its effect on the phase transition temperature of alumina. J. Mater. Chem. 1999, 9, 549–553. [Google Scholar]
- Yen, F.S.; Wang, M.Y.; Chang, J.L. Temperature reduction of θ-to α-phase transformation induced by high-pressure pretreatments of nano-sized alumina powders derived from boehmite. J. Cryst. Growth 2002, 236, 197–209. [Google Scholar] [CrossRef]
- Deneux, V.; Cadoret, Y.; Hervier, S.; Monceau, D. Effect of water vapor on the spallation of thermal barrier coating systems during laboratory cyclic oxidation testing. Oxid. Met. 2010, 73, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Guo, H.B.; Peng, H.; Gong, S.K. Oxidation behaviour of electron beam physical vapour deposition β-NiAlHf coatings at 1100 °C in dry and humid atmospheres. Rare Met. 2015, 35, 513–519. [Google Scholar] [CrossRef]
- Loehman, R.E.; Hosking, F.M.; Gauntt, B.; Kotula, P.G.; Lu, P. Reactions of Hf-Ag and Zr-Ag alloys with Al2O3 at elevated temperatures. J. Mater. Sci. 2005, 40, 2319–2324. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, W.; Ma, Z.; Liu, Y. Study on water corrosion behavior of ZrSiO4 materials. J. Adv. Ceram. 2018, 7, 336–342. [Google Scholar] [CrossRef]
- Yan, K.; He, J.; Guo, H.B. High-temperature oxidation behaviour of minor Hf-doped β-NiAl single crystals in dry and humid atmospheres. Rare Met. 2018, 1–7. [Google Scholar] [CrossRef]
- Appel, O.; Cohen, S.; Beeri, O.; Gelbstein, Y.; Zalkind, S. The initial oxidation of HfNiSn Half-Heusler alloy by oxygen and water vapor. Materials 2021, 14, 3942. [Google Scholar] [CrossRef]
- Huang, W.; Huang, T.; Song, P.; Chen, R.; Lu, J. CrO2(OH)2 volatilization rate and oxidation behaviour prediction of the NiCr coating in Air-H2O environment at 650 °C. Corros. Sci. 2021, 182, 109303. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, B.; Du, C.; Gao, W.; Cao, G. High-temperature oxidation behavior of Fe-10Cr steel under different atmospheres. Materials 2021, 14, 3453. [Google Scholar] [CrossRef]
- Wu, Y.; Narita, T. Oxidation behavior of the single crystal Ni-based superalloy at 900 °C in air and water vapor. Surf. Coat. Technol. 2007, 202, 140–145. [Google Scholar] [CrossRef]
- Appel, O.; Cohen, S.; Beeri, O.; Shamir, N.; Gelbstein, Y.; Zalkind, S. Surface oxidation of TiNiSn (Half-Heusler) alloy by oxygen and water vapor. Materials 2018, 11, 2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Bond Coat Alloys | Experimental Environment | Experimental Conditions | Holding Time |
|---|---|---|---|
| NiCrAlHf bond coat alloys | Air | Stepped heating/Thermostatic oxidation | 24 h |
| NiCrAlHf bond coat alloys | Air | Thermostatic oxidation | 24 h |
| NiCrAlHf bond coat alloys | Water vapor | Stepped heating/Thermostatic oxidation | 24 h |
| NiCrAlHf bond coat alloys | Water vapor | Thermostatic oxidation | 24 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zheng, B.; Song, P.; Huang, T.; Pei, H.; Yang, B.; Shakeel, S. Effects of Stepped Heating on the Initial Growth of Oxide Scales on NiCrAlHf Bond Coat Alloy under Air and Water Vapor Atmospheres. Materials 2022, 15, 2914. https://doi.org/10.3390/ma15082914
He Y, Zheng B, Song P, Huang T, Pei H, Yang B, Shakeel S. Effects of Stepped Heating on the Initial Growth of Oxide Scales on NiCrAlHf Bond Coat Alloy under Air and Water Vapor Atmospheres. Materials. 2022; 15(8):2914. https://doi.org/10.3390/ma15082914
Chicago/Turabian StyleHe, Yang, Biju Zheng, Peng Song, Taihong Huang, Hezhong Pei, Bixiao Yang, and Shakeel Shakeel. 2022. "Effects of Stepped Heating on the Initial Growth of Oxide Scales on NiCrAlHf Bond Coat Alloy under Air and Water Vapor Atmospheres" Materials 15, no. 8: 2914. https://doi.org/10.3390/ma15082914