High Humidity Response of Sol–Gel-Synthesized BiFeO3 Ferroelectric Film
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structure and Morphology of Material
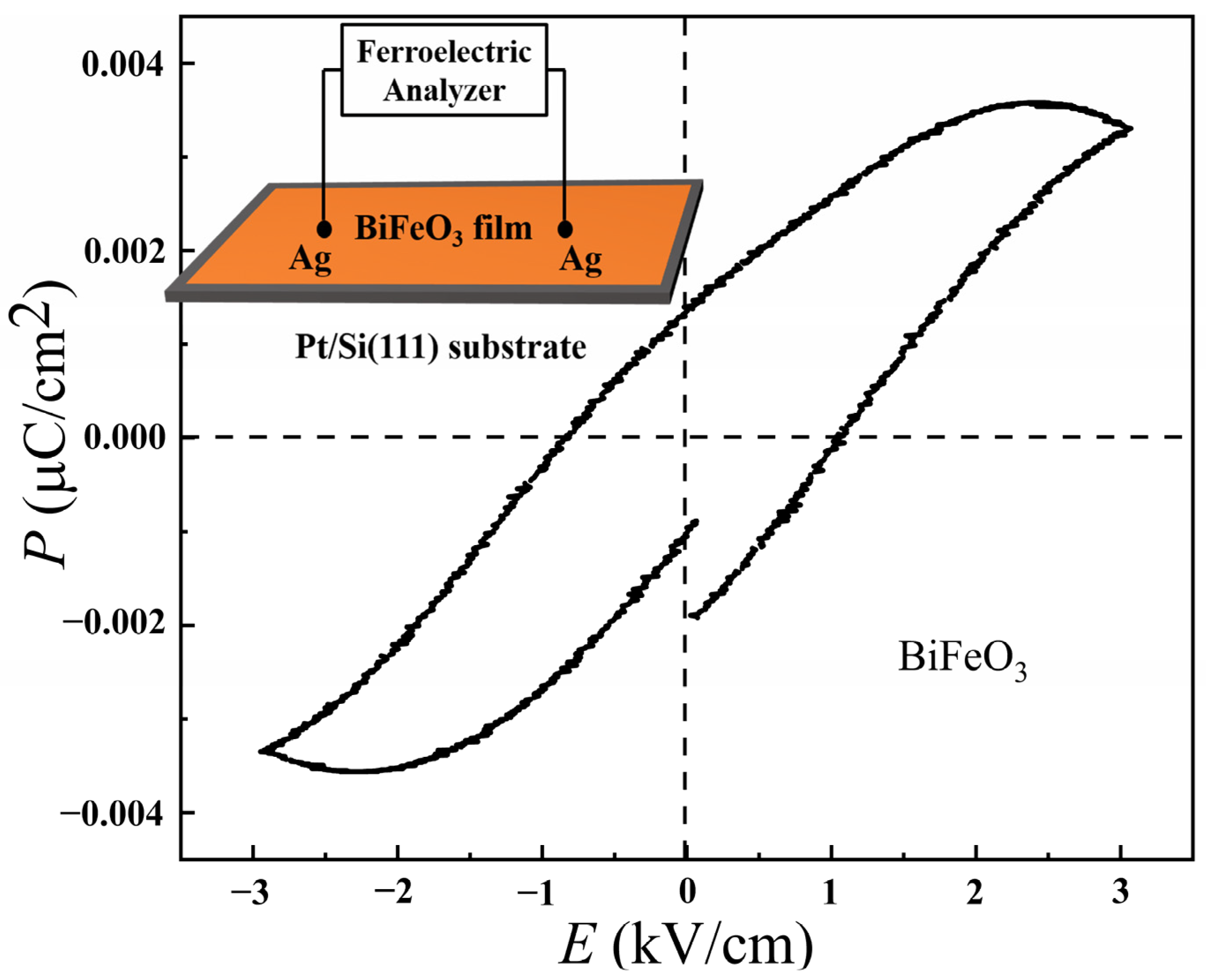
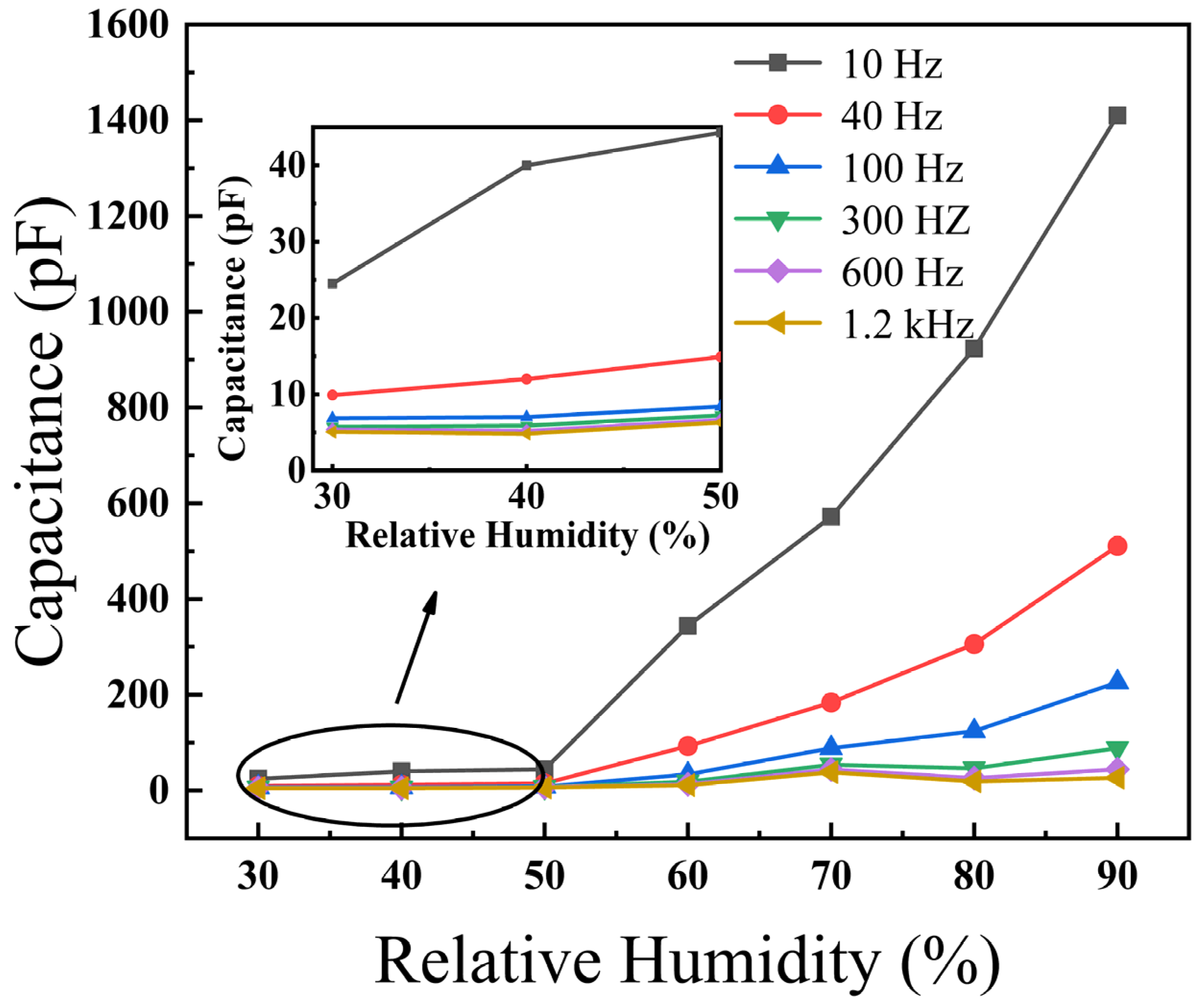
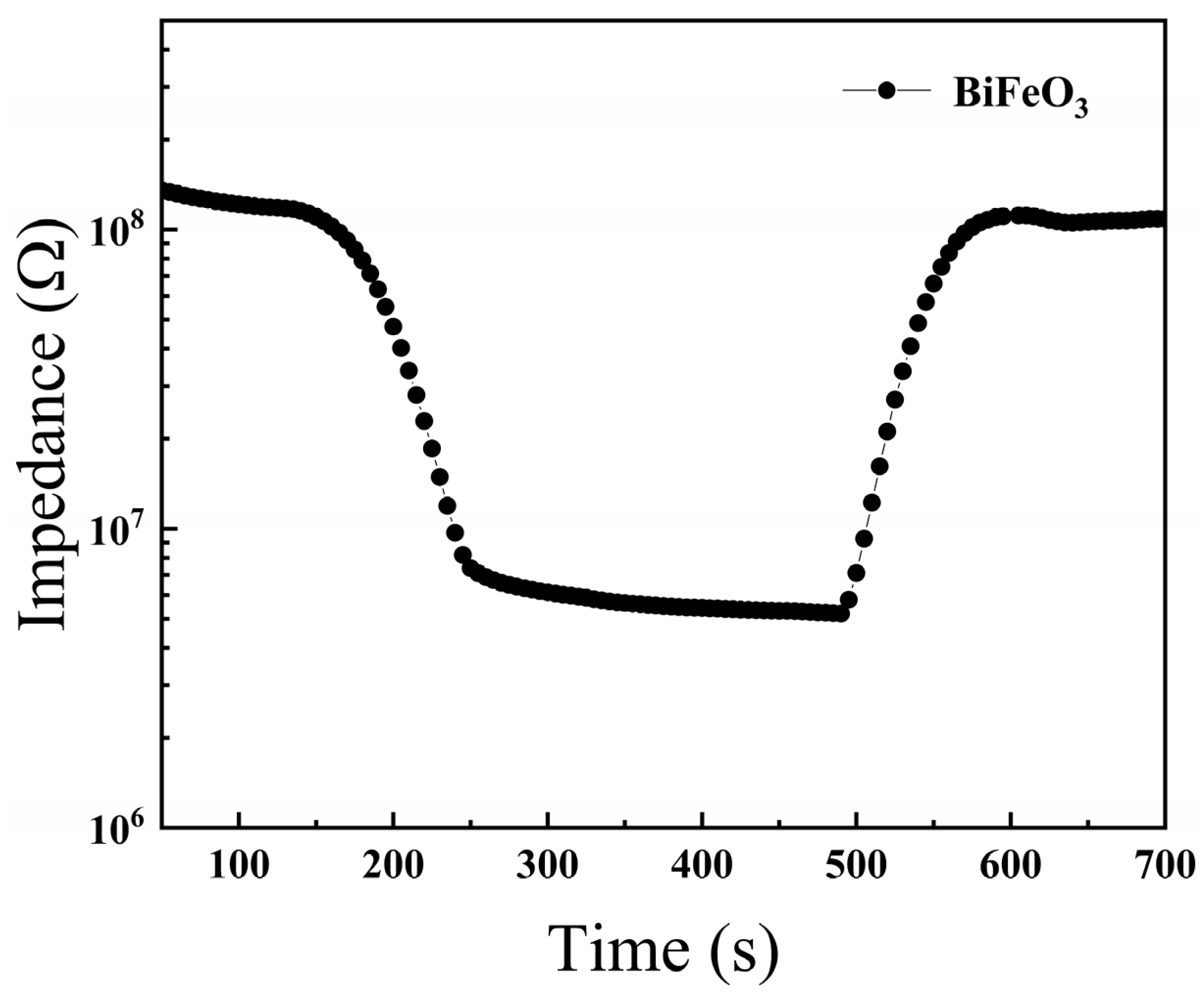
3.2. Humidity-Sensing Properties
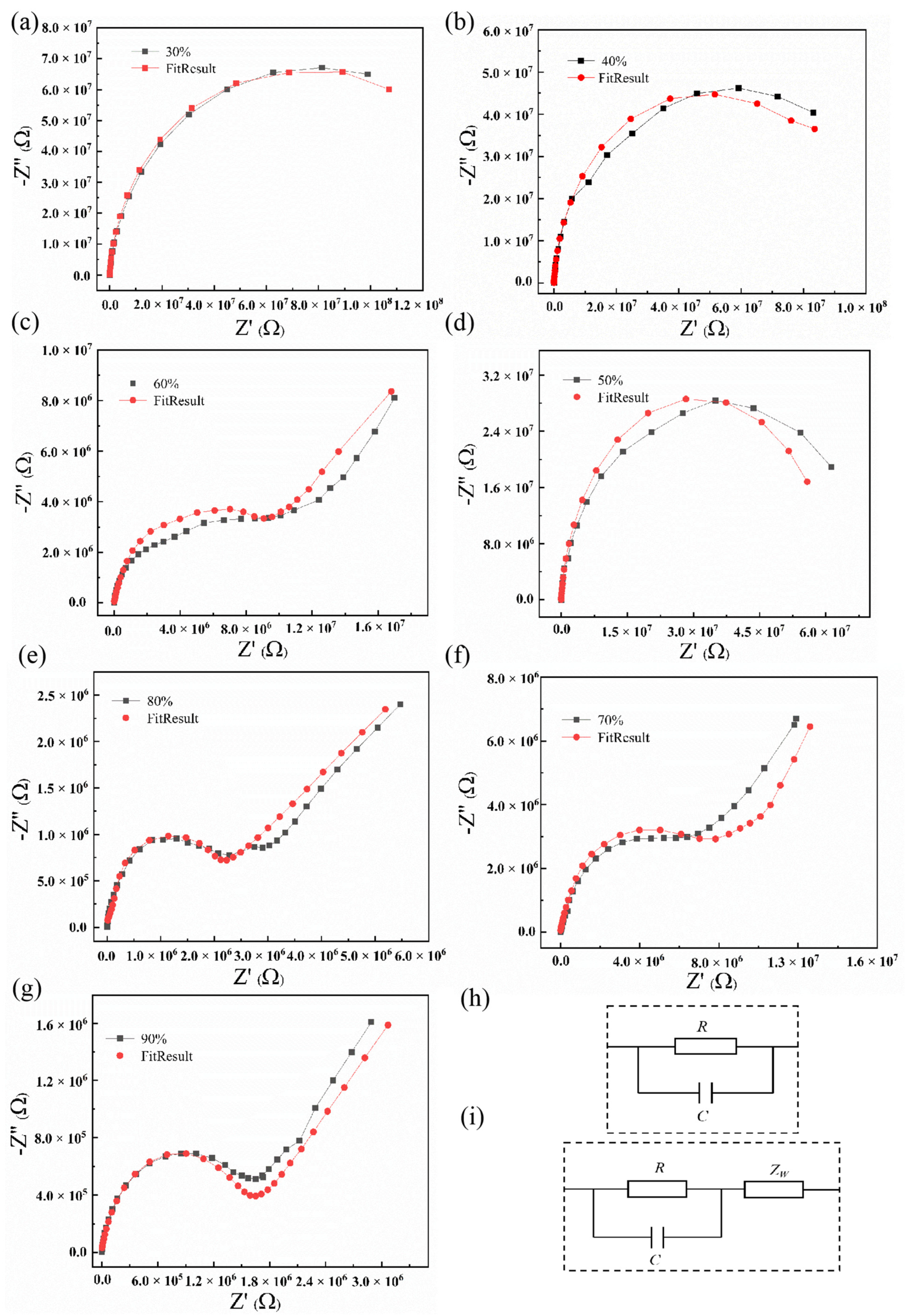
3.3. Humidity-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, R.; Lin, X.; Dai, J.; Zhao, H.; Liu, S.; Fei, T.; Zhang, T. Humidity sensors based on MCM-41/polypyrrole hybrid film via in-situ polymerization. Sens. Actuators B Chem. 2018, 277, 584–590. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, Z.; Zou, H.; Ma, M. Drawn a facile sensor: A fast response humidity sensor based on pencil-trace. Sens. Actuators B Chem. 2018, 261, 345–353. [Google Scholar] [CrossRef]
- Si, R.J.; Li, T.Y.; Sun, J.; Wang, J.; Wang, S.T.; Zhu, G.B.; Wang, C.C. Humidity sensing behavior and its influence on the dielectric properties of (In + Nb) co-doped TiO2 ceramics. J. Mater. Sci. 2019, 54, 14645–14653. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.M.; Wang, S.T.; Tong, L.; Sun, J.; Zhu, G.B.; Wang, C.C. The effect of humidity on the dielectric properties of (In+Nb) co-doped SnO2 ceramics. J. Eur. Ceram. Soc. 2019, 39, 323–329. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, Q.; Wang, S.; Yuan, Z.; Zhang, Y.; Li, X.; Wu, Y.; Jiang, Y.; Tai, H. Novel application of attapulgite on high performance and low-cost humidity sensors. Sens. Actuators B Chem. 2020, 305, 127534. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, J.; Sun, X.; Wu, Y.; Wang, L.; Meng, G.; Kuang, D.; Guo, X.Z.; Qu, W.; Du, B.; et al. An excellent impedance-type humidity sensor based on halide perovskite CsPbBr3 nanoparticles for human respiration monitoring. Sens. Actuators B Chem. 2021, 337, 129772. [Google Scholar] [CrossRef]
- Weng, Z.; Qin, J.; Umar, A.A.; Wang, J.; Zhang, X.; Wang, H.; Cui, X.; Li, X.; Zheng, L.; Zhan, Y. Lead-free Cs2BiAgBr6 double perovskite-based humidity sensor with superfast recovery time. Adv. Funct. Mater. 2019, 29, 1902234. [Google Scholar] [CrossRef]
- Cho, M.-Y.; Kim, S.; Kim, I.-S.; Kim, E.-S.; Wang, Z.-J.; Kim, N.-Y.; Kim, S.-W.; Oh, J.-M. Perovskite-induced ultrasensitive and highly stable humidity sensor systems prepared by aerosol deposition at room temperature. Adv. Funct. Mater. 2020, 30, 1907449. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, T.; Zhao, H.; Fei, T. Preparation of organic-inorganic hybrid polymers and their humidity sensing properties. Sens. Actuators B Chem. 2017, 242, 1108–1114. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Urban, G.A. Investigation of room temperature protonic conduction of perovskite humidity sensors. IEEE Sens. J. 2020, 21, 9657–9666. [Google Scholar] [CrossRef]
- Kumar, A.; Wang, C.; Meng, F.-Y.; Liang, J.-G.; Xie, B.-F.; Zhou, Z.-L.; Zhao, Z.; Kim, N.-Y. Aerosol deposited BaTiO3 film based interdigital capacitor and squared spiral capacitor for humidity sensing application. Ceram. Int. 2021, 47, 510–520. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Li, X.; Lu, G.; You, L.; Liang, X.; Liu, F.; Zhang, T.; Du, Y. Highly sensitive humidity sensor based on high surface area mesoporous LaFeO3 prepared by a nanocasting route. Sens. Actuators B Chem. 2013, 181, 802–809. [Google Scholar] [CrossRef]
- Wang, N.; Luo, X.; Han, L.; Zhang, Z.; Zhang, R.; Olin, H.; Yang, Y. Structure, performance, and application of BiFeO3 nanomaterials. Nano-Micro Lett. 2020, 12, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Y.; Zhang, F.; Xie, X.; Sun, H.; Zhang, L.; Fan, S. Enhanced leakage and ferroelectric properties of Zn-doped BiFeO3 thin films grown by sol-gel method. J. Alloy Compd. 2018, 734, 243–249. [Google Scholar] [CrossRef]
- Chen, M.; Jia, Y.; Li, H.; Wu, Z.; Huang, T.; Zhang, H. Enhanced pyrocatalysis of the pyroelectric BiFeO3/g-C3N4 heterostructure for dye decomposition driven by cold-hot temperature alternation. J. Adv. Ceram 2021, 10, 338–346. [Google Scholar] [CrossRef]
- Wu, J.; Mao, W.; Wu, Z.; Xu, X.; You, H.; Xue, A.; Jia, Y.A.X. Strong pyro-catalysis of pyroelectric BiFeO3 nanoparticles under a room-temperature cold-hot alternation. Nanoscale 2016, 8, 7343–7350. [Google Scholar] [CrossRef]
- You, H.; Jia, Y.; Wu, Z.; Xu, X.; Qian, W.; Xia, Y.; Ismail, M. Strong piezo-electrochemical effect of multiferroic BiFeO3 square micro-sheets for mechanocatalysis. Electrochem. Commun. 2017, 79, 55–58. [Google Scholar] [CrossRef]
- Preethi, A.J.; Ragam, M. Effect of doping in multiferroic BFO: A review. J. Adv. Dielect. 2021, 11, 2130001. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, L.; Haugen, N.O.; Wu, Z.; Jia, Y.; Zhong, W.; Zou, J. High humidity response property of sol-gel synthesized ZnFe2O4 films. Mater. Lett. 2018, 213, 266–268. [Google Scholar] [CrossRef]
- Liang, J.-G.; Kim, E.-S.; Wang, C.; Cho, M.-Y.; Oh, J.-M.; Kim, N.-Y. Thickness effects of aerosol deposited hygroscopic films on ultra-sensitive humidity sensors. Sens. Actuators B Chem. 2018, 265, 632–643. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Guo, Y.; Lv, X.; Dou, X. 2D TiO2 nanosheets for ultrasensitive humidity sensing application benefited by abundant surface oxygen vacancy defects. Sens. Actuators B Chem. 2018, 262, 350–358. [Google Scholar] [CrossRef]
- Rachida, D.; Nouara, L.; M’hand, O.; Malika, S.; Yannick, G.; Ahcène, C.; Bertrand, B. Improvement of humidity sensing performance of BiFeO3 nanoparticles-based sensor by the addition of carbon fibers. Sens. Actuator A Phys. 2020, 307, 111981. [Google Scholar]
- Mahapatra, P.L.; Das, S.; Mondal, P.P.; Das, T.; Saha, D.; Pal, M. Microporous copper chromite thick film based novel and ultrasensitive capacitive humidity sensor. J. Alloy Compd. 2021, 859, 157778. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, Q.; Wang, S.; Huang, Q.; Yuan, Z.; Zhang, Y.; Jiang, Y.; Tai, H. Halloysite nanotubes: Natural, environmental-friendly and low-cost nanomaterials for high-performance humidity sensor. Sens. Actuators B Chem. 2020, 317, 128204. [Google Scholar] [CrossRef]
- Liang, J.-G.; Wang, C.; Yao, Z.; Liu, M.-Q.; Kim, H.-K.; Oh, J.-M.; Kim, N.-Y. Preparation of ultrasensitive humidity-sensing films by aerosol deposition. ACS Appl. Mater. Interfaces 2018, 10, 851–863. [Google Scholar] [CrossRef]
- Zia, T.H.; Ali Ahsh, A.H. Understanding the adsorption of 1 NLB antibody on polyaniline nanotubes as a function of zeta potential and surface charge density for detection of hepatitis C core antigen: A label-free impedimetric immunosensor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127076. [Google Scholar] [CrossRef]
- Li, T.Y.; Si, R.J.; Sun, J.; Wang, S.T.; Wang, J.; Ahmed, R.; Zhu, G.B.; Wang, C.C. Giant and controllable humidity sensitivity achieved in (Na+ Nb) co-doped rutile TiO2. Sens. Actuators B Chem. 2019, 293, 151–158. [Google Scholar] [CrossRef]
- Si, R.; Xie, X.; Li, T.; Zheng, J.; Cheng, C.; Huang, S.; Wang, C. TiO2/(K, Na)NbO3 nanocomposite for boosting humidity-sensing performances. ACS Sens. 2020, 5, 1345–1353. [Google Scholar] [CrossRef]
- Mallick, S.; Ahmad, Z.; Qadir, K.W.; Rehman, A.; Shakoor, R.A.; Touati, F.; Al-Muhtaseb, S.A. Effect of BaTiO3 on the sensing properties of PVDF composite-based capacitive humidity sensors. Ceram. Int. 2020, 46, 2949–2953. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Krstic, J.B.; Labus, N.J.; Lukovic, M.D.; Dojcinovic, M.P.; Radovanovic, M.; Tadic, N.B. Structural, morphological and textural properties of iron manganite (FeMnO3) thick films applied for humidity sensing. Mater. Sci. Eng. B 2020, 257, 114547. [Google Scholar] [CrossRef]
- Ji, G.-J.; Zhang, L.-X.; Zhu, M.-Y.; Li, S.-M.; Yin, J.; Zhao, L.-X.; Fahlman, B.D.; Bie, L.-J. Molten-salt synthesis of Ba5−xSrxNb4O15 solid solutions and their enhanced humidity sensing properties. Ceram. Int. 2018, 44, 477–483. [Google Scholar] [CrossRef]
- Ma, H.; Fang, H.; Wu, W.; Zheng, C.; Wu, L.; Wang, H. A highly transparent humidity sensor with fast response speed based on α-MoO3 thin films. RSC Adv. 2020, 10, 25467–25474. [Google Scholar] [CrossRef]
- Han, J.-W.; Kim, B.; Li, J.; Meyyappan, M. Carbon nanotube based humidity sensor on cellulose paper. J. Phys. Chem. C. 2012, 116, 22094–22097. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, B.; Jia, Y. High Humidity Response of Sol–Gel-Synthesized BiFeO3 Ferroelectric Film. Materials 2022, 15, 2932. https://doi.org/10.3390/ma15082932
Zhang Y, Li B, Jia Y. High Humidity Response of Sol–Gel-Synthesized BiFeO3 Ferroelectric Film. Materials. 2022; 15(8):2932. https://doi.org/10.3390/ma15082932
Chicago/Turabian StyleZhang, Yaming, Bingbing Li, and Yanmin Jia. 2022. "High Humidity Response of Sol–Gel-Synthesized BiFeO3 Ferroelectric Film" Materials 15, no. 8: 2932. https://doi.org/10.3390/ma15082932
APA StyleZhang, Y., Li, B., & Jia, Y. (2022). High Humidity Response of Sol–Gel-Synthesized BiFeO3 Ferroelectric Film. Materials, 15(8), 2932. https://doi.org/10.3390/ma15082932






