Charge Accumulation in the Homo-Crosslinked-Polyethylene Bilayer
Abstract
:1. Introduction
2. Materials and Methods
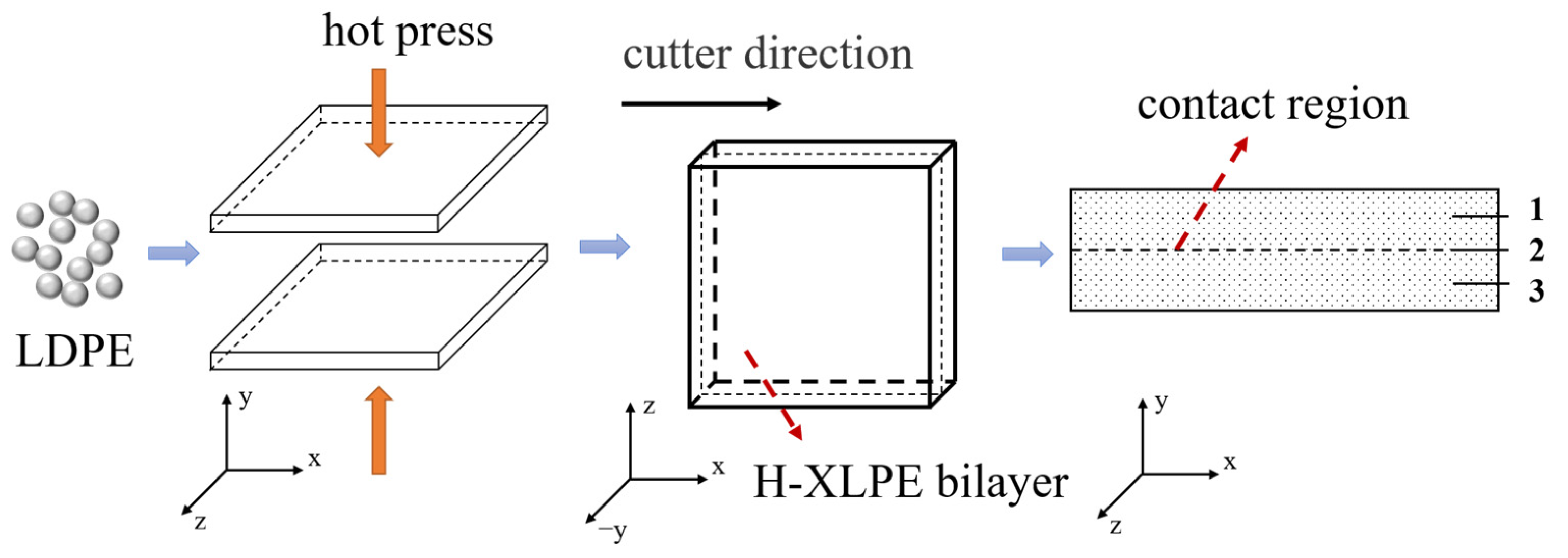
2.1. Material Preparation
2.2. Charge and DC Breakdown Measurement
2.3. Crystal Morphology Characterization
3. Results
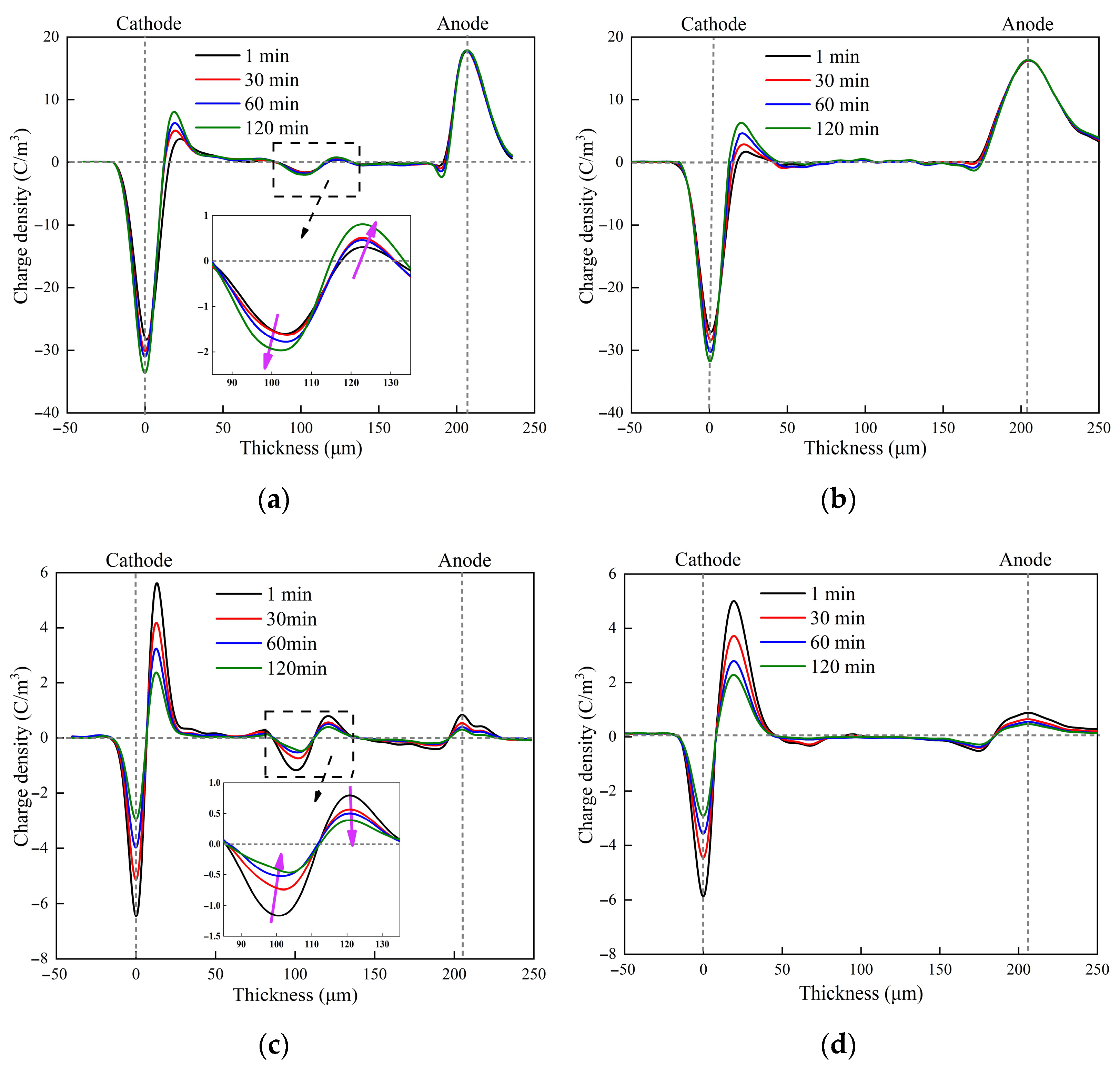
3.1. Charge Characteristics
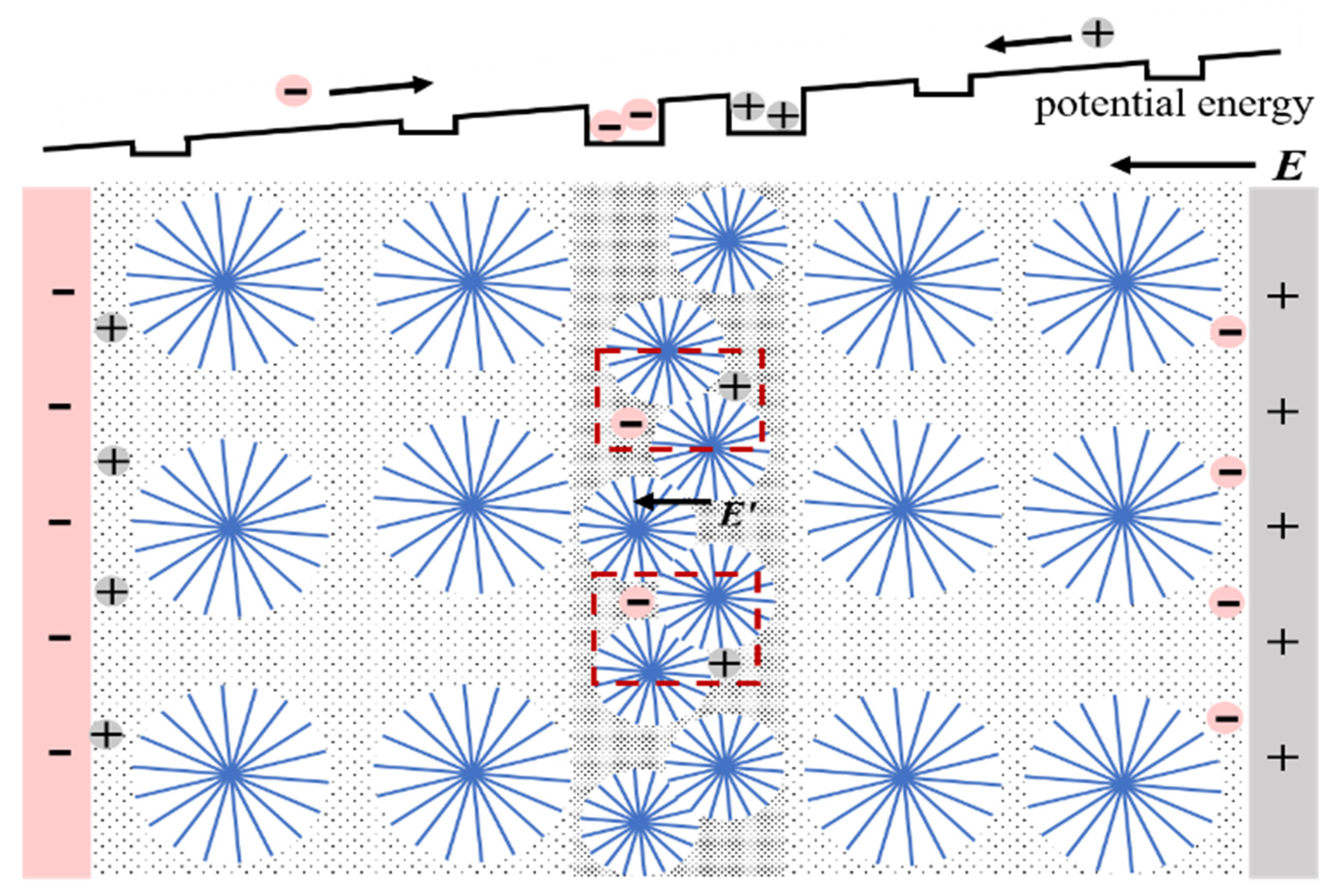
3.2. Electric Field Distribution and Breakdown Characteristics
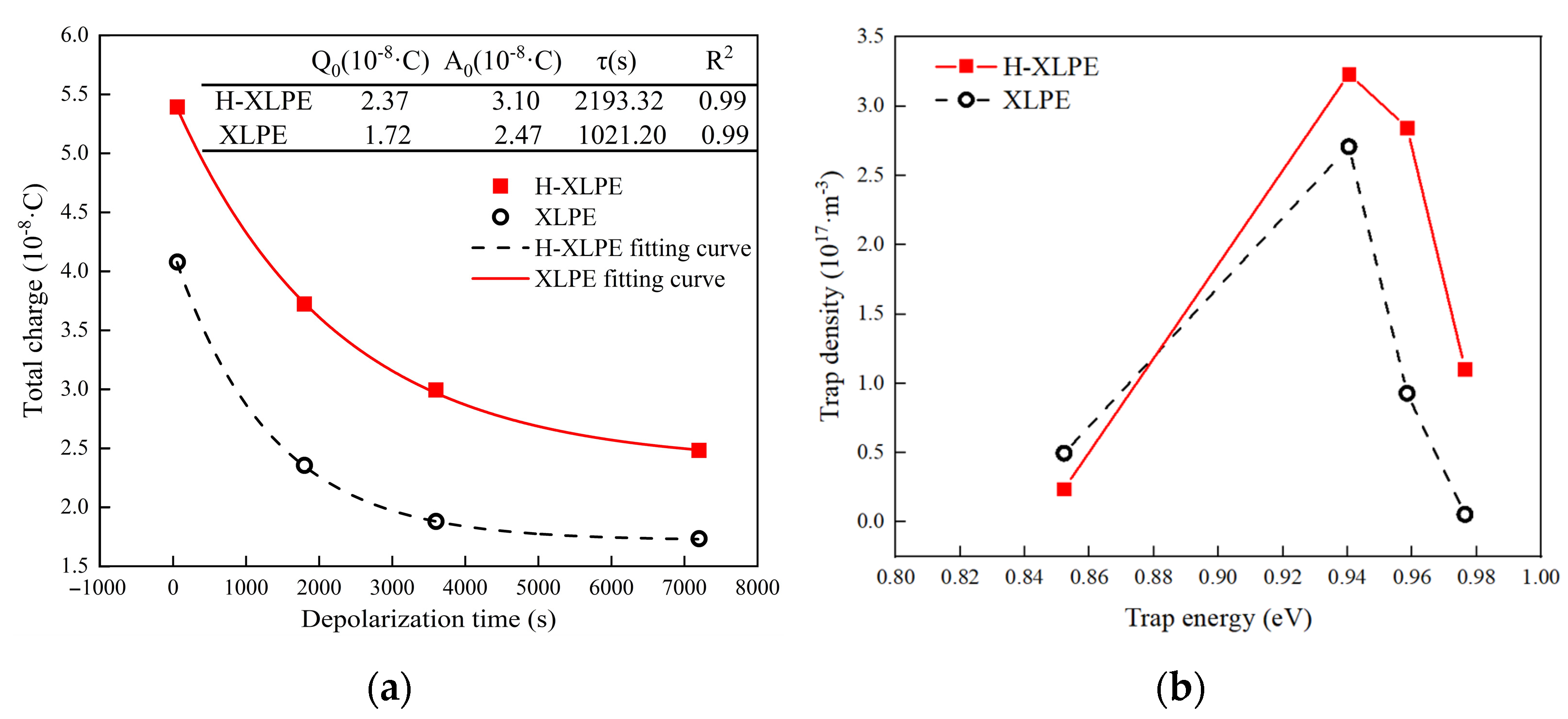
3.3. Trap Characteristics
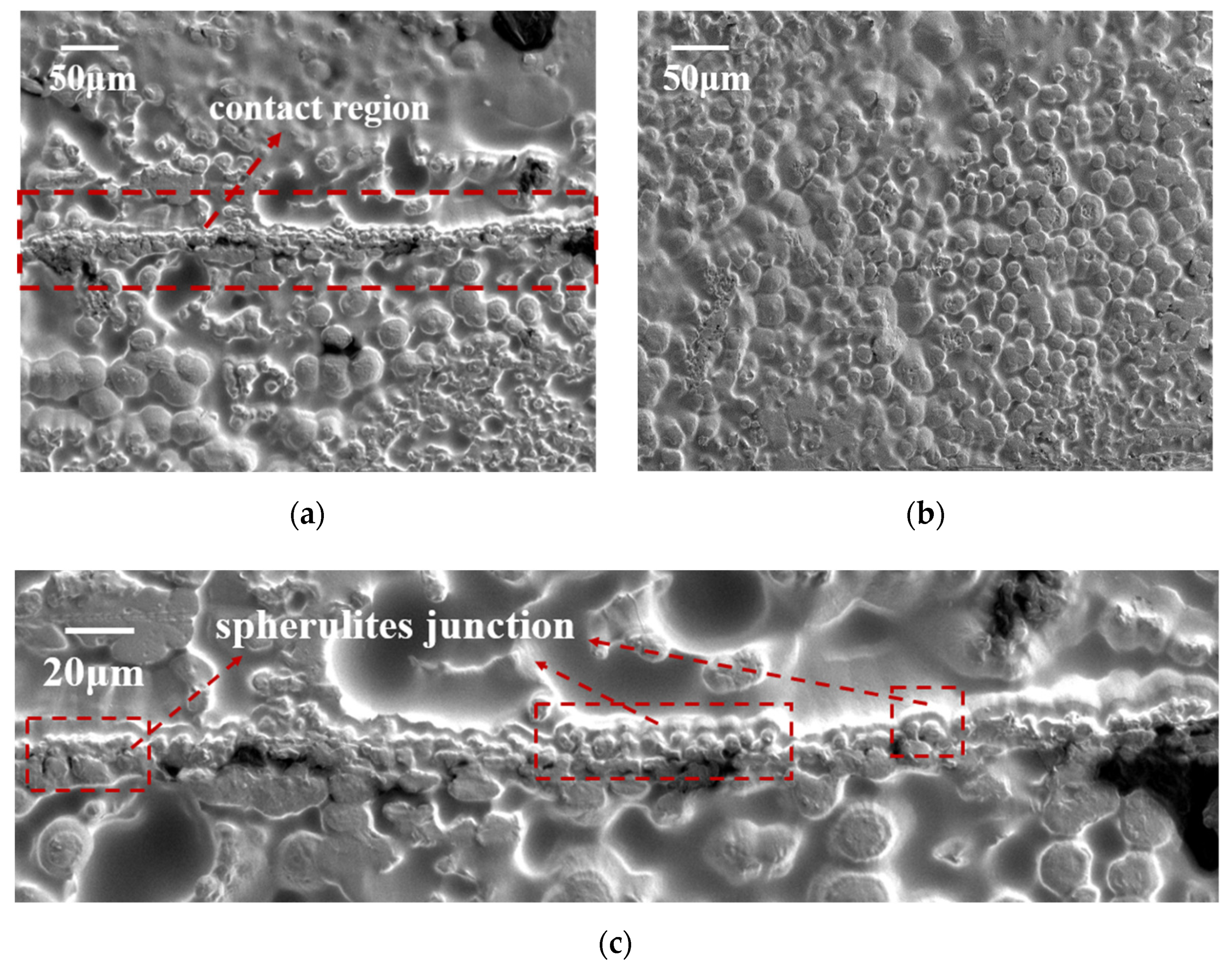
3.4. Spherulite Surface Morphology
3.5. Microcrystal Size and Periodic Crystal Morphology
3.6. Crystallinity and Surface Free Energy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez-Rua, J.; Stolpe, M.; Das, K.; Cutululis, N. Global Optimization of Offshore Wind Farm Collection Systems. IEEE Trans. Power Syst. 2019, 35, 2256–2267. [Google Scholar] [CrossRef]
- Drissi-Habti, M.; Raj-Jiyoti, D.; Vijayaraghavan, S.; Fouad, E. Numerical Simulation for Void Coalescence (Water Treeing) in XLPE Insulation of Submarine Composite Power Cables. Energies 2020, 13, 5472. [Google Scholar] [CrossRef]
- Purvins, A.; Sereno, L.; Ardelean, M.; Covrig, C.F.; Efthimiadis, T.; Minnebo, P. Submarine power cable between Europe and North America: A techno-economic analysis. J. Clean Prod. 2018, 186, 131–145. [Google Scholar] [CrossRef]
- Cao, L.; Zhong, L.; Li, Y.; Zhang, K.; Gao, J.; Chen, G. Enhanced High-Temperature DC Dielectric Performance of Crosslinked Polyethylene with a Polystyrene Pinning Structure. Materials 2019, 12, 1234. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Miao, H.; Xu, Z.; Vaughan, A.; Cao, J.; Wang, H. Review of high voltage direct current cables. CSEE J. Power Energy Syst. 2015, 1, 9–21. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Dong, Y.; Xu, W.; Tao, H.; Wang, J.; Yan, H. Research on the Manufacturing Process of Submarine Cable Joints and Joint Room Design. In Proceedings of the International Conference on Energy, Power, Environment and Computer Application (ICEPECA), Wuhan, China, 20 January 2019; pp. 86–91. [Google Scholar]
- Zhao, W.; Zhong, L.; Yang, X.; Li, Y.; Gao, J. Effects of Revulcanization on XLPE Crystalline Morphology and AC Breakdown Performance. In Proceedings of the 2018 Condition Monitoring and Diagnosis (CMD), Perth, Australia, 23–26 September 2018; pp. 678–684. [Google Scholar]
- Zhang, Z.; Zhao, J.; Zhao, W.; Zhong, L.; Zheng, M. Influence of Morphological Variations in XLPE on the AC Breakdown Performance of Submarine Cable Factory Joint Insulation. High Volt. 2019, 5, 69–75. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, C.; Zheng, M.; Zhao, H.; Zhao, J.; Sun, W.; Chen, J. Interface Damages of Electrical Insulation in Factory Joints of High Voltage Submarine Cables. Energies 2020, 13, 3892. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, L.; Zhao, J.; Zhao, H.; Huang, X.; Zheng, C. Formation Process and Electrical Tree Characteristics of Insulation Recovery Transition Zone of Factory Joints in 500 kV Submarine Cable. High Volt. Eng. 2019, 45, 3413–3420. [Google Scholar]
- Meng, F.; Chen, X.; Dai, C.; Zhang, M. Interfacial microstructure and insulation properties of 500 kV EHVDC XLPE cable factory joint under different roughness and degassing durations. Polym. Degrad. Stabil. 2021, 192, 109688. [Google Scholar] [CrossRef]
- Li, Y.; Yasuda, M.; Takada, T. Pulsed electroacoustic method for measurement of charge accumulation in solid dielectrics. IEEE Trns. Dielectr. Electr. Insul. 1994, 1, 188–195. [Google Scholar]
- Maeno, T.; Fukunaga, K. High-resolution PEA charge distribution measurement system. IEEE Trns. Dielectr. Electr. Insul. 1996, 3, 754–757. [Google Scholar] [CrossRef]
- Chen, G.; Tay, T.; Davies, A. Electrodes and charge injection in low-density polyethylene using pulsed electroacoustic technique. IEEE Trns. Dielectr. Electr. Insul. 2001, 8, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Chen, G.; Liao, R. Charge trapping and detrapping in polymeric materials: Trapping parameters. J. Appl. Phys. 2011, 110, 043724. [Google Scholar] [CrossRef] [Green Version]
- Maeno, T.; Futami, T.; Kushibe, H.; Takada, T.; Cooke, C. Measurement of spatial charge distribution in thick dielectrics using the pulsed electroacoustic method. IEEE Trns. Dielectr. Electr. Insul. 1988, 23, 433–439. [Google Scholar] [CrossRef]
- Choo, W.; Chen, G. Electric field determination in DC polymeric power cable in the presence of space charge. In Proceedings of the 2007 Annual Report—Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Vancouver, BC, Canada, 14–17 October 2008; pp. 489–492. [Google Scholar]
- Zhou, G.; Jiang, T.; Chen, F.; Jin, L.; Man, X. Calculation of initial discharge voltage on insulator surface based on the simulation analysis of distorted electric field. In Proceedings of the 2020 IEEE 3rd International Conference on Dielectrics (ICD), Valencia, Spain, 5–9 July 2020; pp. 158–161. [Google Scholar]
- Yan, Z.; Yang, K.; Zhang, Y.; Wang, S.; Li, J. Crosslinking dependence of trap distribution and breakdown performance of crosslinked polyethylene. J. Mater. Sci.-Mater. Electron. 2019, 30, 20605–20613. [Google Scholar] [CrossRef]
- Meng, F.; Chen, X.; Xu, X.; Dai, C.; Paramane, A.; Tanaka, Y. Effect of degassing treatment durations on physico-chemical and electrical properties of 500 kV extra HVDC XLPE cable insulation. Polym. Degrad. Stabil. 2021, 188, 109566. [Google Scholar]
- Hu, J.; Tashiro, K. Relation between higher-order structure and crystalline phase transition of oriented isotactic polybutene-1 investigated by temperature-dependent time-resolved simultaneous WAXD/SAXS measurements. Polymer 2016, 90, 165–177. [Google Scholar] [CrossRef]
- Hama, H.; Tashiro, K. Structural changes in isothermal crystallization process of polyoxymethylene investigated by time-resolved FTIR, SAXS and WAXS measurements. Polymer 2003, 44, 6973–6988. [Google Scholar] [CrossRef]
- Lusarczyk, C. Time-resolved SAXS investigations of morphological changes in a blend of linear and branched polyethylenes during crystallization and subsequent melting. J. Alloys Compd. 2004, 382, 68–74. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ma, Y.; Yan, Q.; Ouyang, B. The role of thermo-oxidative aging at different temperatures on the crystal structure of crosslinked polyethylene. J. Mater. Sci.-Mater. Electron. 2018, 29, 3696–3703. [Google Scholar] [CrossRef]
- He, P. Structure and Properties of Polymers; Alpha Science International Ltd.: London, UK, 2014. [Google Scholar]
- Lu, X.; Qu, J.; Huang, J. Reactive extrusion of partially crosslinked poly (butylene succinate) via novel vane extruder: Thermal, mechanical and rheological properties. Plast. Rubber Compos. 2016, 45, 261–269. [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Zhu, W.; Zhang, D.; Xiao, Y. Ultraviolet-induced crosslinking of poly (butylene succinate) and its thermal property, dynamic mechanical property, and biodegradability. Polym. Adv. Technol. 2011, 22, 648–656. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, W.S.; Lee, D.H.; Min, K.E.; Seo, K.H. Modification of poly (butylene succinate) with peroxide: Crosslinking, physical and thermal properties, and biodegradation. J. Appl. Polym. Sci. 2010, 81, 1115–1124. [Google Scholar] [CrossRef]
- Cheng, J.; Polak, M.; Penlidis, A. Influence of micromolecular structure on environmental stress cracking resistance of high density polyethylene. Tunn. Undergr. Space Technol. 2011, 26, 582–593. [Google Scholar] [CrossRef]
- Fu, M.; Chen, G.; Dissado, L.A.; Fothergill, J.C. Influence of thermal treatment and residues on space charge accumulation in XLPE for DC power cable application. IEEE Trns. Dielectr. Electr. Insul. 2007, 14, 53–64. [Google Scholar] [CrossRef] [Green Version]


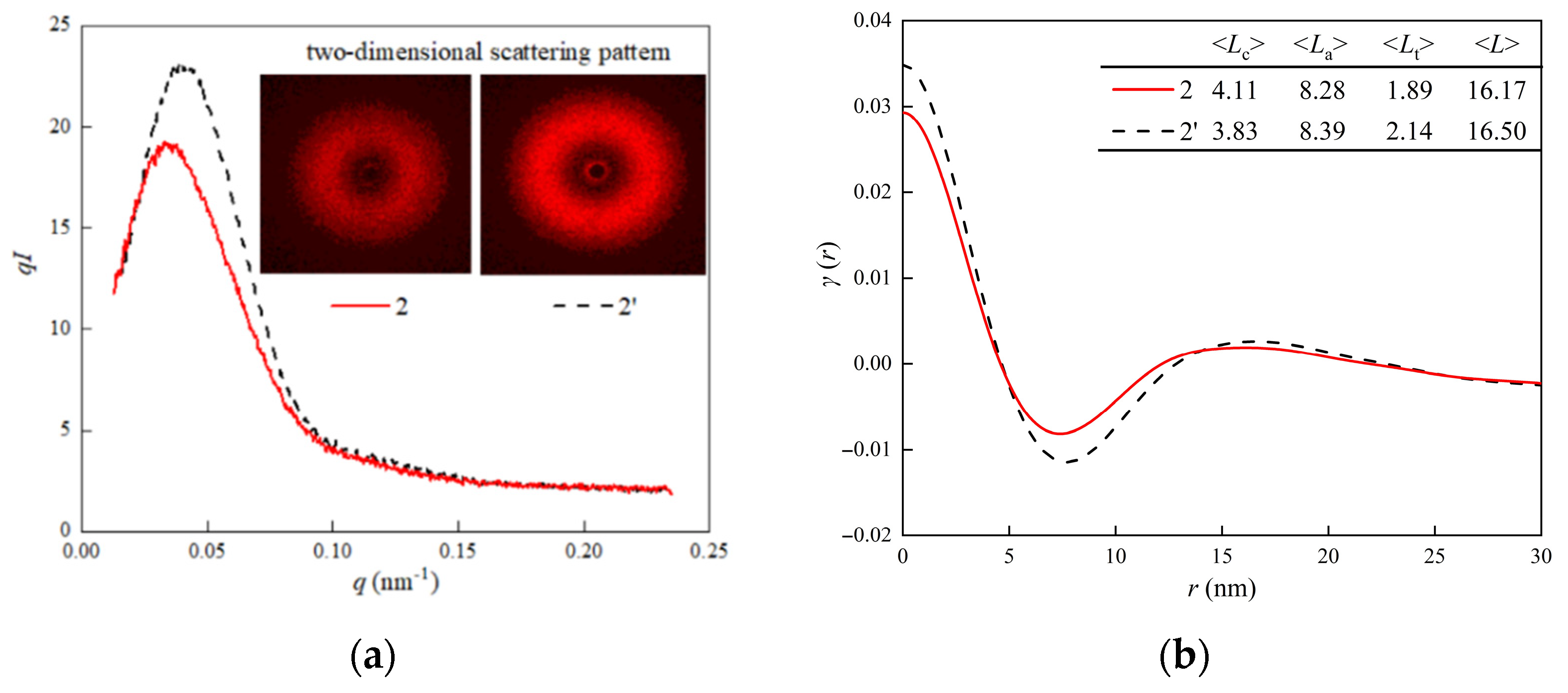


| Specimen | H-XLPE | XLPE | |||||
|---|---|---|---|---|---|---|---|
| Lattice Orientation | 1 | 2 | 3 | 1’ | 2’ | 3’ | |
| (110) | 16.48 | 17.37 | 16.44 | 16.44 | 16.38 | 16.41 | |
| (200) | 7.70 | 8.19 | 7.69 | 7.67 | 7.64 | 7.70 | |
| Specimen | H-XLPE | XLPE | |||||
|---|---|---|---|---|---|---|---|
| Lattice Orientation | 1 | 2 | 3 | 1’ | 2’ | 3’ | |
| (110) | 161.54 | 193.77 | 157.70 | 151.13 | 146.70 | 145.92 | |
| (200) | 76.00 | 91.31 | 73.70 | 70.50 | 68.45 | 68.47 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Li, H.; Li, W.; Chen, X.; Zhong, L.; Gao, J. Charge Accumulation in the Homo-Crosslinked-Polyethylene Bilayer. Materials 2022, 15, 3024. https://doi.org/10.3390/ma15093024
Zhao W, Li H, Li W, Chen X, Zhong L, Gao J. Charge Accumulation in the Homo-Crosslinked-Polyethylene Bilayer. Materials. 2022; 15(9):3024. https://doi.org/10.3390/ma15093024
Chicago/Turabian StyleZhao, Wei, Huaqiang Li, Wenpeng Li, Xin Chen, Lisheng Zhong, and Jinghui Gao. 2022. "Charge Accumulation in the Homo-Crosslinked-Polyethylene Bilayer" Materials 15, no. 9: 3024. https://doi.org/10.3390/ma15093024







