XPS Study on Calcining Mixtures of Brucite with Titania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methods
2.3.1. Characterization by Spectrometry of X-ray-Induced Photoelectrons (XPS)
2.3.2. X-ray Diffraction Characterization
3. Results and Discussion
3.1. Analysis of Chemical State by XPS
3.2. Microstructural Analysis by XRD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landy, R. Magnesia Refractories. In Refractories Handbook, 2nd ed.; Schacht, C., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2004; Volume 1, pp. 109, 112. [Google Scholar]
- Shand, M.A. Formation and Ocurrence of Magnesite and Brucite. In The Chemistry and Technology of Magnesia, 1st ed.; Shand, M.A., Ed.; John Wiley & Sons, Inc. Publication: Hoboken, NJ, USA, 2006; Volume 1, pp. 12, 33–35, 39. [Google Scholar]
- FDMINERALS. Available online: https://www.fdminerals.es/2018/04/21/brucita/ (accessed on 21 January 2022).
- Hernández Reséndiz, M. Estudio Comparativo Sobre los Efectos en las Propiedades Microestructurales de la Magnesia Sinterizada con Adiciones de Nanopartículas de Titania Partiendo de Precursores de Mg(OH)2 y MgO Cáustico de Origen Sintético en México. Master´s Thesis, Universidad Autónoma de Nuevo León, San Nicolas de los Garza, México, 22 July 2022. [Google Scholar]
- Anwar, M.; Ali, M.S.A.; Masood, U.K.; Hassan, M.; Hussain, A.K.; Muchtar, A. A Review of X-ray Photoelectron Spectroscopy Technique to Analyze the Stability and Degradation Mechanism of Solid Oxide Fuel Cell Cathode Materials. Materials 2022, 15, 2540. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.V.; Mendivil, M.I.; Das-Roy, T.K.; Castillo, G.A.; Shaji, S. Laser sintering of magnesia with nanoparticles of iron oxide and aluminum oxide. Appl. Surf. Sci. 2015, 336, 59–66. [Google Scholar] [CrossRef]
- García-Quiñonez, L.V.; Mendivil-Palma, M.I.; Das-Roy, T.K.; Castillo-Rodríguez, G.A.; Gómez-Rodríguez, C.; Fernández-González, D.; Shaji, S. Effects of irradiation energy and nanoparticle concentrations on the structure and morphology of laser sintered magnesia with alumina and iron oxide nanoparticles. Ceram. Int. 2020, 46, 7850–7860. [Google Scholar] [CrossRef]
- Khairallah, F.; Glisentia, A. XPS Study of MgO Nanopowders Obtained by Different Preparation Procedures. Surf. Sci. Spectra 2007, 13, 58–71. [Google Scholar] [CrossRef]
- Sun, N.Y.; Zhang, Y.Q.; Fu, H.R.; Che, W.R.; You, C.Y.; Shan, R. Perpendicular magnetic anisotropy in Mn2CoAl thin film. AIP Adv. 2016, 6, 15006–15012. [Google Scholar] [CrossRef] [Green Version]
- Nobre, J.; Ahmed, H.; Bravo, M.; Evangelista, L.; Brito, J. Magnesia (MgO) Production and Characterization, and Its Influence on the Performance of Cementitious Materials: A Review. Materials 2020, 13, 4752. [Google Scholar] [CrossRef]
- Castillo Rodríguez, G.A. Fusión de Magnesia por Horno de Arco Eléctrico para la Industria Refractaria. Master’s Thesis, Universidad Autónoma de Nuevo León, San Nicolas de los Garza, México, 1992. [Google Scholar]
- Crist, B.V. Handbooks of Monochromatic XPS Spectra, 1st ed.; XPS International, LLC.: Mountain View, CA, USA, 2004. [Google Scholar]
- Blasco, T.; Camblor, M.A.; Corma, A.; Perez-Pariente, J. The state of Ti in titanoaluminosilicates isomorphous with zeolite β. J. Am. Chem. Soc. 1993, 115, 11806–11813. [Google Scholar] [CrossRef]
- Cardinaud, C.; Lemperiere, G.; Peignon, M.C.; Jouan, P.Y. Characterisation of TiN coatings and of the TiN/Si interface by X-ray photoelectron spectroscopy and Auger electron spectroscopy. App. Surf. Sci. 1993, 68, 595–603. [Google Scholar]
- Nawaz, R.; Kait, C.F.; Chia, H.Y.; Isa, M.H.; Huei, L.W. Glycerol-Mediated Facile Synthesis of Colored Titania Nanoparticles for Visible Light Photodegradation of Phenolic Compounds. Nanomaterials 2019, 9, 1586. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Zhang, Y.; Jiang, J.; Rong, Y.; Wang, Y.; Wu, Y.; Pan, C. Characterization of Oxygen Vacancy Associates within Hydrogenated TiO2: A Positron Annihilation Study. J. Phys. Chem. C 2012, 116, 22619–22624. [Google Scholar] [CrossRef]
- Hsieh, P.T.; Chen, Y.C.; Kao, K.S.; Wang, C.M. Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl. Phys. A 2008, 90, 317–321. [Google Scholar] [CrossRef]
- Huravlev, J.F.; Kuznetsov, M.V.; Gubanov, V.A. XPS analysis of adsorption of oxygen molecules on the surface of Ti and TiNx films in vacuum. J. Electron Spectrosc. Relat. Phenom. 1992, 38, 169–176. [Google Scholar]
- Szörényi, T.; Laude, L.D.; Bertóti, I.; Kántor, Z.; Geretovszky, Z. Excime laser processing of indium-tin-oxide films: An optical investigation. J. Appl. Phys. 1995, 78, 6211–6219. [Google Scholar] [CrossRef]
- Casagrande, A.; Glisenti, A.; Lanzoni, E.; Tondello, E.; Mirenghi, L.; Casarin, M.; Bertoncello, R. TiN TiC and Ti(C,N) film characterization and its relationship to tribological behavior. Surf. Interface Anal. 1992, 18, 525–531. [Google Scholar]
- Barr, T.L. The nature of the relative bonding chemistry in zeolites: An XPS study. J. Phys. Chem. 1990, 10, 760–765. [Google Scholar] [CrossRef]
- Wagner, C.D.; Zatko, D.A.; Raymond, R.H. Use of the oxygen KLL Auger lines in identification of surface chemical states by electron spectroscopy for chemical analysis. Anal. Chem. 1980, 52, 1445–1451. [Google Scholar] [CrossRef]
- Gómez-Rodriguez, C.R.; García-Quiñonez, L.V.G.; Aguilar-Martinez, J.A.; Castillo-Rodriguez, G.A.; Rodríguez-Castellanos, E.A.; López-Perales, J.F.; Mendivil-Palma, M.I.; Verdeja, L.F.; Fernández-Gonzalez, D. MgO–ZrO2 Ceramic Composites for Silicomanganese Production. Materials 2022, 15, 2421. [Google Scholar] [CrossRef]
- Wu, P.Y.; Jiang, Y.P.; Zhang, Q.Y.; Jia, Y.; Peng, D.Y.; Xu, W. Comparative study on arsenate removal mechanism of MgO and MgO/TiO2 composites: FTIR and XPS analisis. New J. Chem. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Burke, P.J.; Bayindir, Z.; Kipouros, G.J. X-ray Photoelectron Spectroscopy (XPS) Investigation of the Surface Film on Magnesium Powders. Appl. Spectrosc. 2012, 66, 510–518. [Google Scholar] [CrossRef]
- Santamaria, M.; di Quarto, F.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef] [Green Version]
- Fournier, V.; Marcus, P.; Olefjord, I. Oxidation of magnesium. Surf. Interface Anal. 2002, 34, 494–497. [Google Scholar] [CrossRef]
- Feliu, S., Jr.; Merino, M.C.; Arrabal, R.; Coy, A.E.; Matykina, E. XPS study of the effect of aluminium on the atmospheric corrosion of the AZ31 magnesium alloy. Surf. Interface Anal. 2009, 41, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Fotea, C.; Callaway, J.; Alexander, M.R. Characterisation of the surface chemistry of magnesium exposed to the ambient atmosphere. Surf. Interface Anal. 2006, 38, 1578–1587. [Google Scholar] [CrossRef]
- Rheinheimer, V.; Unluer, C.; Liu, J.; Ruan, S.; Pan, J.; Monteiro, P. XPS Study on the Stability and Transformation of Hydrate and Carbonate Phases within MgO Systems. Materials 2017, 10, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haycock, D.E.; Nicholls, E.J.; Urch, D.S.; Webber, M.J.; Wiech, G.I. The electronic structure of magnesium hydroxide (brucite) using x-ray emission, X-ray photoelectron, and auger spectroscopy. J. Chern. Soc. Dalton Trans. 1978, 12, 1791–1798. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Peng, S.; Wang, J.; Ji, D.; Yan, W.; Ramakrishna, S. Fabrication of MgTiO3 nanofibers by electrospinning and their photocatalytic water splitting activity. Int. J. Hydrogen Energy 2017, 42, 25882–25890. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Naeem, R.; McKee, V.; Rehman, A.; Hakeem, A.; Mazhar, M. Fabrication of photoactive CaTiO3–TiO2 composite thin film electrodes via facile single step aerosol assisted chemical vapor deposition route. J. Mater. Sci. Mater. Electron. 2019, 30, 1411–1424. [Google Scholar] [CrossRef]
- Shawky, A.; Alhaddad, M.; Al-Namshah, K.S.; Mohamed, R.M.; Awwad, N.S. Synthesis of Pt-decorated CaTiO3 nanocrystals for efficient photoconversion of nitrobenzene to aniline under visible light. J. Mol. Liq. 2020, 304, 112704. [Google Scholar] [CrossRef]
- The American Ceramic Society and the National Institute of Standards and Technology. NIST Standard Reference Database 31. Figure Number 92-003. 2014. Available online: www.nist.gov/srd/nist31.cfm (accessed on 25 January 2022).















| MgO (% Weight) | CaO (% Weight) | SiO2 (% Weight) | Fe2O3 (% Weight) | Al2O3 (% Weight) | LOI (% Weight) |
|---|---|---|---|---|---|
| 46.00 | 0.31 | 0.04 | 0.02 | 0.04 | 53.59 |
| Compound | Molar Weight gr | % Weight |
|---|---|---|
| Mg(OH)2 | 58.3197 | 42.20 |
| TiO2 | 79.8658 | 57.80 |
| Total | 138.1855 | 100 |
| Mg(OH)2 % Mol | TiO2 % Mol | Calcined Brucite | |
|---|---|---|---|
| M4 (not calcined) | 100 | 0 | 0 |
| M3 (not calcined) | 0 | 100 | 0 |
| M2 (calcined) | 0 | 0 | 100 |
| M1 (calcined) | 50 | 50 | 0 |
| Ion | Peak Binding Energy eV | FWHM eV | Area CPS eV | % Ti3+ | % Ti4+ | O/Ti | % O | % Vacancies of O |
|---|---|---|---|---|---|---|---|---|
| Ti4+ | •458.53 | 1.87 | 58,355.26 | 4% | 96% | 1.979966759 | 99% | 1% |
| Ti3+ | •457.08 | 0.62 | 2338.09 |
| Sample | Ion | Peak Binding Energy eV | Area CPS eV | Area Ratio | FWHM eV |
|---|---|---|---|---|---|
| High-purity titania | •Ti4+ | 458.24 | 63,407.41 | 1 | 1.27 |
| •Ti4+ | 463.68 | 30,579.04 | 0.48 | 2.18 | |
| •Ti3+ | 457.08 | 3991 | 0.06 | 0.84 | |
| •Ti3+ | 463.28 | 2056.9 | 0.03 | 0.84 | |
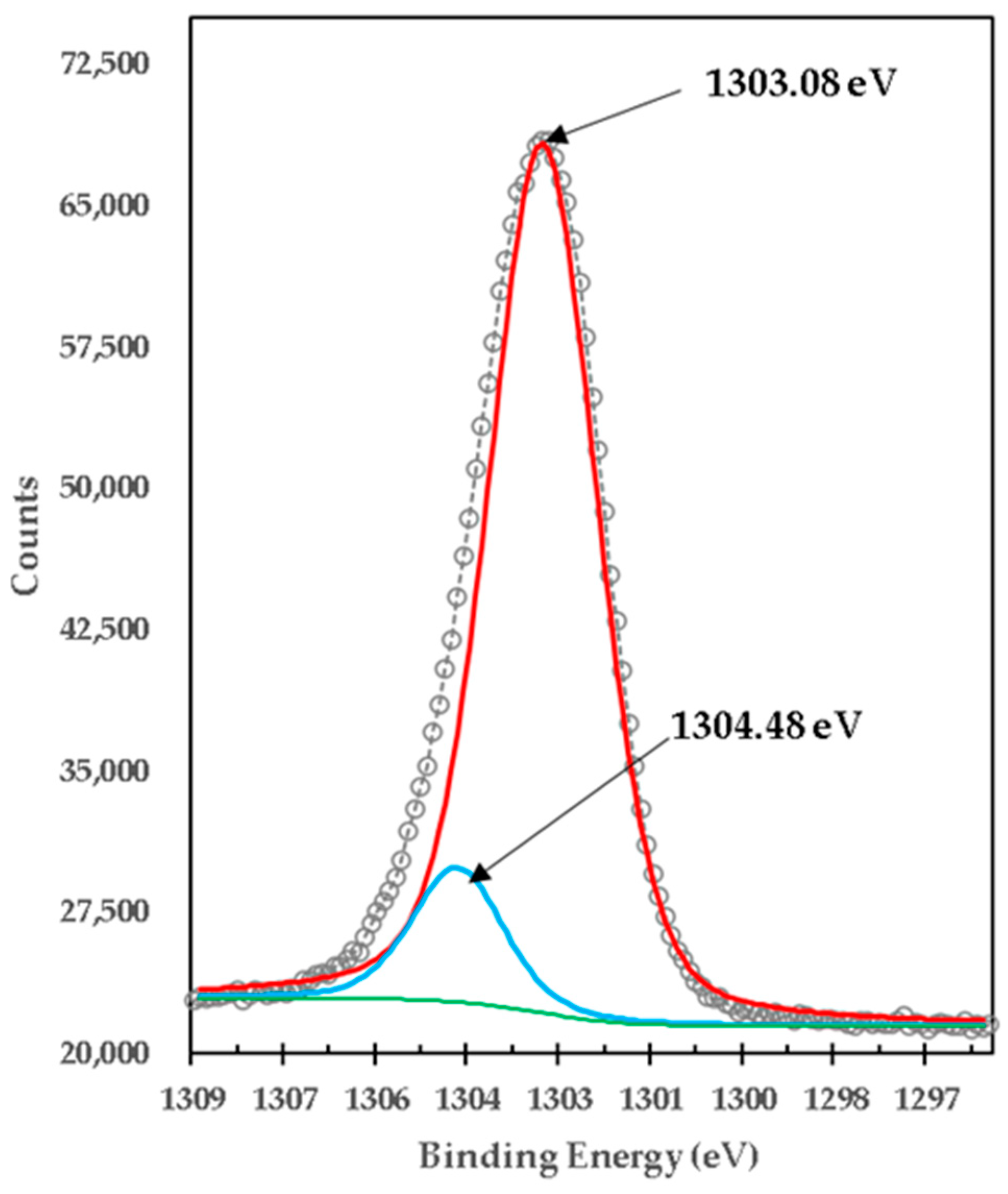
| Brucite + TiO2 mixture calcined at 1100 °C 1 h (1:1 Molar MgO:TiO2) | •Ti3+ | 457.68 | 20,655.55 | 1 | 1.29 |
| •Ti3+ | 463.41 | 9098.07 | 0.44 | 2.04 | |
| •Ti4+ | 459.28 | 465.95 | 0.02 | 0.46 | |
| •Ti4+ | 464.48 | 730.39 | 0.04 | 0.52 |
| Sample | Ion | Peak Bond Energy Ev | Area CPS Ev | Area Ratio | FWHM Tight Ev |
|---|---|---|---|---|---|
| High-purity titania | •O1s (TiO2) | 530.68 | 80,898.97 | 1 | 1.45 |
| •O1s (O2 deficiencies in TiO2) | 529.48 | 1592.77 | 0.02 | 0.7 | |
| •O1s (Ti2O3) | 532.38 | 9340.62 | 0.12 | 1.1 | |
| •O1s (OH) | 533.38 | 6457.01 | 0.08 | 1.46 | |
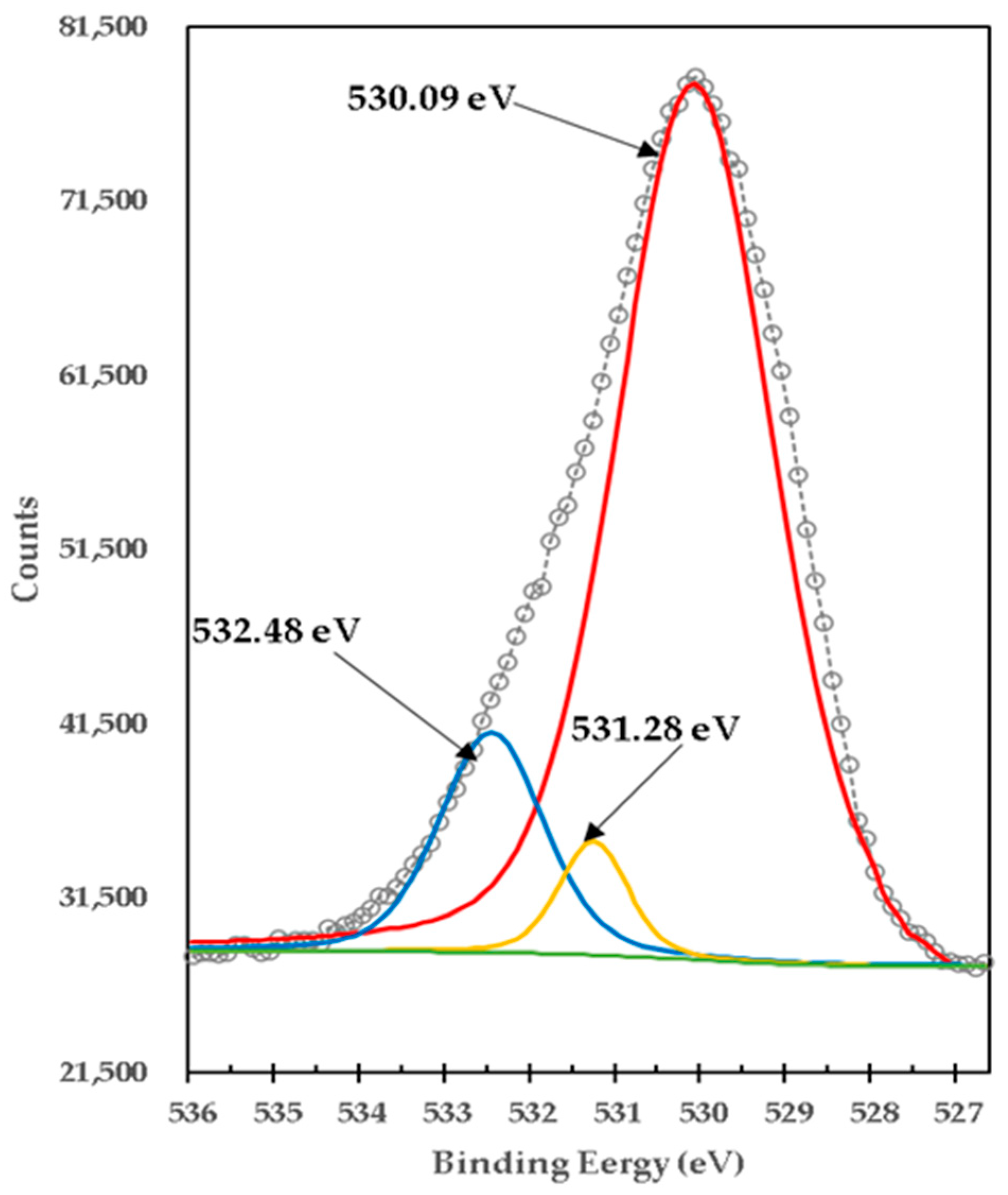
| Brucite + TiO2 mixture treated at 1100 °C 1 h (1:1 Molar MgO:TiO2) | •O1s (Varied Ti compounds) | 530.09 | 167,660.41 | 1 | 2.9 |
| •O1s (MgO) | 531.28 | 121.15 | 0.00 | 0.94 | |
| •O1s (varied compounds Mg-Ti-O) | 532.48 | 9048.83 | 0.05 | 1.46 |
| Sample | Compound | Crystal Structure | Space Group | a Å | b Å | c Å | α ° | β ° | γ ° |
|---|---|---|---|---|---|---|---|---|---|
| M2 | MgO | Cubic | F m −3 m (225) | 4.213313 | 4.213313 | 4.213313 | 90 | 90 | 90 |
| M1 | MgO | Cubic | F m −3 m (225) | 4.21156 | 4.21156 | 4.21156 | 90 | 90 | 90 |
| MgTiO3 | Rhombohedral | R −3 (148) | 5.0549 | 5.0549 | 13.8939 | 90 | 90 | 90 | |
| MgTi2O5 | Orthorhombic | C m c m (63) | 3.7428 | 9.7387 | 9.9976 | 90 | 90 | 90 | |
| TiO2 | Tetragonal | P 42/m n m (136) | 4.59327 | 4.59327 | 2.95892 | 90 | 90 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Zambrano, K.S.; Hernández-Reséndiz, M.; Gómez-Rodríguez, C.; García-Quiñonez, L.V.; Aguilar-Martínez, J.A.; Rodríguez-Castellanos, E.A.; Verdeja, L.F.; Fernández-González, D.; Castillo-Rodríguez, G.A. XPS Study on Calcining Mixtures of Brucite with Titania. Materials 2022, 15, 3117. https://doi.org/10.3390/ma15093117
Sánchez-Zambrano KS, Hernández-Reséndiz M, Gómez-Rodríguez C, García-Quiñonez LV, Aguilar-Martínez JA, Rodríguez-Castellanos EA, Verdeja LF, Fernández-González D, Castillo-Rodríguez GA. XPS Study on Calcining Mixtures of Brucite with Titania. Materials. 2022; 15(9):3117. https://doi.org/10.3390/ma15093117
Chicago/Turabian StyleSánchez-Zambrano, Karla Sofía, Marina Hernández-Reséndiz, Cristian Gómez-Rodríguez, Linda Viviana García-Quiñonez, Josué Amilcar Aguilar-Martínez, Edén Amaral Rodríguez-Castellanos, Luis Felipe Verdeja, Daniel Fernández-González, and Guadalupe Alan Castillo-Rodríguez. 2022. "XPS Study on Calcining Mixtures of Brucite with Titania" Materials 15, no. 9: 3117. https://doi.org/10.3390/ma15093117
APA StyleSánchez-Zambrano, K. S., Hernández-Reséndiz, M., Gómez-Rodríguez, C., García-Quiñonez, L. V., Aguilar-Martínez, J. A., Rodríguez-Castellanos, E. A., Verdeja, L. F., Fernández-González, D., & Castillo-Rodríguez, G. A. (2022). XPS Study on Calcining Mixtures of Brucite with Titania. Materials, 15(9), 3117. https://doi.org/10.3390/ma15093117










