Facile Preparation of a Novel Vanillin-Containing DOPO Derivate as a Flame Retardant for Epoxy Resins
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of AVD
2.3. Preparation of EP and Flame Retardant EPs (FREPs)
2.4. Characterization
3. Results and Discussion
3.1. Characterization of AVD
3.2. Curing Behaviors
3.3. Thermal Stability
3.4. Flame Retardancy of EP and FREPs
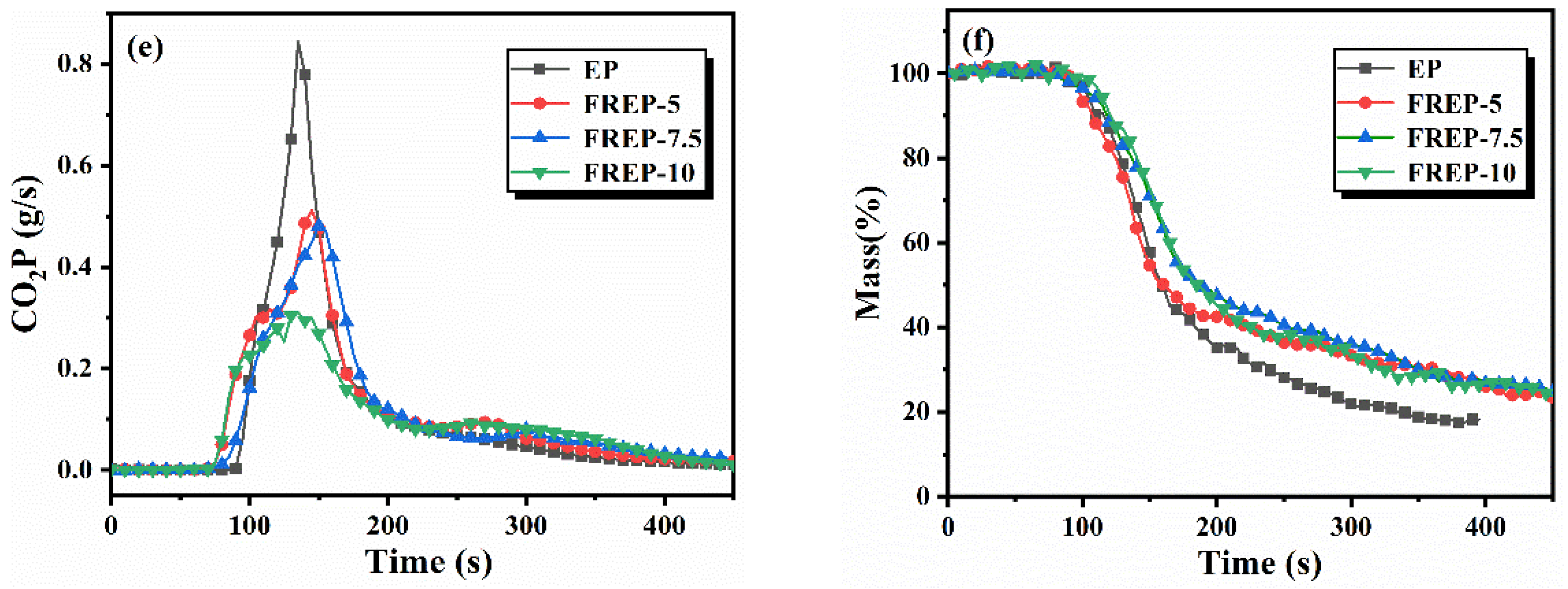
3.5. Analysis of Fire Behaviors
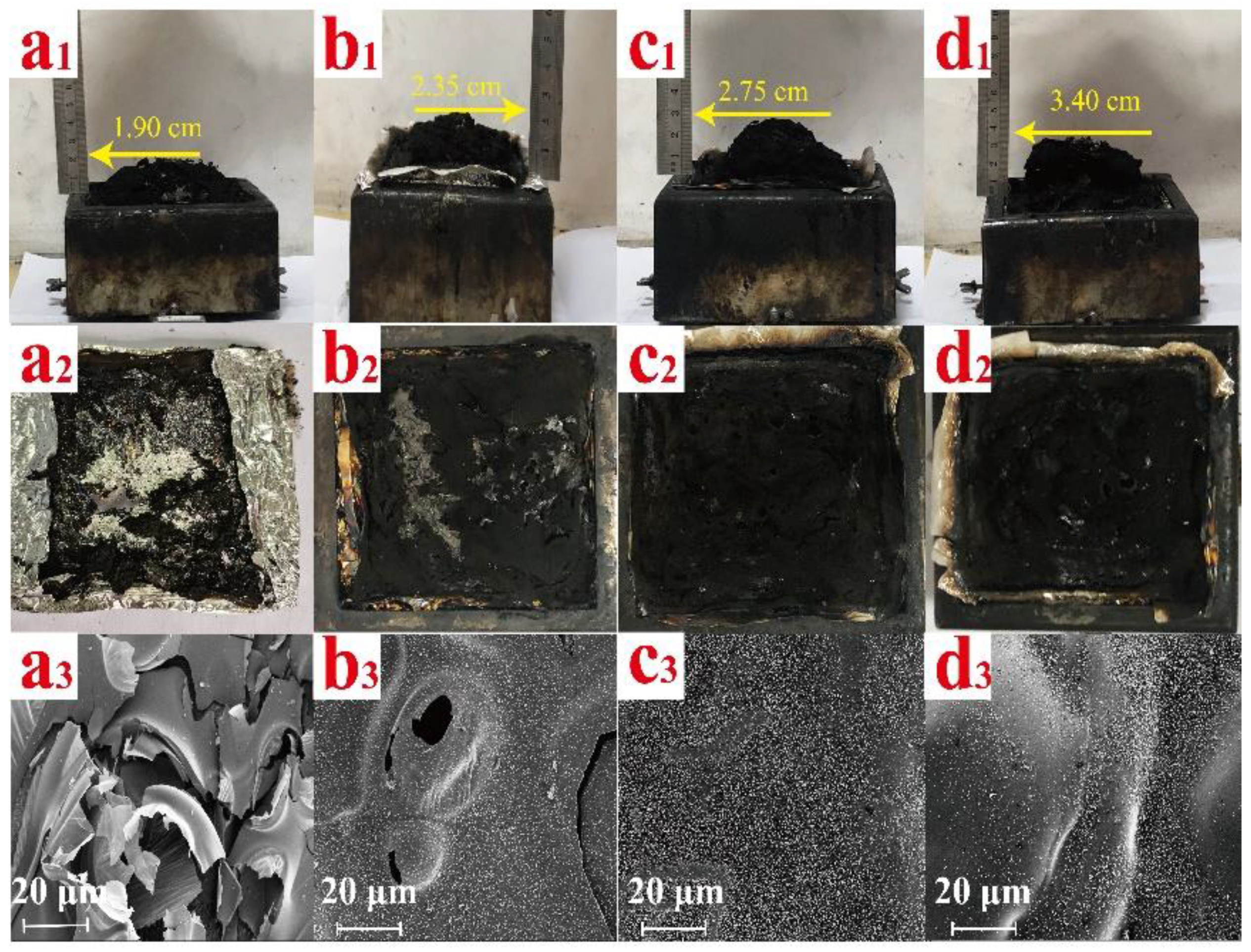
3.6. Morphology of Char Residues
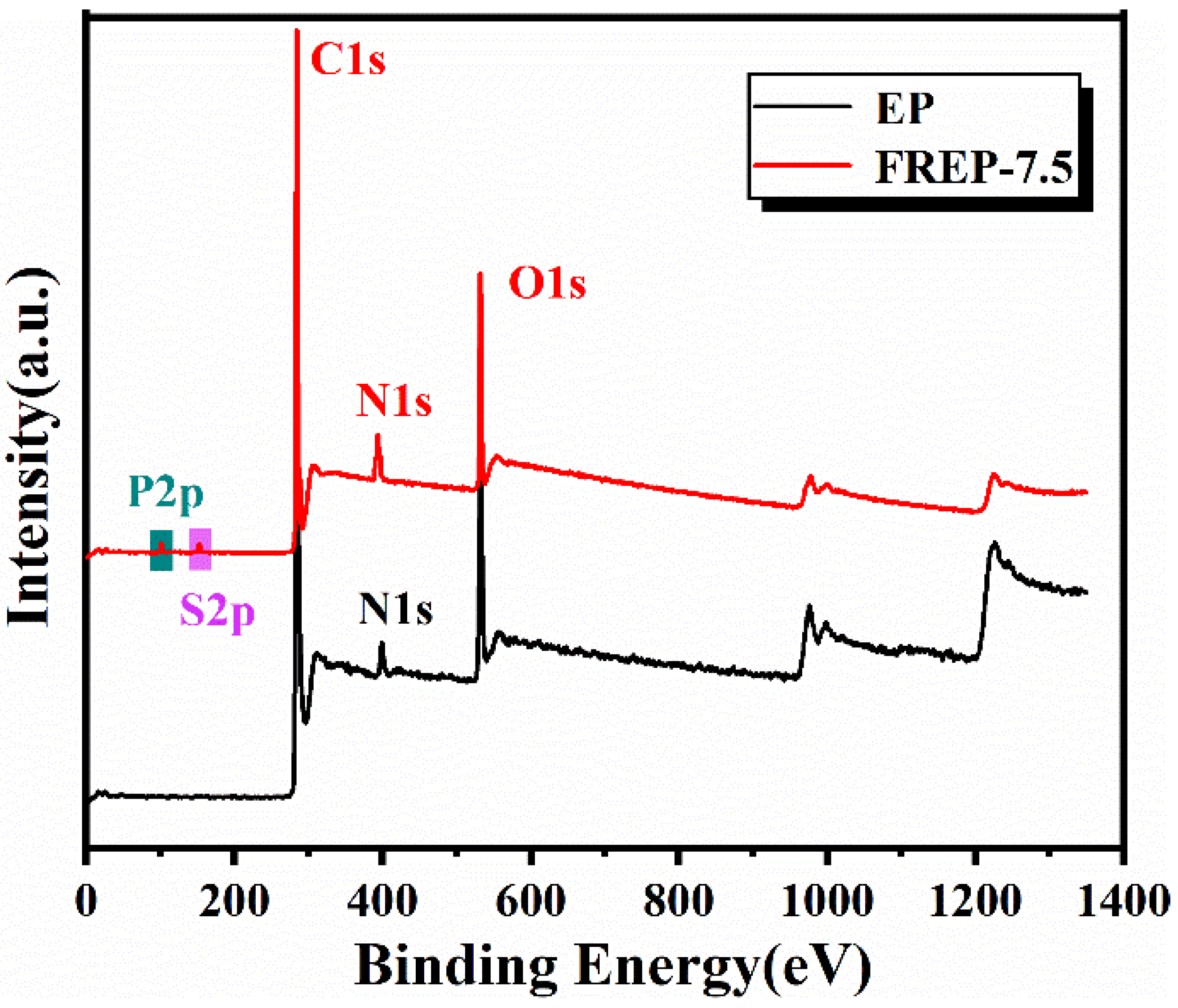
3.7. Chemical Component of Residual Char
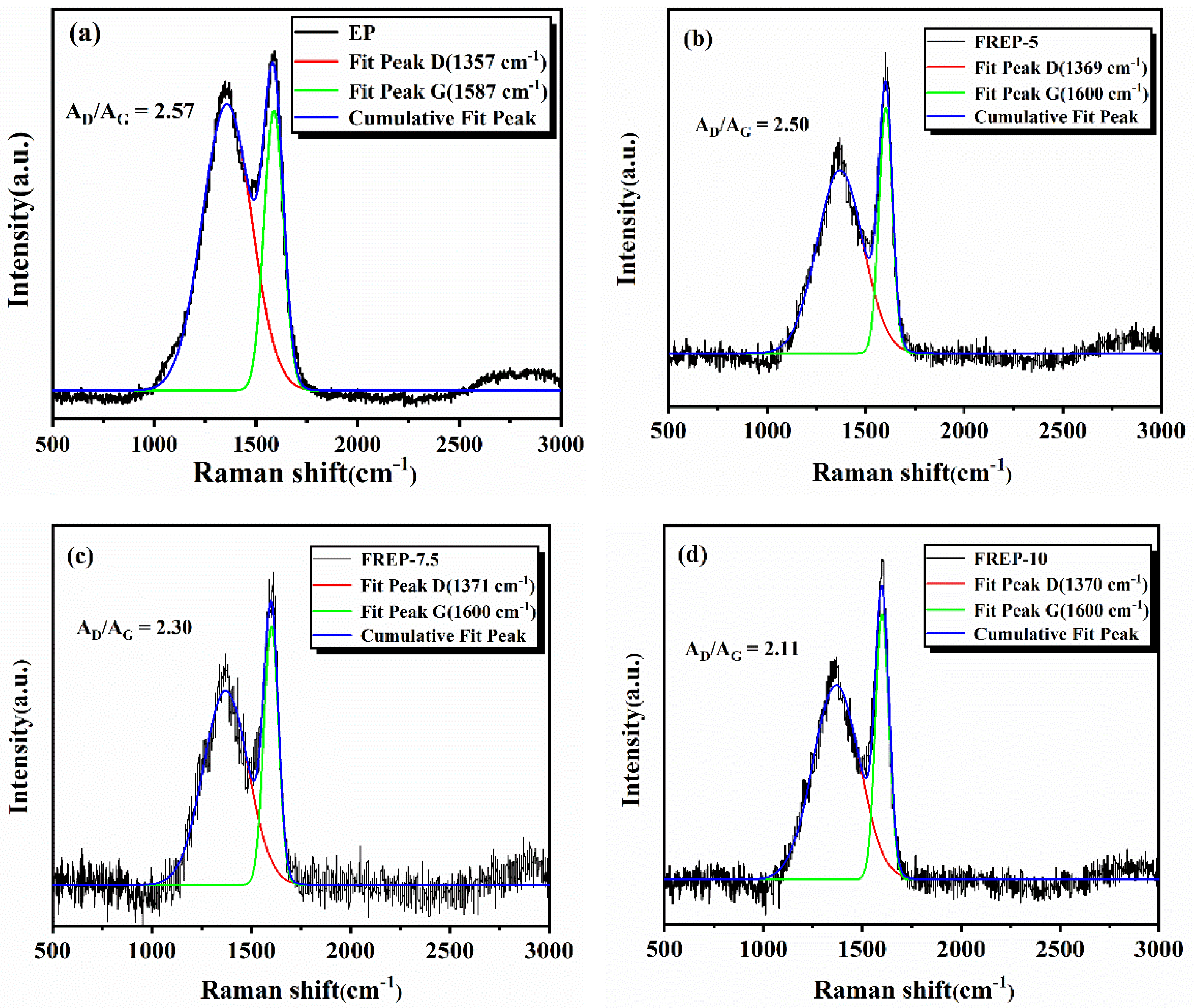
3.8. Raman Characterization of the Char Residues
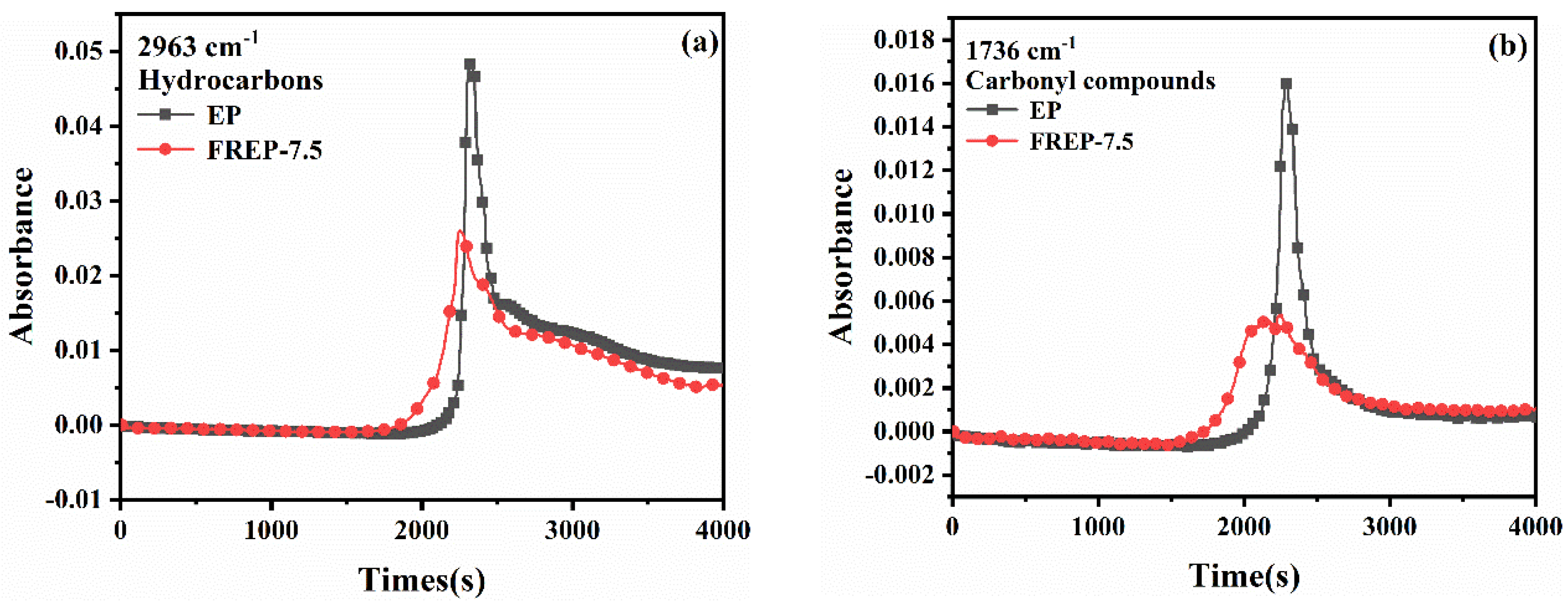
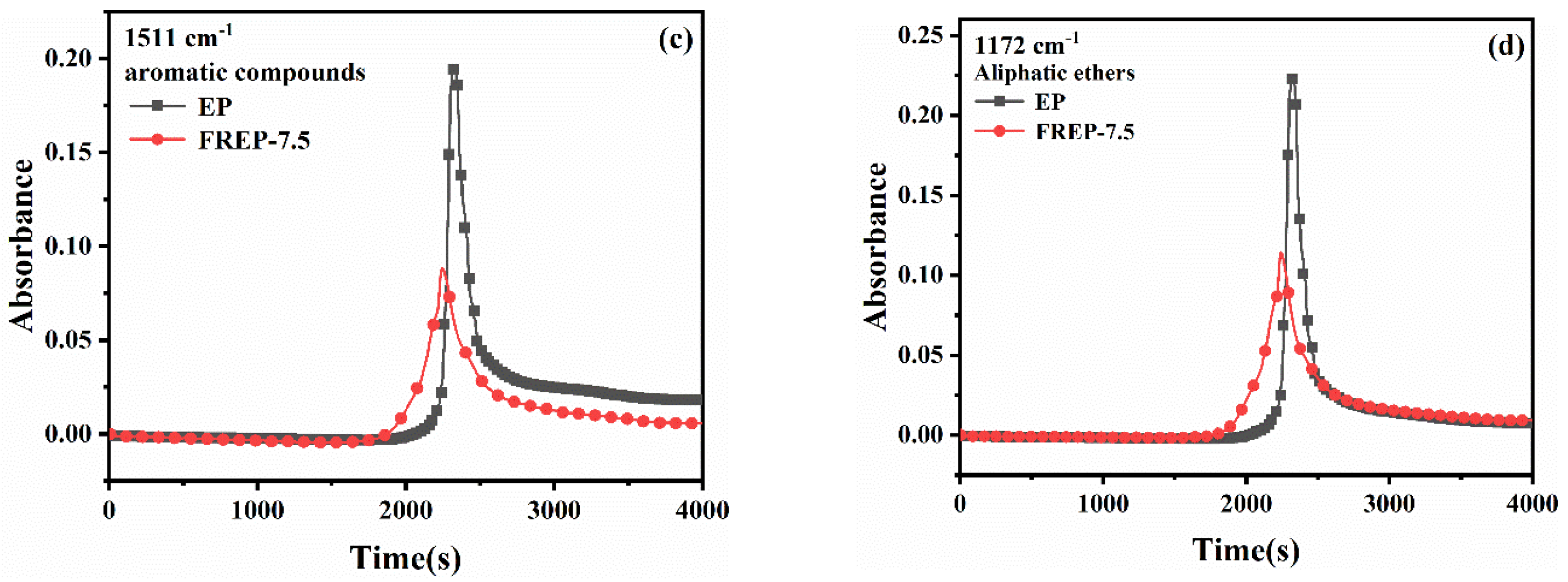
3.9. Analysis of Gaseous Products of Pyrolysis of EP Composites
3.10. Potential Flame-Retardant Mode of Action
3.11. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karaer Özmen, F.; Üreyen, M.E.; Koparal, A.S. Cleaner production of flame-retardant-glass reinforced epoxy resin composite for aviation and reducing smoke toxicity. J. Clean. Prod. 2020, 276, 124065. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, Y.; Yu, B.; Zhan, J.; Zhang, Y.; Song, L.; Ma, C.; Hu, Y. Facile synthesis of aluminum branched oligo(phenylphosphonate) submicro-particles with enhanced flame retardance and smoke toxicity suppression for epoxy resin composites. J. Hazard. Mater. 2020, 381, 121233. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kan, Y.; Yu, B.; Pan, Y.; Liew, K.M.; Song, L.; Hu, Y. Synthesis of MnO2 nanoparticles with different morphologies and application for improving the fire safety of epoxy. Compos. Part A Appl. Sci. Manuf. 2017, 95, 173–182. [Google Scholar] [CrossRef]
- Zhang, Y. Synthesis and characterization of PEDSCD and its application as a flame retardant in epoxy resins. RSC Adv. 2021, 11, 34849–34859. [Google Scholar] [CrossRef]
- Jin, S.; Qian, L.; Qiu, Y.; Chen, Y.; Xin, F. High-efficiency flame retardant behavior of bi-DOPO compound with hydroxyl group on epoxy resin. Polym. Degrad. Stab. 2019, 166, 344–352. [Google Scholar] [CrossRef]
- Xu, Y.; Nie, S.; Dai, G.; Yang, J.; Dong, X.; Feng, X. Effect of phosphorus-modified nickel phyllosilicates on the thermal stability, flame retardancy and mechanical property of epoxy composites. J. Polym. Res. 2022, 29, 1–15. [Google Scholar] [CrossRef]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Yang, S.; Cheng, J.; Ding, G.; Hu, Y.; Huo, S. Synthesis of a P/N/S-based flame retardant and its flame retardant effect on epoxy resin. Fire Saf. J. 2020, 113, 102994. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, X.; Huang, S.; Tian, X.; Song, L.; Yu, Q.; Wang, Z. Performance comparison of flame retardant epoxy resins modified by DPO-PHE and DOPO-PHE. Polym. Degrad. Stab. 2018, 156, 89–99. [Google Scholar] [CrossRef]
- Shen, D.; Xu, Y.J.; Long, J.W.; Shi, X.H.; Chen, L.; Wang, Y.Z. Epoxy resin flame-retarded via a novel melamine-organophosphinic acid salt: Thermal stability, flame retardance and pyrolysis behavior. J. Anal. Appl. Pyrolysis 2017, 128, 54–63. [Google Scholar] [CrossRef]
- Fang, Y.; Miao, J.; Yang, X.; Zhu, Y.; Wang, G. Fabrication of polyphosphazene covalent triazine polymer with excellent flame retardancy and smoke suppression for epoxy resin. Chem. Eng. J. 2020, 385, 123830. [Google Scholar] [CrossRef]
- Wagner, J.; Deglmann, P.; Fuchs, S.; Ciesielski, M.; Fleckenstein, C.A.; Döring, M. A flame retardant synergism of organic disulfides and phosphorous compounds. Polym. Degrad. Stab. 2016, 129, 63–76. [Google Scholar] [CrossRef]
- Jian, R.; Wang, P.; Xia, L.; Zheng, X. Effect of a novel P/N/S-containing reactive flame retardant on curing behavior, thermal and flame-retardant properties of epoxy resin. J. Anal. Appl. Pyrolysis 2017, 127, 360–368. [Google Scholar] [CrossRef]
- Qiu, Y.; Qian, L.; Feng, H.; Jin, S.; Hao, J. Toughening Effect and Flame-Retardant Behaviors of Phosphaphenanthrene/Phenylsiloxane Bigroup Macromolecules in Epoxy Thermoset. Macromolecules 2018, 51, 9992–10002. [Google Scholar] [CrossRef]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y.W. Improving thermal and flame retardant properties of epoxy resin by functionalized graphene containing phosphorous, nitrogen and silicon elements. Compos. Part A Appl. Sci. Manuf. 2017, 103, 74–83. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.; Qiu, Y.; Dong, Y. High-performance flame retardant epoxy resin based on a bi-group molecule containing phosphaphenanthrene and borate groups. Polym. Degrad. Stab. 2018, 153, 210–219. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Hu, Y. Synthesis of a novel flame retardant containing phosphorus, nitrogen and boron and its application in flame-retardant epoxy resin. Polym. Degrad. Stab. 2016, 133, 358–366. [Google Scholar] [CrossRef]
- Wu, Q.; Andersson, R.L.; Holgate, T.; Johansson, E.; Gedde, U.W.; Olsson, R.T.; Hedenqvist, M.S. Highly porous flame-retardant and sustainable biofoams based on wheat gluten and in situ polymerized silica. J. Mater. Chem. A. 2014, 2, 20996–21009. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Dai, J.; Wang, S.; Peng, Y.; Cao, L.; Liu, X. Facile synthesis of bio-based reactive flame retardant from vanillin and guaiacol for epoxy resin. Compos. Part B Eng. 2020, 190, 107926. [Google Scholar] [CrossRef]
- Howell, B.A.; Lazar S, T. Isosorbide as a platform for the generation of new biobased organophosphorus flame retardants. Ind. Eng. Chem. Res. 2020, 1, 1–3. [Google Scholar] [CrossRef]
- Howell, B.A.; Sun, W. Biobased flame retardants from tartaric acid and derivatives. Polym. Degrad. Stab. 2018, 157, 199–211. [Google Scholar] [CrossRef]
- Chen, R.; Luo, Z.J.; Yu, X.J.; Tang, H.; Zhou, Y.; Zhou, H. Synthesis of chitosan-based flame retardant and its fire resistance in epoxy resin. Carbohydr. Polym. 2020, 245, 116530. [Google Scholar] [CrossRef] [PubMed]
- Howell, B.A.; Han, X.R. Effective biobased phosphorus flame retardants from starch-derived bis-2,5-(Hydroxymethyl)furan. Molecules 2020, 25, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, B.A.; Ostrander, E.A.; Oberdorfer, K.L. Phosphorus flame retardants from crop plant phenolic acids. In Sustainability & Green Polymer Chemistry Volume 1: Green Products and Processes; American Chemical Society: Washington, DC, USA, 2020; Volume 1372, pp. 199–208. [Google Scholar] [CrossRef]
- Howell, B.A.; Lazar, S.T. Biobased plasticizers from glycerol/adipic acid hyperbranched poly(ester)s. Ind. Eng. Chem. Res. 2019, 58, 17227–17234. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Phosphorus flame retardants from isosorbide bis-acrylate. Polym. Degrad. Stab. 2018, 156, 14–21. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Batool, S.; Song, L.; Hu, Y. A phosphaphenanthrene-containing vanillin derivative as co-curing agent for flame-retardant and antibacterial epoxy thermoset. Polymers 2021, 217, 123460. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, J.; Yang, S.; Zhang, Q.; Hu, Y.; Ding, G.; Huo, S. Aminobenzothiazole-substituted cyclotriphosphazene derivative as reactive flame retardant for epoxy resin. React. Funct. Polym. 2020, 146, 104412. [Google Scholar] [CrossRef]
- Wang, P.; Xia, L.; Jian, R.; Ai, Y.; Zheng, X.; Chen, G.; Wang, J. Flame-retarding epoxy resin with an efficient P/N/S-containing flame retardant: Preparation, thermal stability, and flame retardance. Polym. Degrad. Stab. 2018, 149, 69–77. [Google Scholar] [CrossRef]
- Zou, J.; Duan, H.; Zhang, J.; Cao, J.; Zhang, C.; Wan, C.; Ma, H. Multi-element heterocyclic compound derived from DOPO and thiadiazole toward flame-retardant epoxy resin with satisfactory mechanical properties. J. Appl. Polym. Sci. 2021, 139, 52036. [Google Scholar] [CrossRef]
- Zou, J.; Duan, H.; Chen, Y.; Ji, S.; Cao, J.; Ma, H. A P/N/S-containing high-efficiency flame retardant endowing epoxy resin with excellent flame retardance, mechanical properties and heat resistance. Compos. Part B Eng. 2020, 199, 108228. [Google Scholar] [CrossRef]
- Wang, P.; Chen, L.; Xiao, H. Flame retardant effect and mechanism of a novel DOPO based tetrazole derivative on epoxy resin. J. Anal. Appl. Pyrolysis 2019, 139, 104–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.; Feng, G.; Liu, R.; Yang, K.; Feng, W.; Zhang, S.; He, C. Non-aromatic Si, P, N-containing hyperbranched flame retardant on reducing fire hazards of epoxy resin with desirable mechanical properties and lower curing temperature. Compos. Part B Eng. 2021, 222, 109043. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, Z.Y.; Deng, C.; Lu, P.; Zhao, P.P.; He, S.; Chen, S.W.; Lin, W. Facile synthesis of phytic acid and aluminum hydroxide chelate-mediated hybrid complex toward fire safety of ethylene-vinyl acetate copolymer. Polym. Degrad. Stab. 2021, 190, 109659. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Cui, J.; Guo, J.; Zhang, H.; Yang, B. Effect of ionic liquid octyltriphenylphosphonium-chelated orthoborates on flame retardance of epoxy. Polym. Adv. Technol. 2021, 32, 1579–1596. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, X.; Wu, J.; Wei, M.; Yu, Q.; Xiujuan, T.; Wang, Z. Performance comparison of epoxy resins modified with diphenylphosphine oxide and DOPO. Fire Mater. 2019, 43, 892–902. [Google Scholar] [CrossRef]
- Chu, F.; Ma, C.; Zhang, T.; Xu, Z.; Mu, X.; Cai, W.; Zhou, X.; Ma, S.; Zhou, Y.; Hu, W.; et al. Renewable vanillin-based flame retardant toughening agent with ultra-low phosphorus loading for the fabrication of high-performance epoxy thermoset. Compos. Part B Eng. 2020, 190, 107925. [Google Scholar] [CrossRef]
- Luo, H.; Rao, W.; Zhao, P.; Wang, L.; Liu, Y.; Yu, C. An efficient organic/inorganic phosphorus–nitrogen–silicon flame retardant towards low-flammability epoxy resin. Polym. Degrad. Stab. 2020, 178, 109195. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q.; Peng, X.; Liu, C.; Li, Z.; Li, Z.; Wang, R.; Zheng, P.; Zhang, H. High-efficiency phosphorus/nitrogen-containing flame retardant on epoxy resin. Polym. Degrad. Stab. 2021, 187, 109544. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Q.; Luo, Z.; Wang, B. Facile synthesis of a reactive P/N/S-containing compound toward highly effective flame retardancy of epoxy resin with high transparency and improved mechanical strength. Fire Saf. J. 2021, 126, 103472. [Google Scholar] [CrossRef]
- Kong, F.B.; He, Q.L.; Peng, W.; Nie, S.B.; Dong, X.; Yang, J. Eco-friendly flame retardant poly(lactic acid) composites based on banana peel powders and phytic acid: Flame retardancy and thermal property. J. Polym. Res. 2020, 27. [Google Scholar] [CrossRef]
- Rao, W.; Zhao, P.; Yu, C.; Zhao, H.B.; Wang, Y.Z. High strength, low flammability, and smoke suppression for epoxy thermoset enabled by a low-loading phosphorus-nitrogen-silicon compound. Compos. Part B Eng. 2021, 211, 108640. [Google Scholar] [CrossRef]
- Varganici, C.D.; Rosu, L.; Lehner, S.; Hamciuc, C.; Jovic, M.; Rosu, D.; Mustata, F.; Gaan, S. Semi–interpenetrating networks based on epoxy resin and oligophosphonate: Comparative effect of three hardeners on the thermal and fire properties. Mater. Design. 2021, 212, 110237. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, H.; Ji, S.; Ma, H. Novel phosphorus/nitrogen/boron-containing carboxylic acid as co-curing agent for fire safety of epoxy resin with enhanced mechanical properties. J. Hazard. Mater. 2021, 402, 123769. [Google Scholar] [CrossRef] [PubMed]

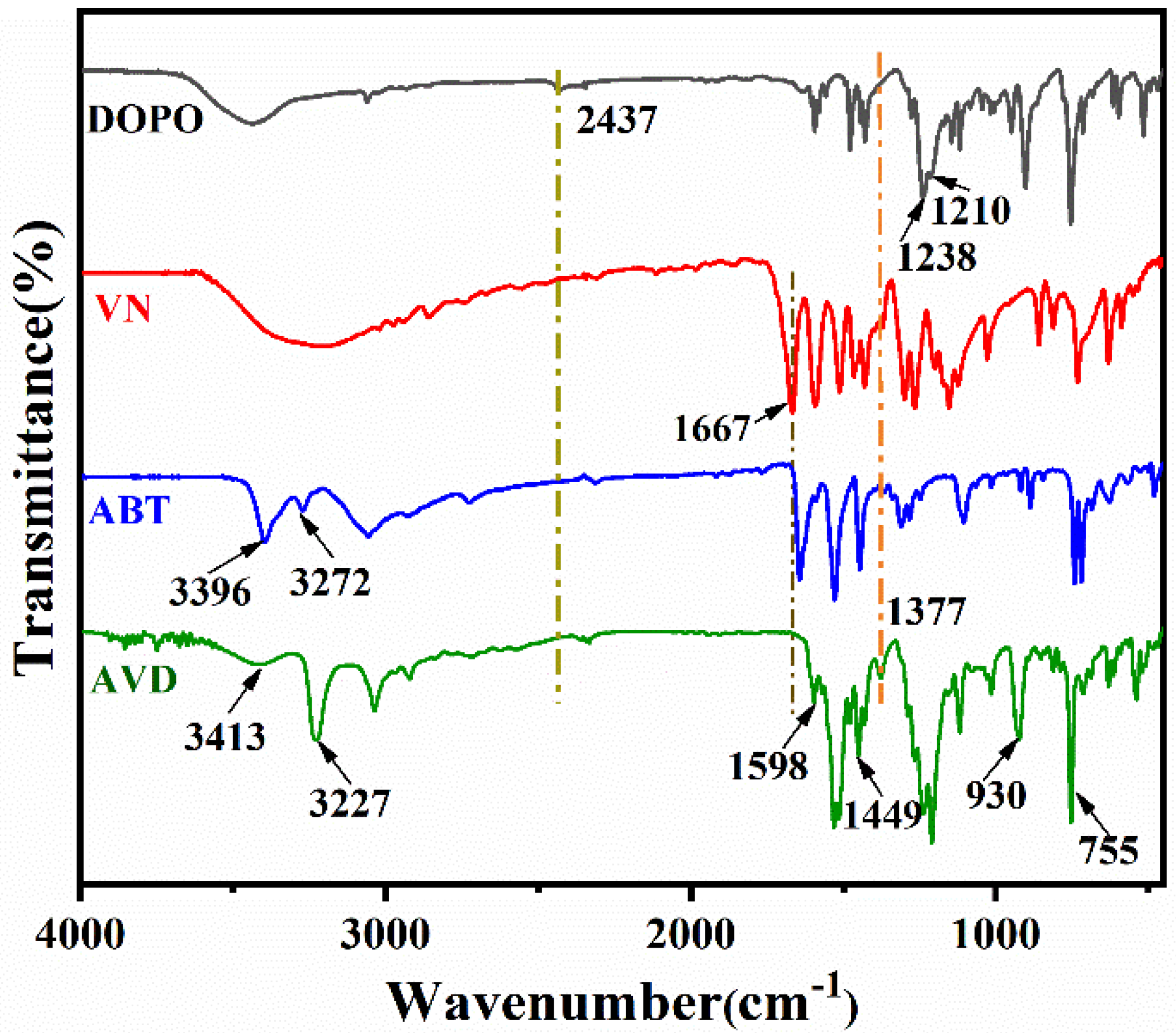


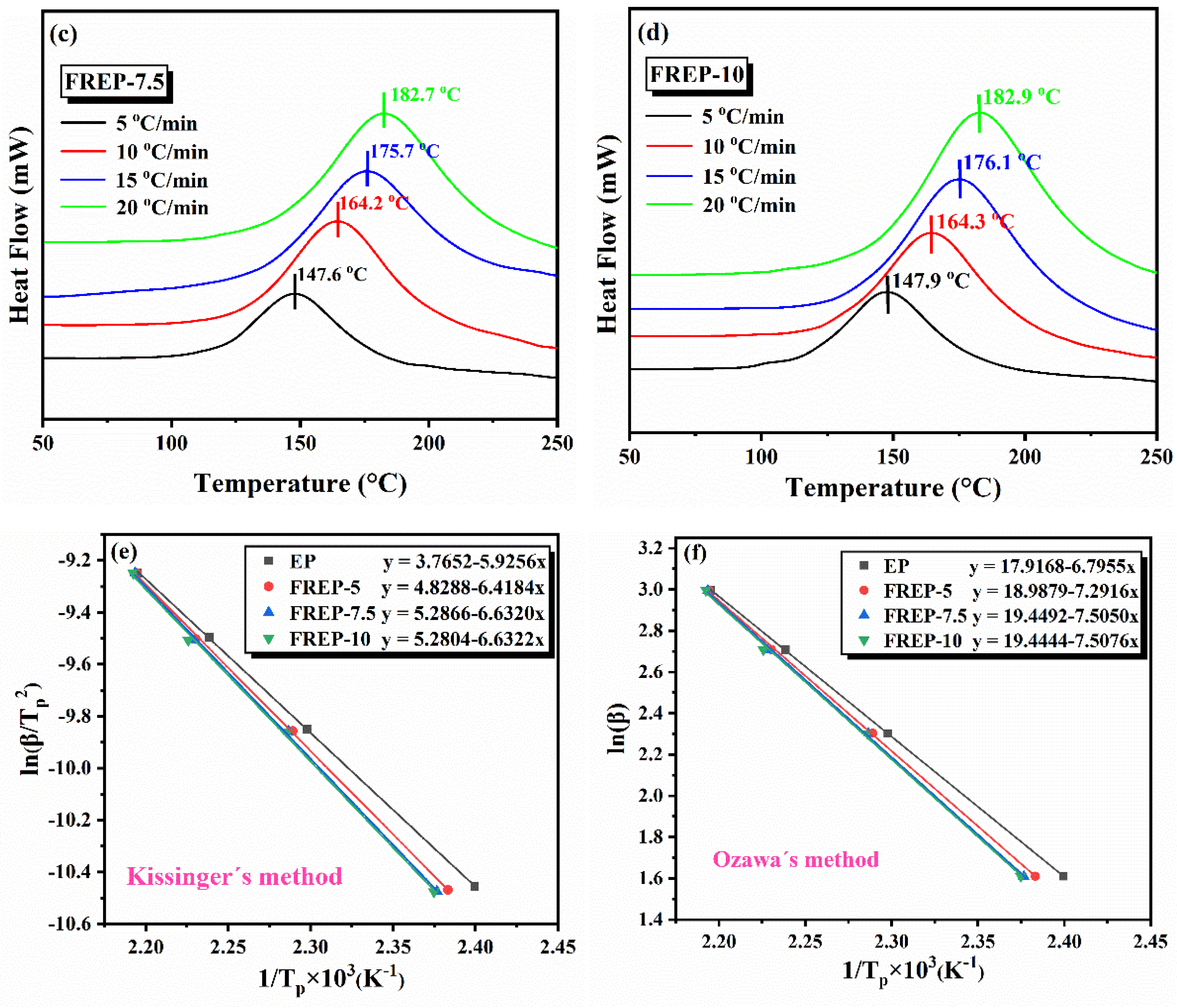
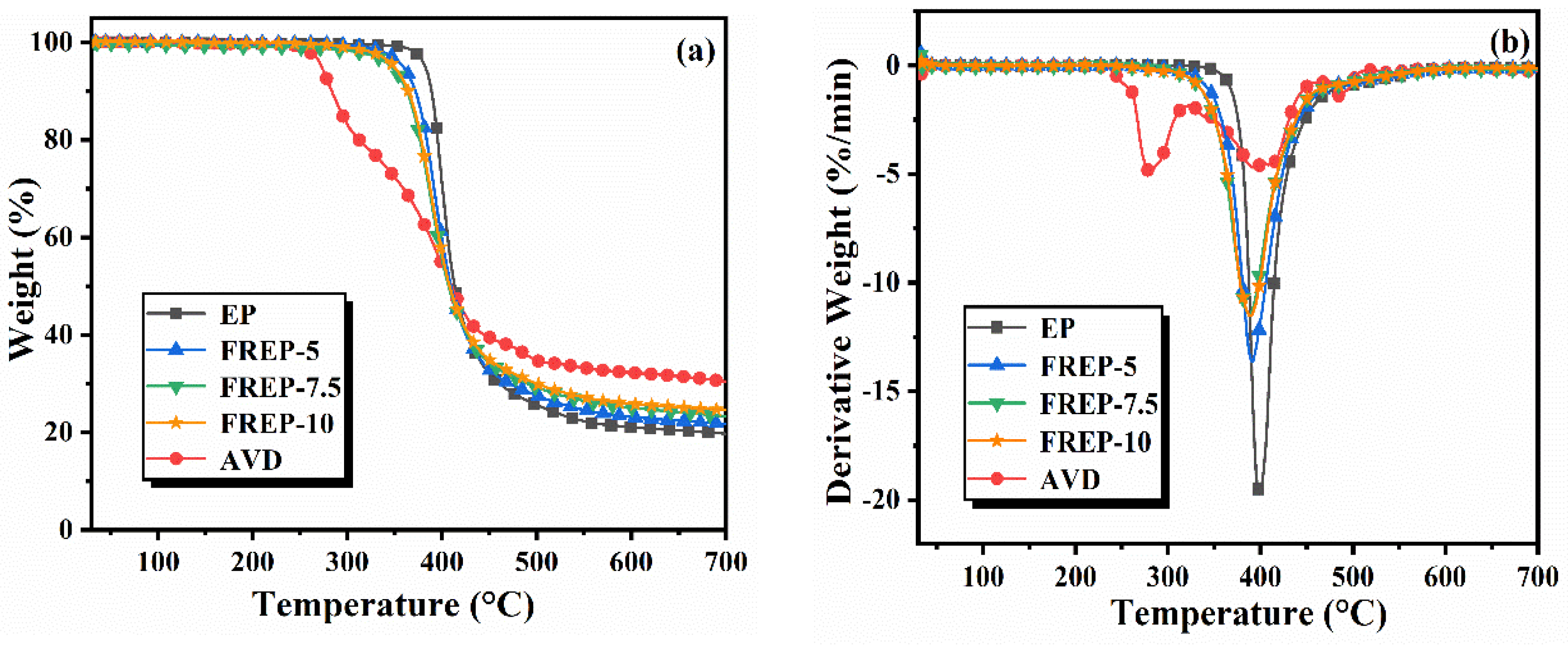
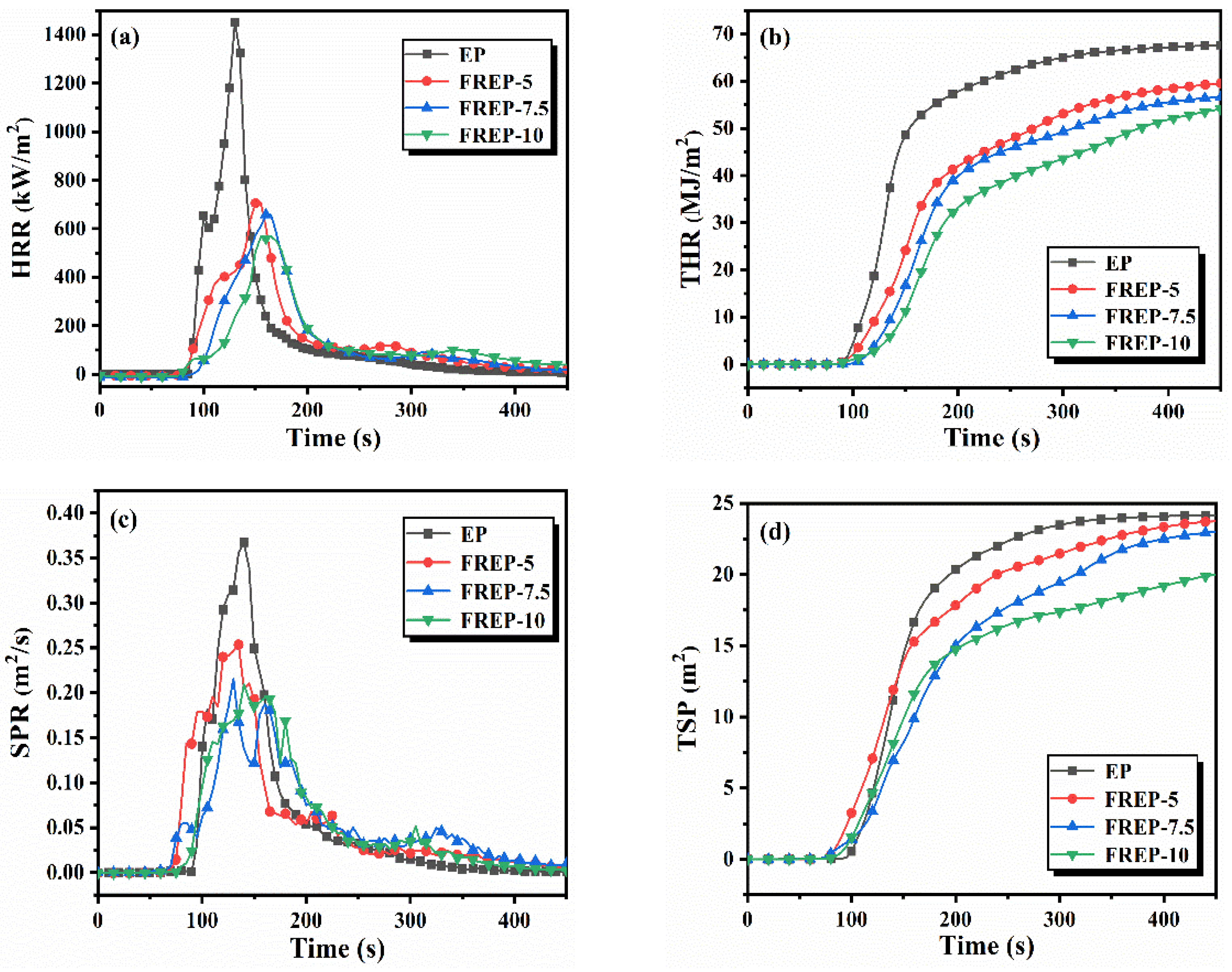




| Sample | EP (g) | DDM (g) | AVD (g) | AVD (wt%) | P (wt%) |
|---|---|---|---|---|---|
| EP | 40 | 10.11 | 0 | 0 | 0 |
| FREP-5 | 40 | 9.54 | 2.61 | 5 | 0.34 |
| FREP-7.5 | 40 | 9.36 | 4.00 | 7.5 | 0.52 |
| FREP-10 | 40 | 8.92 | 5.43 | 10 | 0.69 |
| Sample | Kissinger’s Method Ea (kJ mol−1) | Ozawa’s Method Ea (kJ mol−1) |
|---|---|---|
| EP | 49.27 | 53.71 |
| FREP-5 | 53.36 | 57.63 |
| FREP-7.5 | 55.14 | 59.31 |
| FREP-10 | 55.14 | 59.33 |
| Sample | T5% (°C) | Tmax (°C) | Rate (%·min−1) | CR700 (wt%) |
|---|---|---|---|---|
| EP | 382.3 | 399.0 | 18.8 | 19.7 |
| FREP-5 | 359.1 | 391.0 | 12.5 | 21.7 |
| FREP-7.5 | 349.5 | 388.7 | 10.7 | 23.3 |
| FREP-10 | 346.3 | 388.3 | 10.7 | 24.5 |
| AVD | 272.8 | 279.3, 410.6 | 3.5, 2.7 | 30.1 |
| Sample | P (wt%) | LOI (%) | UL-94, 3 mm bar | ||
|---|---|---|---|---|---|
| (b) t1 + t2 (s) | Dripping | Rating | |||
| EP | 0 | 25 ± 0.1 | (a) BC | Yes | No |
| FREP-5 | 0.34 | 30.0 ± 0.2 | 5.6 + 5.8 | No | V-1 |
| FREP-7.5 | 0.52 | 31.3 ± 0.1 | 4.3 + 2.7 | No | V-0 |
| FREP-10 | 0.69 | 32.5 ± 0.3 | 2 + 2 | No | V-0 |
| Sample | EP | FREP-5 | FREP-7.5 | FREP-10 |
|---|---|---|---|---|
| TTI (s) | 91 ± 2 | 73 ± 5 | 68 ± 3 | 56 ± 1 |
| PHRR (kW/m2) | 1453 ± 20 | 708 ± 14 | 658 ± 9 | 568 ± 16 |
| THR (MJ/m2) | 67.3 ± 2.1 | 59.7 ± 1.9 | 57.4 ± 2.4 | 55.8 ± 2.2 |
| FIGRA (kW/m2·s) | 11.2 ± 0.3 | 4.57 ± 0.6 | 4.11 ± 0.2 | 3.44 ± 0.4 |
| TSP (m2) | 24.1 ± 0.7 | 23.8 ± 0.3 | 23.1 ± 0.8 | 20.4 ± 0.5 |
| av-EHC (MJ/kg) | 22.1 ± 1.1 | 15.0 ± 0.7 | 14.3 ± 0.9 | 12.7 ± 1.3 |
| av-COY (kg/kg) | 0.28 ± 0.05 | 0.52 ± 0.07 | 0.58 ± 0.1 | 0.62 ± 0.04 |
| av-CO2Y (kg/kg) | 1.80 ± 0.07 | 1.36 ± 0.03 | 1.29 ± 0.08 | 1.27 ± 0.02 |
| Char residue (%) | 18.1 ± 0.1 | 20.1 ± 0.7 | 22.5 ± 0.3 | 23.1 ± 0.4 |
| Sample | C (At%) | O (At%) | N (At%) | P (At%) | S (At%) |
|---|---|---|---|---|---|
| EP | 81.71 | 14.37 | 3.92 | 0 | 0 |
| FREP-7.5 | 83.18 | 12.17 | 4.24 | 0.23 | 0.18 |
| Sample | Tg (°C) | E′ at 50 °C (MPa) | E′ at Tg + 40 °C (MPa) | υe (mol·m−3) |
|---|---|---|---|---|
| EP | 170.8 | 2786 | 63.56 | 5265 |
| FREP-5 | 157.3 | 3157 | 58.91 | 5021 |
| FREP-7.5 | 145.0 | 3216 | 52.40 | 4586 |
| FREP-10 | 143.1 | 3790 | 51.42 | 4516 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Luo, Z.; Wang, B. Facile Preparation of a Novel Vanillin-Containing DOPO Derivate as a Flame Retardant for Epoxy Resins. Materials 2022, 15, 3155. https://doi.org/10.3390/ma15093155
Chen L, Luo Z, Wang B. Facile Preparation of a Novel Vanillin-Containing DOPO Derivate as a Flame Retardant for Epoxy Resins. Materials. 2022; 15(9):3155. https://doi.org/10.3390/ma15093155
Chicago/Turabian StyleChen, Liping, Zhonglin Luo, and Biaobing Wang. 2022. "Facile Preparation of a Novel Vanillin-Containing DOPO Derivate as a Flame Retardant for Epoxy Resins" Materials 15, no. 9: 3155. https://doi.org/10.3390/ma15093155






