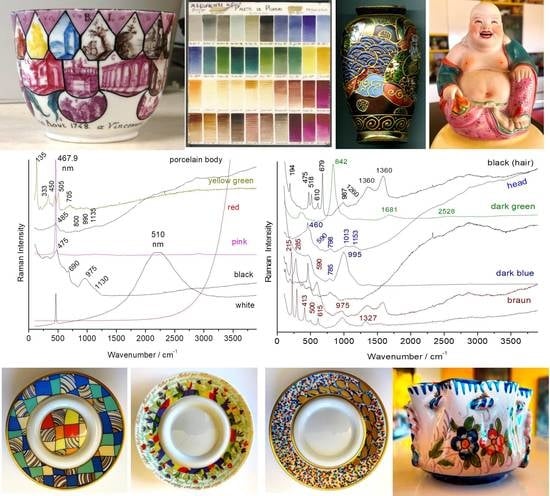
Full Spectral Range Raman Signatures Related to Changes in Enameling Technologies from the 18th to the 20th Century: Guidelines, Effectiveness and Limitations of the Raman Analysis
Abstract
Share and Cite
Colomban, P. Full Spectral Range Raman Signatures Related to Changes in Enameling Technologies from the 18th to the 20th Century: Guidelines, Effectiveness and Limitations of the Raman Analysis. Materials 2022, 15, 3158. https://doi.org/10.3390/ma15093158
Colomban P. Full Spectral Range Raman Signatures Related to Changes in Enameling Technologies from the 18th to the 20th Century: Guidelines, Effectiveness and Limitations of the Raman Analysis. Materials. 2022; 15(9):3158. https://doi.org/10.3390/ma15093158
Chicago/Turabian StyleColomban, Philippe. 2022. "Full Spectral Range Raman Signatures Related to Changes in Enameling Technologies from the 18th to the 20th Century: Guidelines, Effectiveness and Limitations of the Raman Analysis" Materials 15, no. 9: 3158. https://doi.org/10.3390/ma15093158
APA StyleColomban, P. (2022). Full Spectral Range Raman Signatures Related to Changes in Enameling Technologies from the 18th to the 20th Century: Guidelines, Effectiveness and Limitations of the Raman Analysis. Materials, 15(9), 3158. https://doi.org/10.3390/ma15093158