Obtaining and Characterization of Highly Crystalline Recycled Graphites from Different Types of Spent Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining of Recycled Graphites
2.2. Characterization
3. Results and Discussion
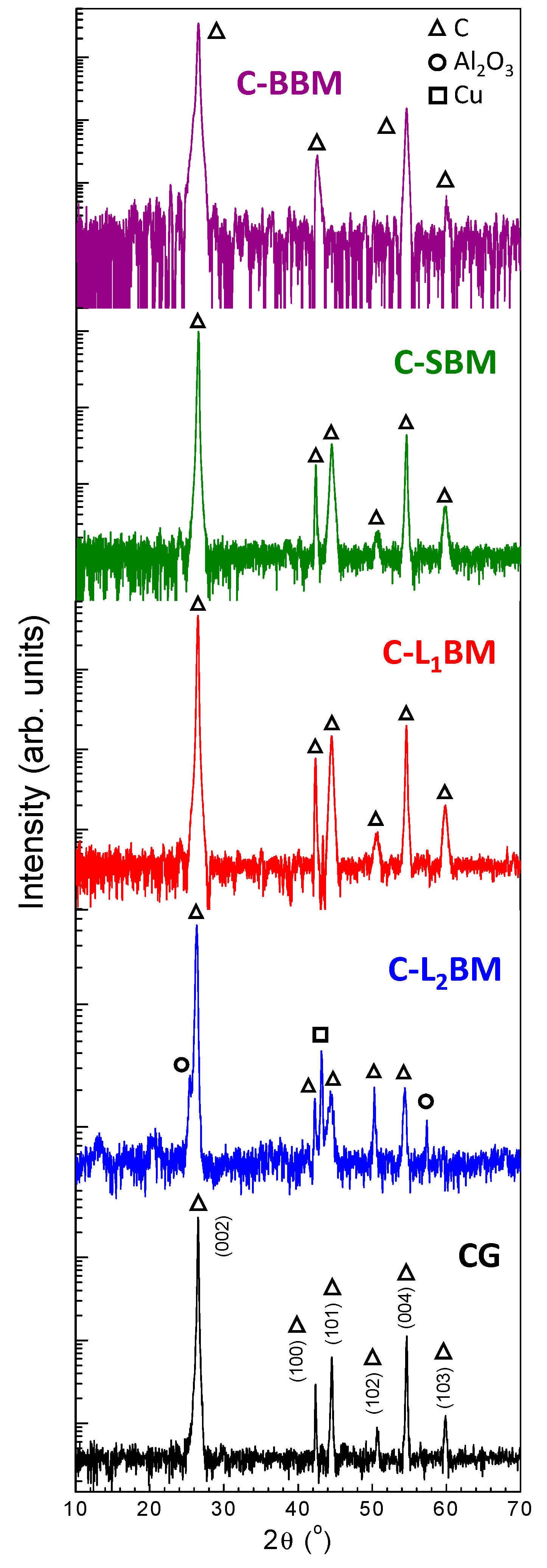
3.1. X-ray Fluorescence (XRF)
3.2. X-ray Diffraction (XRD)
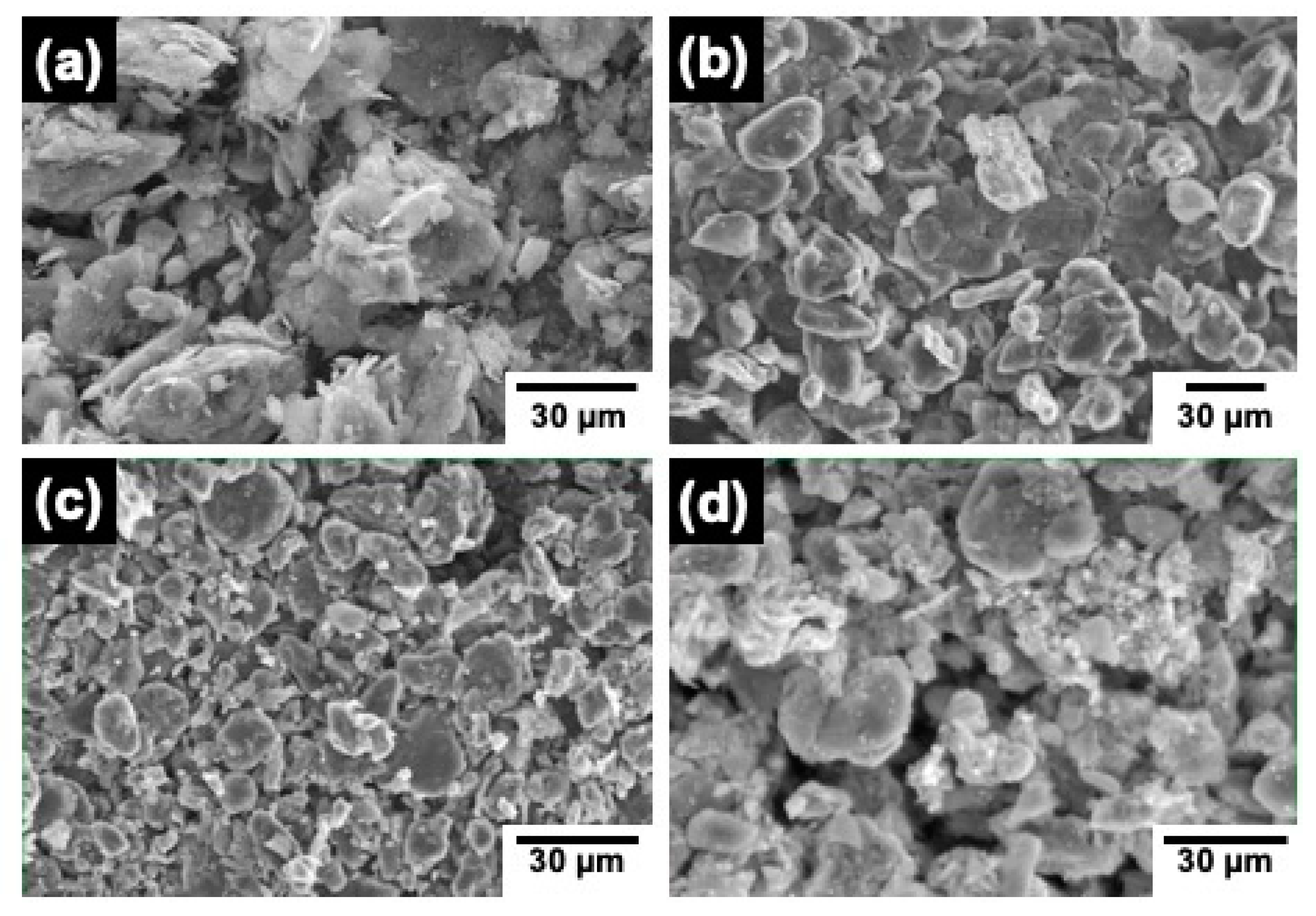
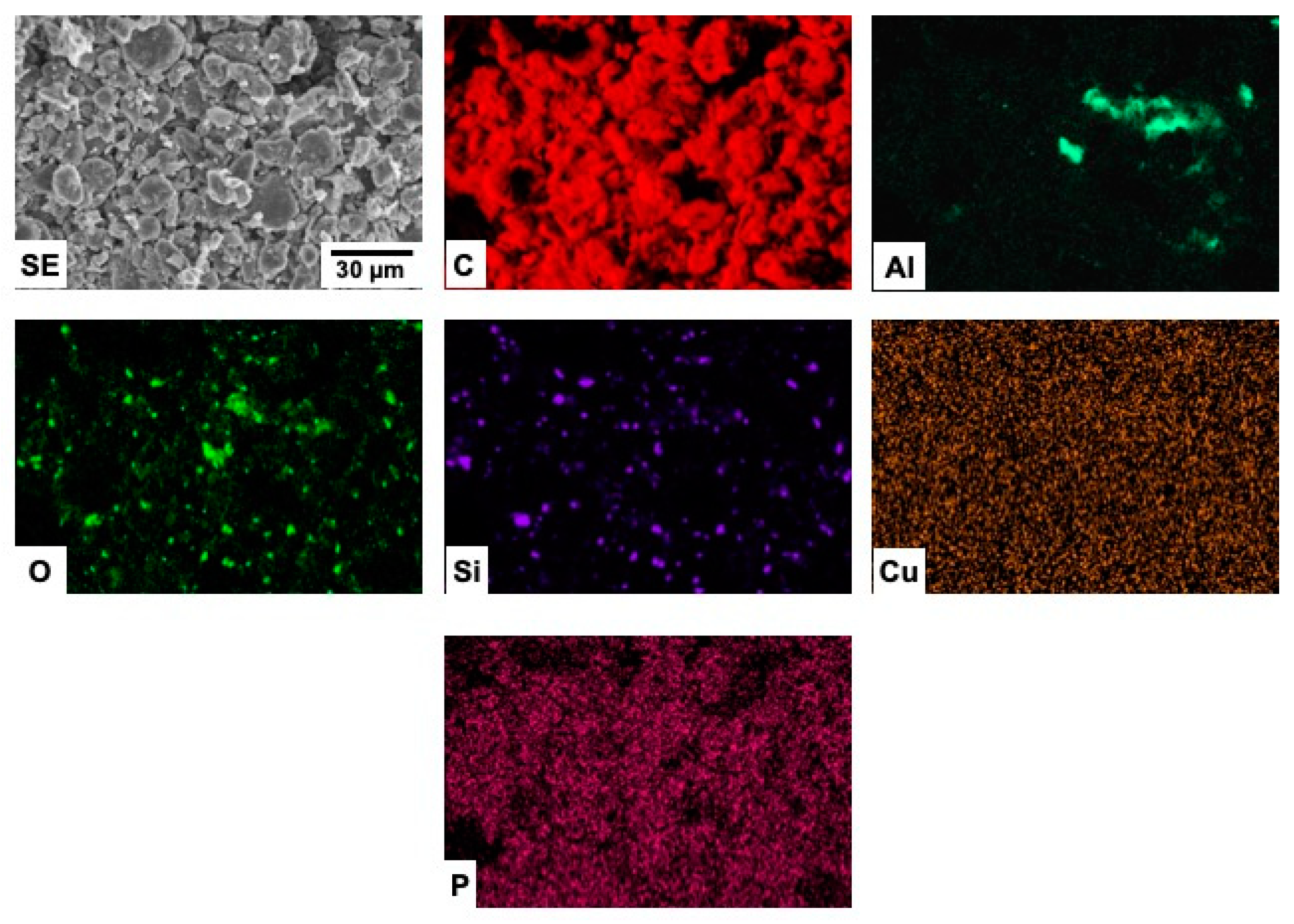
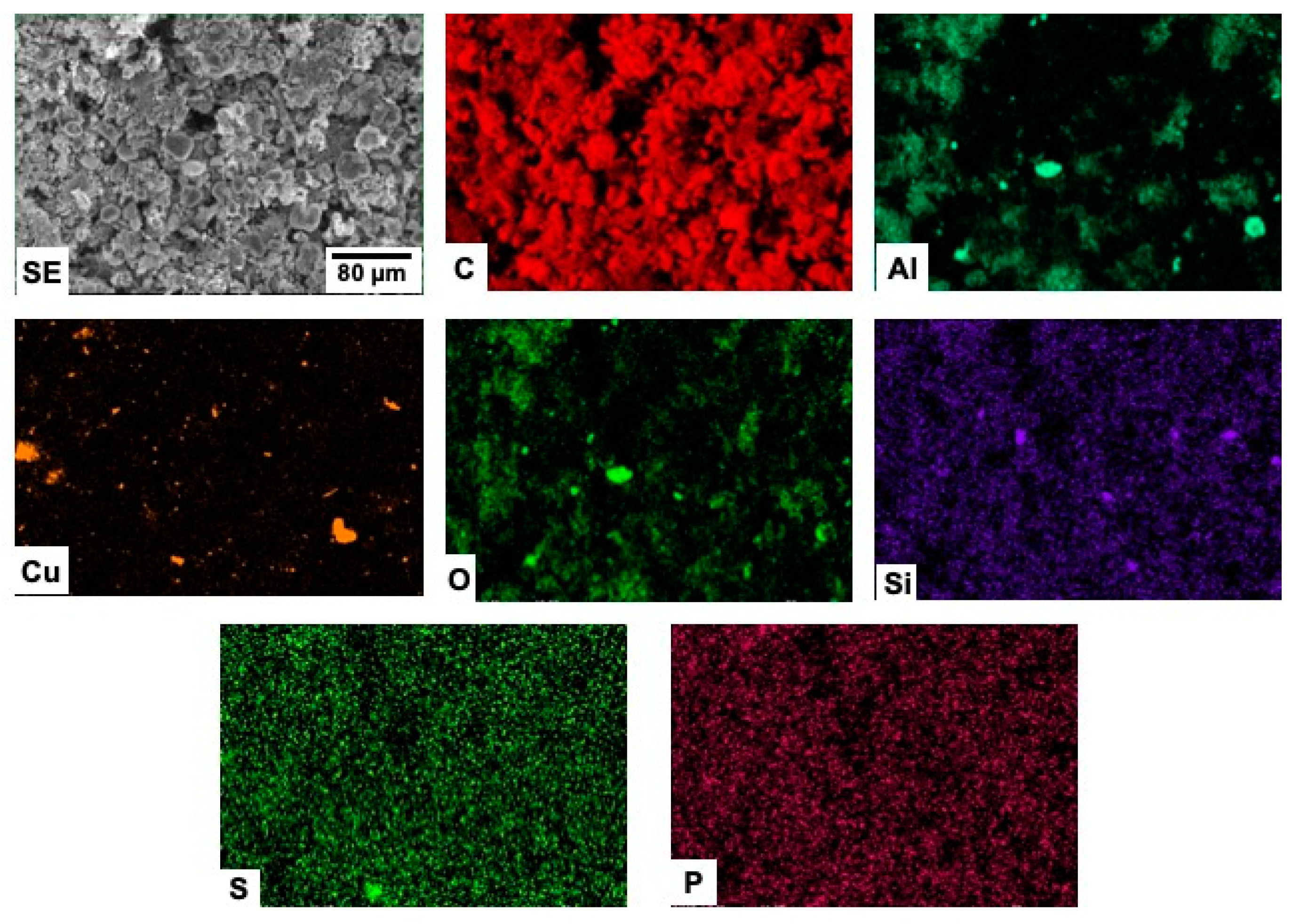
3.3. Scanning Electron Microscopy (SEM)
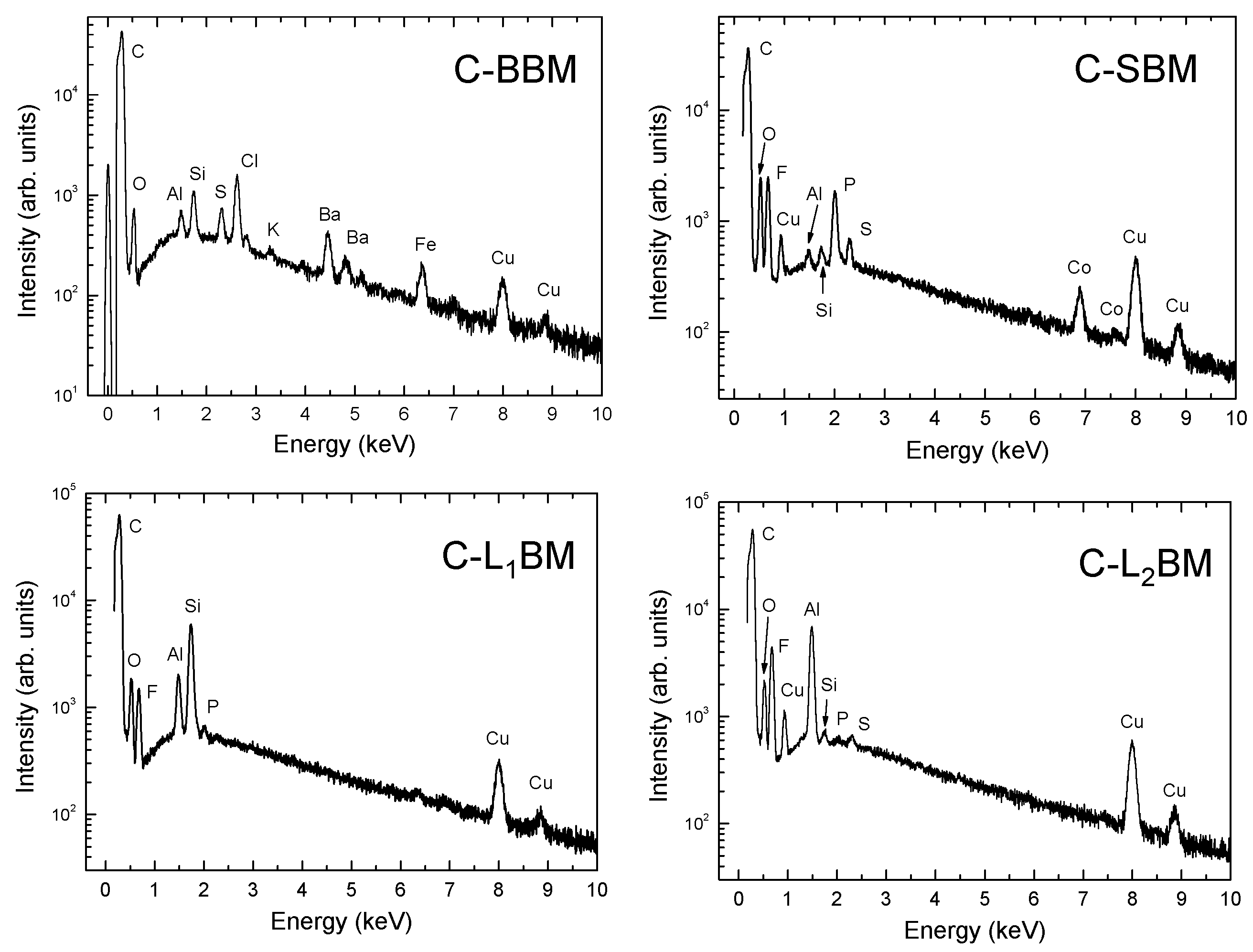
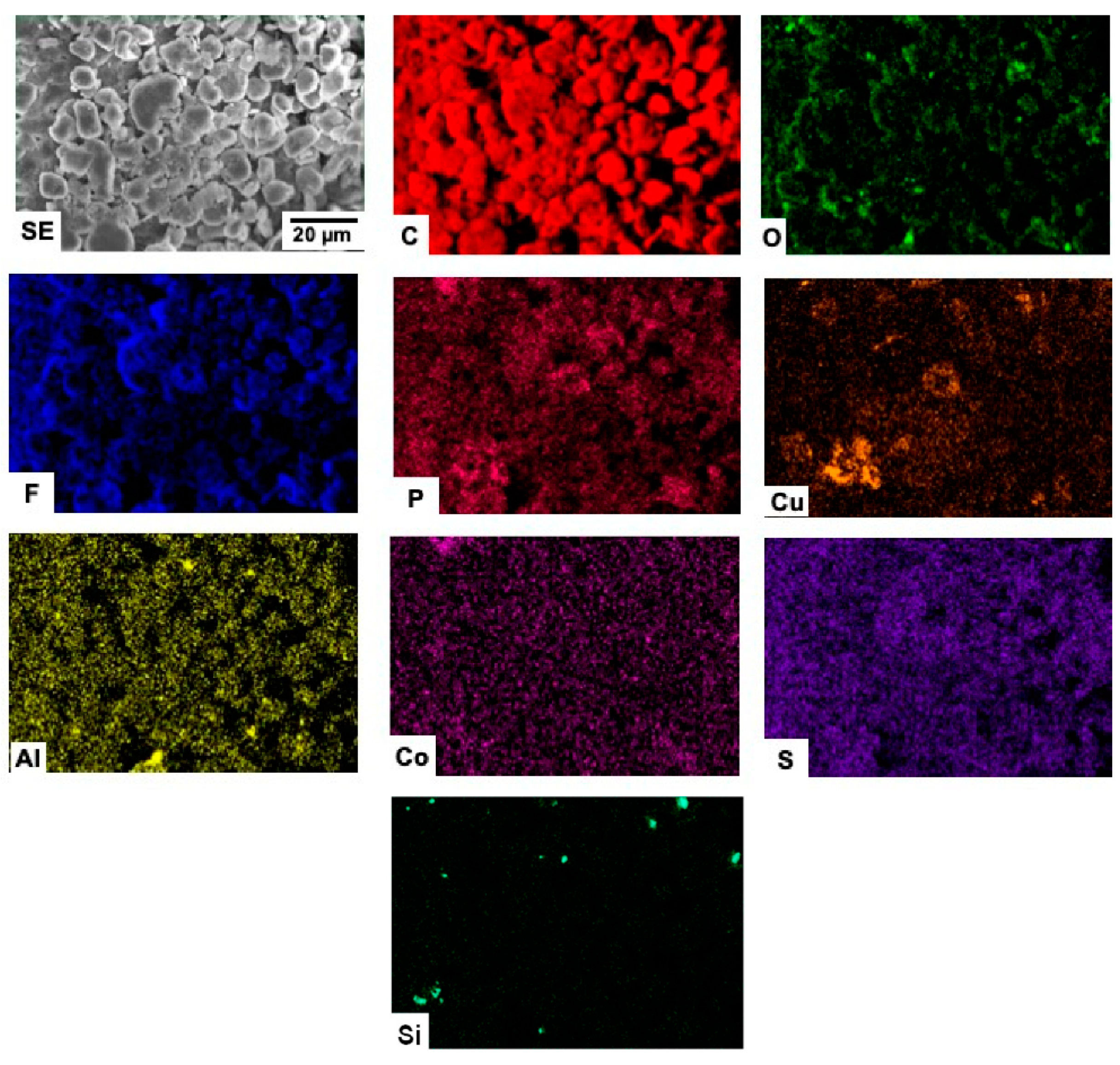
3.4. EDX Microanalyses
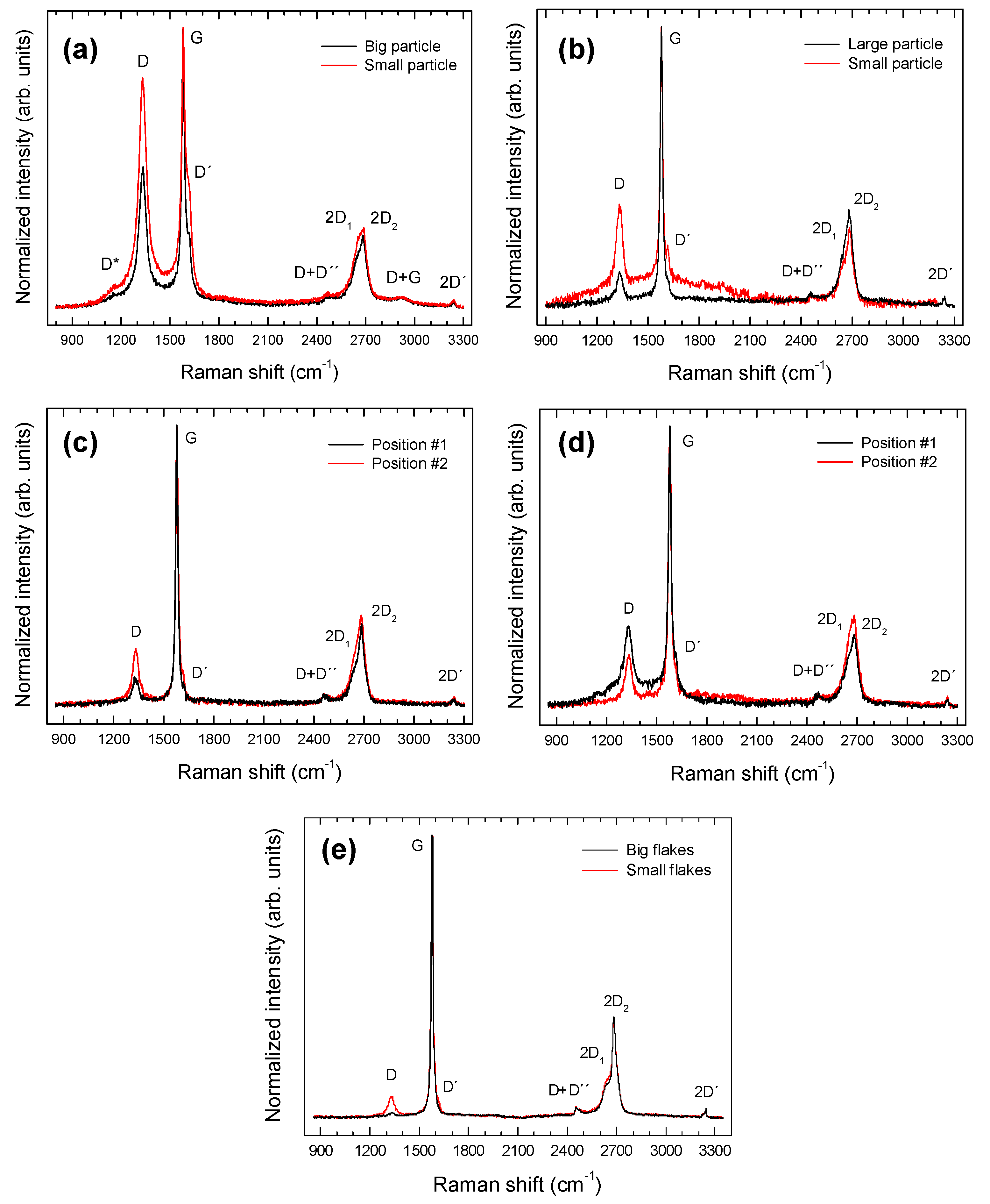
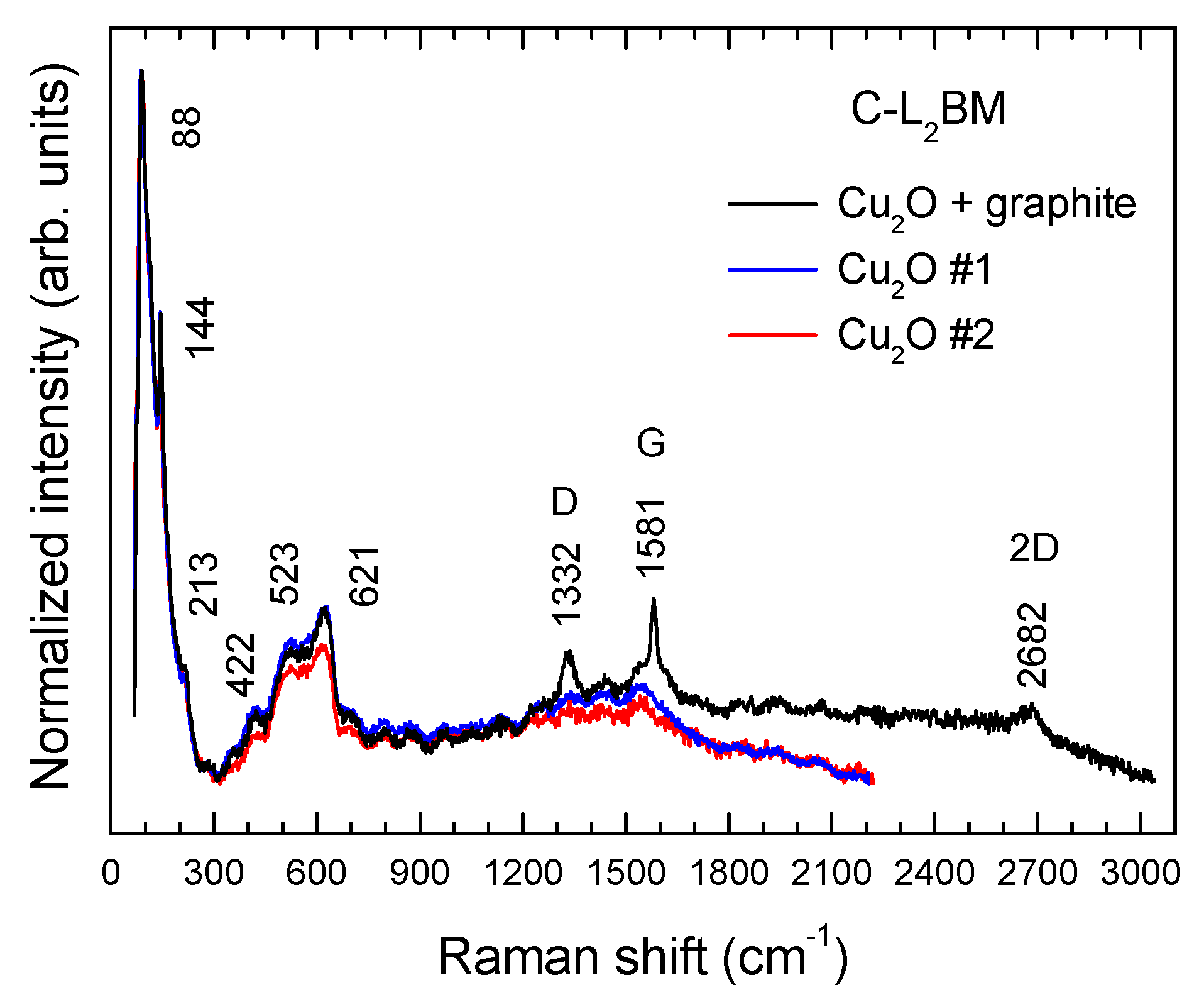
3.5. Micro-Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moradi, B.; Botte, G.G. Recycling of graphite anodes for the next generation of lithium ion batteries. J. Appl. Electrochem. 2016, 46, 123–148. [Google Scholar] [CrossRef]
- El-Hout, S.I.; Attia, S.Y.; Mohamed, S.G.; Abdelbasir, S.M. From waste to value-added products: Evaluation of activated carbon generated from leather waste for supercapacitor applications. J. Environ. Manag. 2022, 304, 114222. [Google Scholar] [CrossRef]
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling End-of-Life Electric Vehicle Lithium-Ion Batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Kang, D.H.P.; Chen, M.; Ogunseitan, O.A. Potential Environmental and Human Health Impacts of Rechargeable Lithium Batteries in Electronic Waste. Environ. Sci. Technol. 2013, 47, 5495–5503. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Hu, X.; Geng, Y.; Yang, L.; Shao, P.; Luo, X. Critical strategies for recycling process of graphite from spent lithium-ion batteries: A review. Sci. Total Environ. 2021, 816, 151621. [Google Scholar] [CrossRef] [PubMed]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Freitas, M.B.J.G.; Freitas, J.C.C. Recycling of graphite and metals from spent Li-ion batteries aiming the production of graphene/CoO-based electrochemical sensors. J. Environ. Chem. Eng. 2021, 9, 104689. [Google Scholar] [CrossRef]
- Yu, J.; He, Y.; Ge, Z.; Li, H.; Xie, W.; Wang, S. A promising physical method for recovery of LiCoO2 and graphite from spent lithium-ion batteries: Grinding flotation. Sep. Purif. Technol. 2018, 190, 45–52. [Google Scholar] [CrossRef]
- Rey, I.; Vallejo, C.; Santiago, G.; Iturrondobeitia, M.; Lizundia, E. Environmental Impacts of Graphite Recycling from Spent Lithium-Ion Batteries Based on Life Cycle Assessment. ACS Sustain. Chem. Eng. 2021, 9, 14488–14501. [Google Scholar] [CrossRef]
- Ma, X.; Chen, M.; Chen, B.; Meng, Z.; Wang, Y. High-Performance Graphite Recovered from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 19732–19738. [Google Scholar] [CrossRef]
- Rui, X.; Geng, Y.; Zhuang, M.; Xiao, S.; Sun, X. Emergy-based environmental accounting of graphite anode material production. J. Clean. Prod. 2022, 339, 130705. [Google Scholar] [CrossRef]
- Robshaw, T.J.; Atkinson, D.; Howse, J.R.; Ogden, M.D.; Cumming, D.J. Recycling graphite from waste aluminium smelter Spent Pot Lining into lithium-ion battery electrode feedstock. Clean. Prod. Lett. 2022, 2, 100004. [Google Scholar] [CrossRef]
- Bobba, S.; Carrara, S.; Huisman, J.; Mathieux, F.; Pavel, C. Critical Raw Materials for Strategic Technologies and Sectors in the EU—A Foresight Study; Technical Report; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Yang, Y.; Song, S.; Lei, S.; Sun, W.; Hou, H.; Jiang, F.; Ji, X.; Zhao, W.; Hu, Y. A process for combination of recycling lithium and regenerating graphite from spent lithium-ion battery. Waste Manag. 2019, 85, 529–537. [Google Scholar] [CrossRef]
- Badawy, S.M. Synthesis of high-quality graphene oxide from spent mobile phone batteries. Environ. Prog. Sustain. Energy 2016, 35, 1485–1491. [Google Scholar] [CrossRef]
- Aravindan, V.; Jayaraman, S.; Tedjar, F.; Madhavi, S. From Electrodes to Electrodes: Building High-Performance Li-Ion Capacitors and Batteries from Spent Lithium-Ion Battery Carbonaceous Materials. ChemElectroChem 2019, 6, 1407–1412. [Google Scholar] [CrossRef]
- Alcaraz Romo, L.; López-Fernández, A.; García-Díaz, I.; Fernández, P.; Urbieta, A.; López, F.A. From spent alkaline batteries to ZnxMn3−xO4 by a hydrometallurgical route: Synthesis and characterization. RSC Adv. 2018, 8, 33496–33505. [Google Scholar] [CrossRef] [Green Version]
- Qiu, T.; Yang, J.G.; Bai, X.J.; Wang, Y.L. The preparation of synthetic graphite materials with hierarchical pores from lignite by one-step impregnation and their characterization as dye absorbents. RSC Adv. 2019, 9, 12737–12746. [Google Scholar] [CrossRef] [Green Version]
- Alcaraz, L.; García-Díaz, I.; González, L.; Rabanal, M.E.; Urbieta, A.; Fernández, P.; López, F.A. New photocatalytic materials obtained from the recycling of alkaline and Zn/C spent batteries. J. Mater. Res. Technol. 2019, 8, 2809–2818. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, S.; Song, S.; Sun, W.; Wang, L. Stepwise recycling of valuable metals from Ni-rich cathode material of spent lithium-ion batteries. Waste Manag. 2020, 102, 131–138. [Google Scholar] [CrossRef]
- Gerold, E.; Schinnerl, C.; Antrekowitsch, H. Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries. Recycling 2022, 7, 4. [Google Scholar] [CrossRef]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Lithium Carbonate Recovery from Cathode Scrap of Spent Lithium-Ion Battery: A Closed-Loop Process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, W. Recovery of Co, Li, and Ni from Spent Li-Ion Batteries by the Inorganic and/or Organic Reducer Assisted Leaching Method. Minerals 2020, 10, 555. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Maire, J.; Mering, J. Chemistry and Physics of Carbon; Walker, P.L., Jr., Ed.; Marcel Dekker: New York, NY, USA, 1970; Volume 6, p. 125. [Google Scholar]
- Franklin, R.E. The structure of graphitic carbons. Acta Crystallogr. 1951, 4, 253–261. [Google Scholar] [CrossRef]
- Franklin, R.E. Crystallite growth in graphitizing and non-graphitizing carbons. Proc. R. Soc. Lond. A 1951, 209, 196–218. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef] [Green Version]
- Lavi, O.; Luski, S.; Shpigel, N.; Menachem, C.; Pomerantz, Z.; Elias, Y.; Aurbach, D. Electrolyte Solutions for Rechargeable Li-Ion Batteries Based on Fluorinated Solvents. ACS Appl. Energy Mater. 2020, 3, 7485–7499. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C 2015, 1, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Zhao, X.; Li, T.; Lv, Z.; Lv, S.; Han, H.; Yin, Y.; Wang, H. First-principles investigation in the Raman and infrared spectra of sp3 carbon allotropes. Carbon N. Y. 2014, 78, 70–78. [Google Scholar] [CrossRef]
- Ergun, S. Structure of carbon. Carbon N. Y. 1968, 6, 141–159. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Jorio, A. Raman Spectroscopy in Graphene-Based Systems: Prototypes for Nanoscience and Nanometrology. ISRN Nanotechnol. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [Green Version]
- May, P.; Lazzeri, M.; Venezuela, P.; Herziger, F.; Callsen, G.; Reparaz, J.S.; Hoffmann, A.; Mauri, F.; Maultzsch, J. Signature of the two-dimensional phonon dispersion in graphene probed by double-resonant Raman scattering. Phys. Rev. B 2013, 87, 075402. [Google Scholar] [CrossRef] [Green Version]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Origin of the 1150 cm−1 Raman mode in nanocrystalline diamond. Phys. Rev. B 2001, 63, 121405. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Pimenta, M.A. Measuring the absolute Raman cross section of nanographites as a function of laser energy and crystallite size. Phys. Rev. B 2007, 76, 064304. [Google Scholar] [CrossRef]
- Yoshida, A.; Kaburagi, Y.; Hishiyama, Y. Full width at half maximum intensity of the G band in the first order Raman spectrum of carbon material as a parameter for graphitization. Carbon N. Y. 2006, 44, 2333–2335. [Google Scholar] [CrossRef]
- Singhal, A.; Pai, M.R.; Rao, R.; Pillai, K.T.; Lieberwirth, I.; Tyagi, A.K. Copper(I) Oxide Nanocrystals—One Step Synthesis, Characterization, Formation Mechanism, and Photocatalytic Properties. Eur. J. Inorg. Chem. 2013, 2013, 2640–2651. [Google Scholar] [CrossRef]
- Zhang, X.; Mauger, A.; Lu, Q.; Groult, H.; Perrigaud, L.; Gendron, F.; Julien, C.M. Synthesis and characterization of LiNi1/3Mn1/3Co1/3O2 by wet-chemical method. Electrochim. Acta 2010, 55, 6440–6449. [Google Scholar] [CrossRef]
- Dawson, P.; Hargreave, M.M.; Wilkinson, G.R. The dielectric and lattice vibrational spectrum of cuprous oxide. J. Phys. Chem. Solids 1973, 34, 2201–2208. [Google Scholar] [CrossRef]
- Shi, M.; Tai, Z.; Li, N.; Zou, K.; Chen, Y.; Sun, J.; Liu, Y. Spherical graphite produced by waste semi-coke with enhanced properties as anode material for Li-ion batteries. Sustain. Energy Fuels 2019, 3, 3116–3127. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]

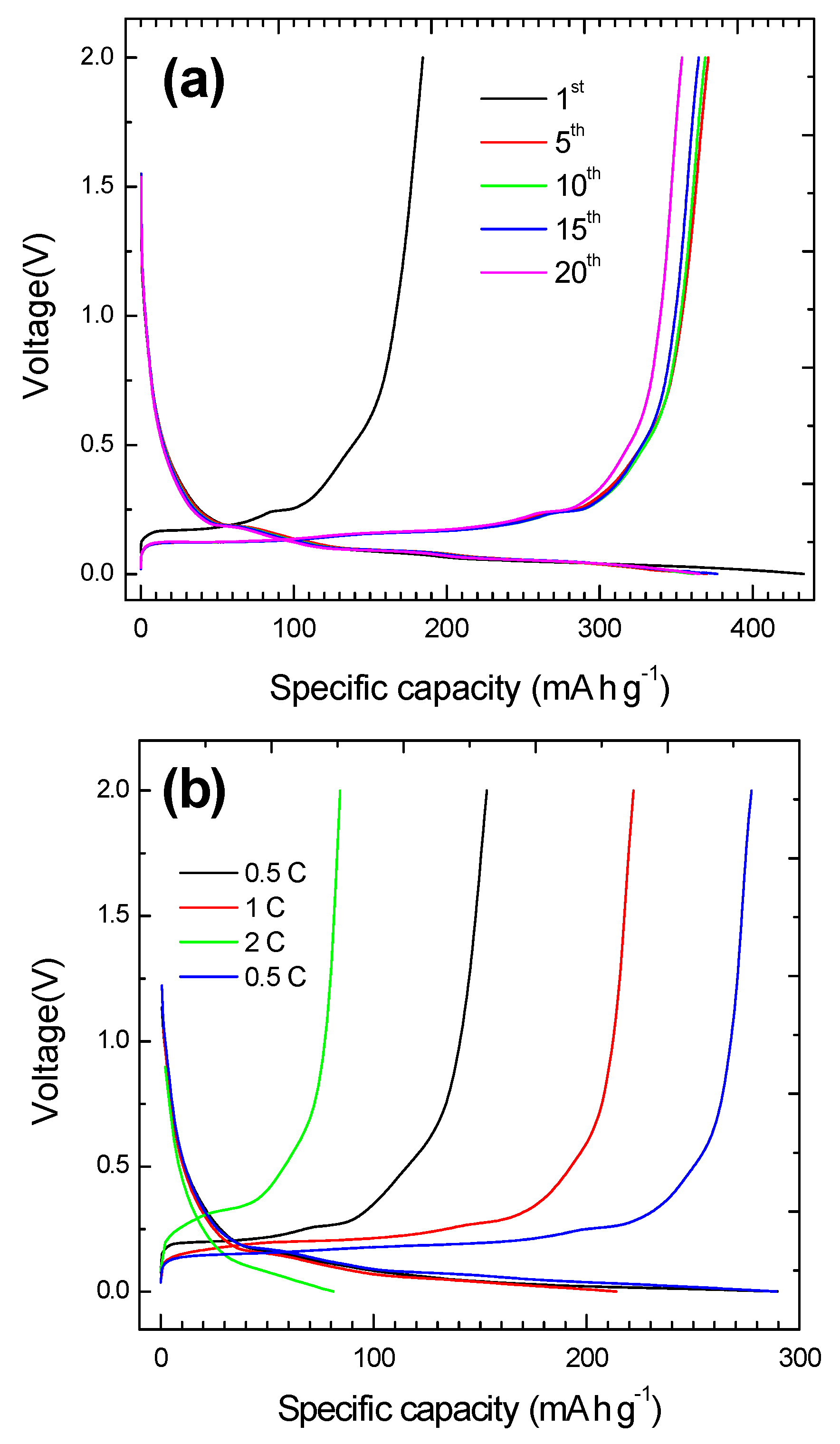
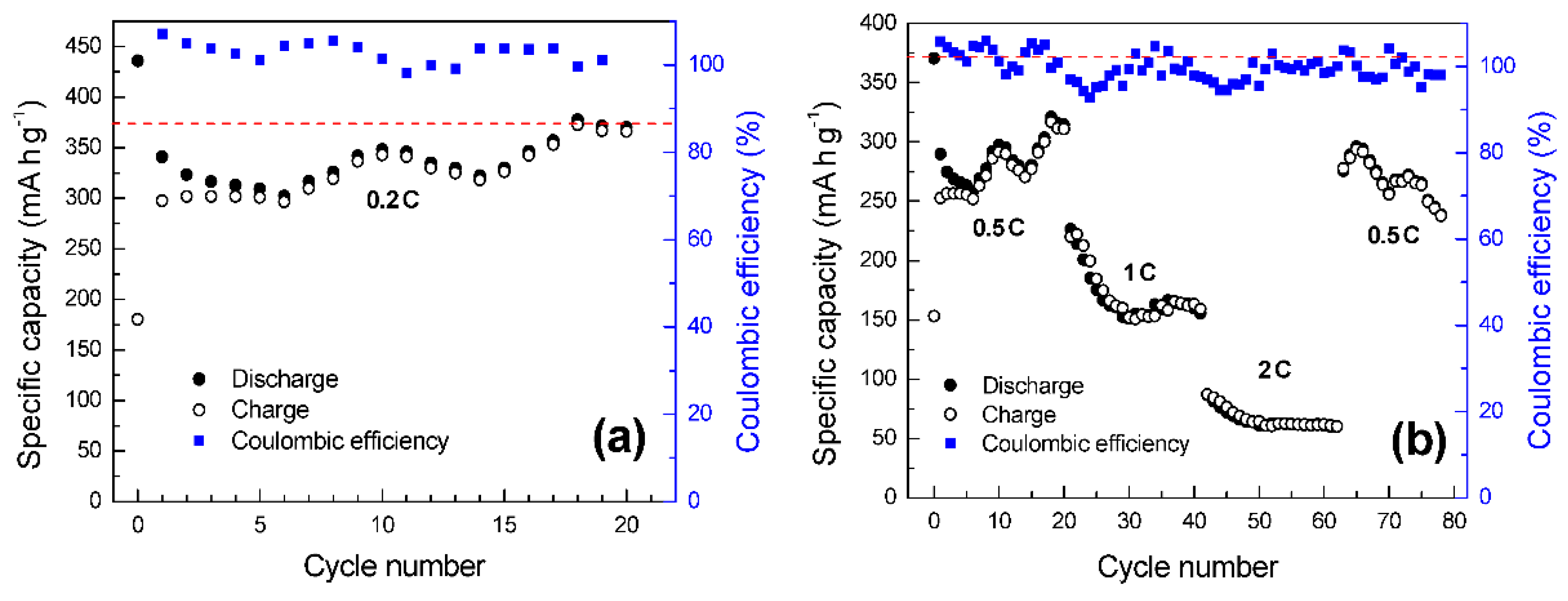
| Sample Notation | Black Mass | Leaching Conditions |
|---|---|---|
| C-BBM | Black mass from spent Zn/C alkaline batteries | 6 M HCl/H2O2 (25% v/v) |
| C-SBM | Black mass from spent smartphone lithium-ion batteries | Untreated |
| C-L1BM | Black mass from spent vehicles lithium-ion batteries | 2 M H2SO4/H2O2 (5 % v/v) |
| C-L2BM | Black mass from spent vehicles lithium-ion batteries | 1.25 M C6H8O7/H2O2 (1 % v/v) |
| Compound | C-BBM | C-SBM | C-LBM |
|---|---|---|---|
| Na2O | 7.75 | 0.06 | - |
| MgO | 0.13 | - | - |
| Al2O3 | 0.46 | 0.04 | 2.25 |
| SiO2 | 0.19 | 0.08 | 0.06 |
| P2O5 | 0.99 | 0.75 | 1.08 |
| SO3 | 0.64 | 0.15 | 0.21 |
| Cl | 1.76 | - | 0.04 |
| K2O | 6.70 | - | - |
| CaO | 0.36 | - | 0.07 |
| TiO2 | 0.16 | - | 0.01 |
| MnO | 41.40 | 0.04 | 6.45 |
| Fe2O3 | 1.42 | 0.02 | 0.03 |
| Co3O4 | 0.03 | 0.20 | 4.43 |
| NiO | 0.18 | - | 6.84 |
| CuO | 0.08 | 0.94 | 1.17 |
| ZnO | 26.88 | - | 0.95 |
| SrO | 0.04 | - | - |
| ZrO2 | 0.01 | - | 0.14 |
| CdO | 0.01 | - | - |
| SnO2 | 0.03 | - | 0.01 |
| PbO | 0.04 | - | - |
| Others | 10.74 | 97.92 | 76.26 |
| C | 5.42 | 85.59 | 33.87 |
| Sample | d002 (nm) | g (%) | Lc (nm) |
|---|---|---|---|
| C-BBM | 0.3363 | 87 | 30.47 |
| C-SBM | 0.3358 | 92 | 40.62 |
| C-L1BM | 0.3356 | 96 | 44.90 |
| C-L2BM | 0.3360 | 91 | 32.81 |
| CG | 0.3355 | 97 | 45.14 |
| Element | C-BBM | C-SBM | C-L1BM | C-L2BM |
|---|---|---|---|---|
| Carbon | 80.97 | 56.4 | 73.65 | 55.4 |
| Oxygen | 14.77 | 15.1 | 14.86 | 9.523 |
| Barium | 1.29 | - | - | - |
| Chlorine | 1.20 | - | - | - |
| Iron | 0.49 | - | - | - |
| Silicon | 0.427 | 0.1 | 1.88 | 0.054 |
| Sulfur | 0.279 | 0.2 | - | 0.079 |
| Aluminum | 0.191 | 0.1 | 0.81 | 3.02 |
| Potassium | 0.082 | - | - | - |
| Copper | 0.3 | 2.4 | 0.8 | 3.81 |
| Fluorine | - | 54 | 7.85 | 28 |
| Cobalt | - | 0.59 | - | - |
| Phosphorus | - | 1.07 | 0.045 | 0.066 |
| Band | C-BBM | C-SBM | C-L1BM | C-L2BM | CG |
|---|---|---|---|---|---|
| D* | ~1155 | n.o. | n.o. | n.o. | n.o. |
| D | 1332 | 1332 | 1332 | 1333 | 1330 |
| G | 1581 | 1579 | 1579 | 1578 | 1580 |
| D′ | 1615 | 1617 | 1619 | 1616 | 1616 |
| D + D″ | ~2470 | ~2456 | ~2458 | ~2467 | 2467 |
| 2D1 | 2647 | 2646 | 2646 | 2653 | 2654 |
| 2D2 | 2686 | 2686 | 2686 | 2685 | 2687 |
| D + G | 2922 | n.o. | n.o. | n.o. | n.o. |
| 2D′ | 3241 | 3242 | 3240 | 3241 | 3241 |
| Intensity Ratio/Sample | C-BBM | C-SBM | C-L1BM | C-L2BM | CG |
|---|---|---|---|---|---|
| (ID/IG) | 0.50–0.82 | 0.1–0.3 | 0.11–0.21 | 0.19–0.29 | 0.02–0.27 |
| (ID´/IG) | 0.27–0.48 | 0.03–0.09 | 0.03–0.05 | 0.04–0.08 | 0.01–0.08 |
| FWMH (cm−1) | 19.6–24.9 | 16.7–17.4 | 16.5–17.7 | 19–20 | 13.6–18.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaraz, L.; Díaz-Guerra, C.; Calbet, J.; López, M.L.; López, F.A. Obtaining and Characterization of Highly Crystalline Recycled Graphites from Different Types of Spent Batteries. Materials 2022, 15, 3246. https://doi.org/10.3390/ma15093246
Alcaraz L, Díaz-Guerra C, Calbet J, López ML, López FA. Obtaining and Characterization of Highly Crystalline Recycled Graphites from Different Types of Spent Batteries. Materials. 2022; 15(9):3246. https://doi.org/10.3390/ma15093246
Chicago/Turabian StyleAlcaraz, Lorena, Carlos Díaz-Guerra, Joaquín Calbet, María Luisa López, and Félix A. López. 2022. "Obtaining and Characterization of Highly Crystalline Recycled Graphites from Different Types of Spent Batteries" Materials 15, no. 9: 3246. https://doi.org/10.3390/ma15093246
APA StyleAlcaraz, L., Díaz-Guerra, C., Calbet, J., López, M. L., & López, F. A. (2022). Obtaining and Characterization of Highly Crystalline Recycled Graphites from Different Types of Spent Batteries. Materials, 15(9), 3246. https://doi.org/10.3390/ma15093246








