Influence of Annealing Treatment on Microstructure and Properties of Ni-Rich NiTi Alloy Coating Prepared by Laser Cladding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Sample Preparation
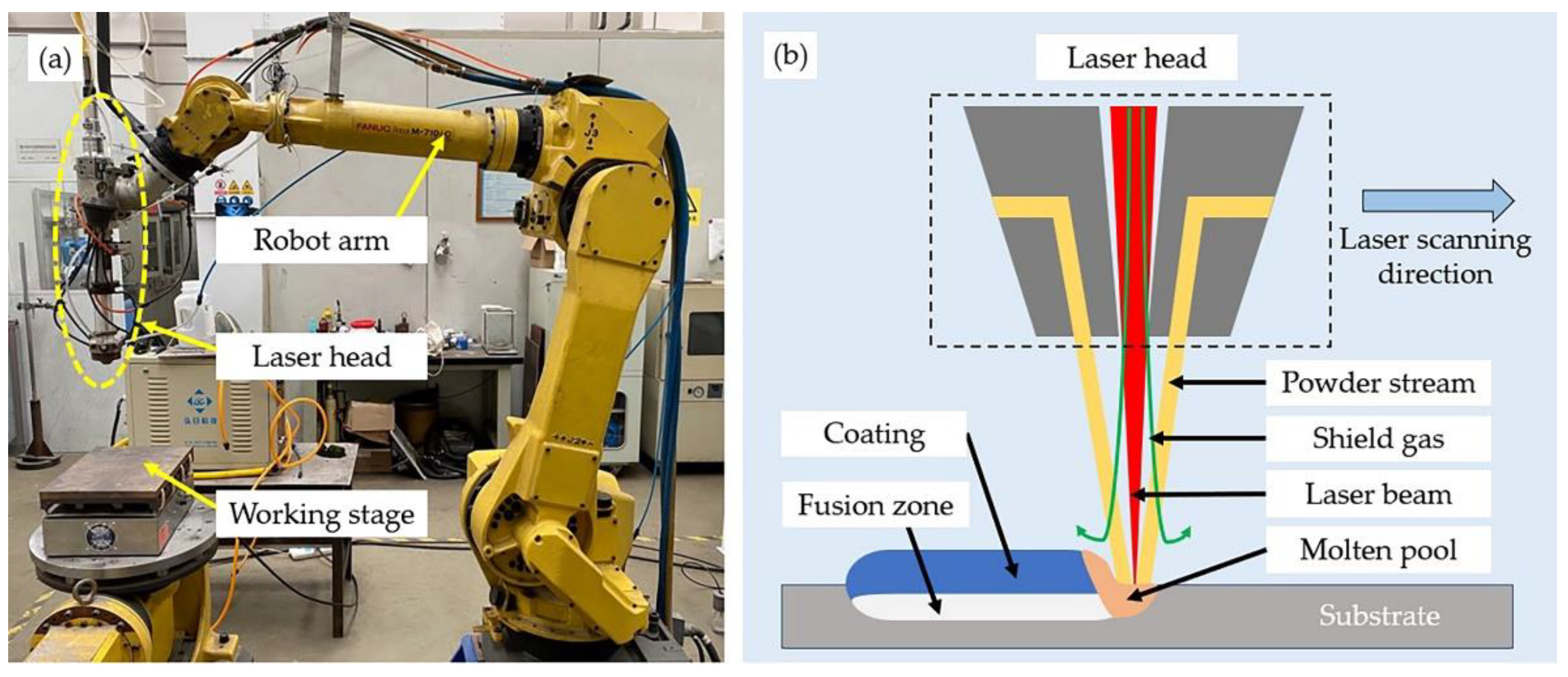
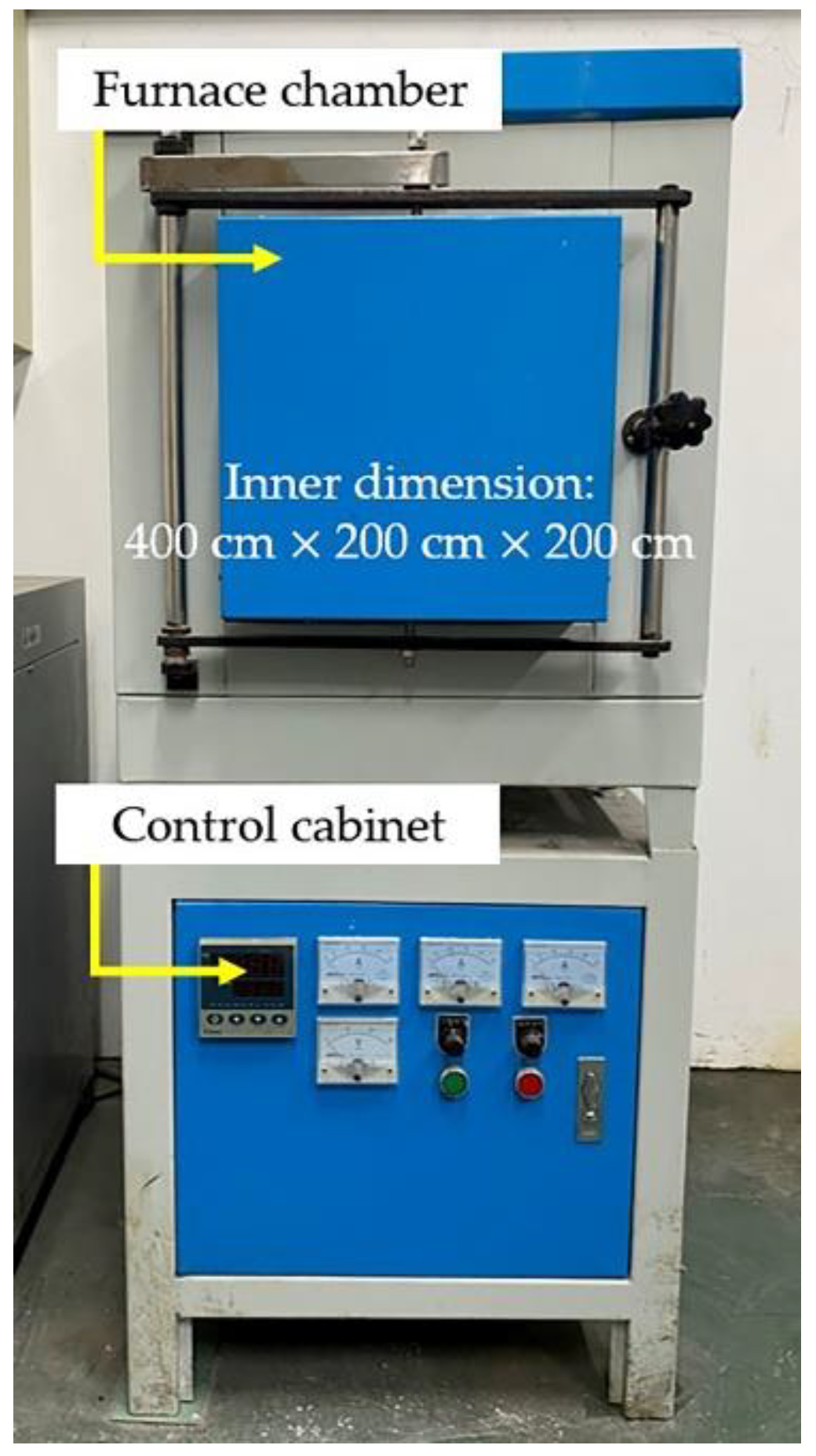
2.2. Experimental Procedure
2.3. Sample Characterization
3. Results and Discussions
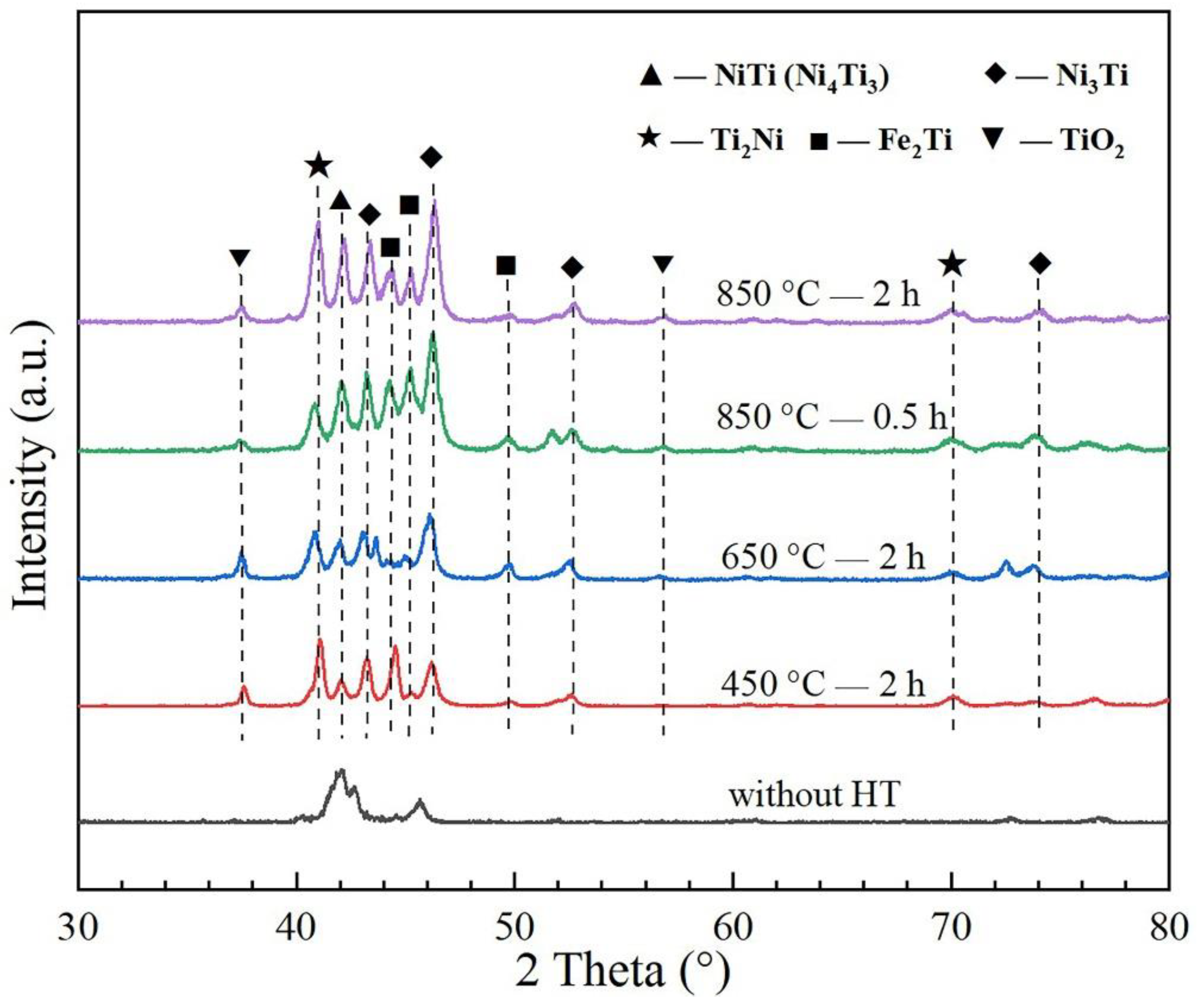
3.1. Coating Phase Composition Analysis
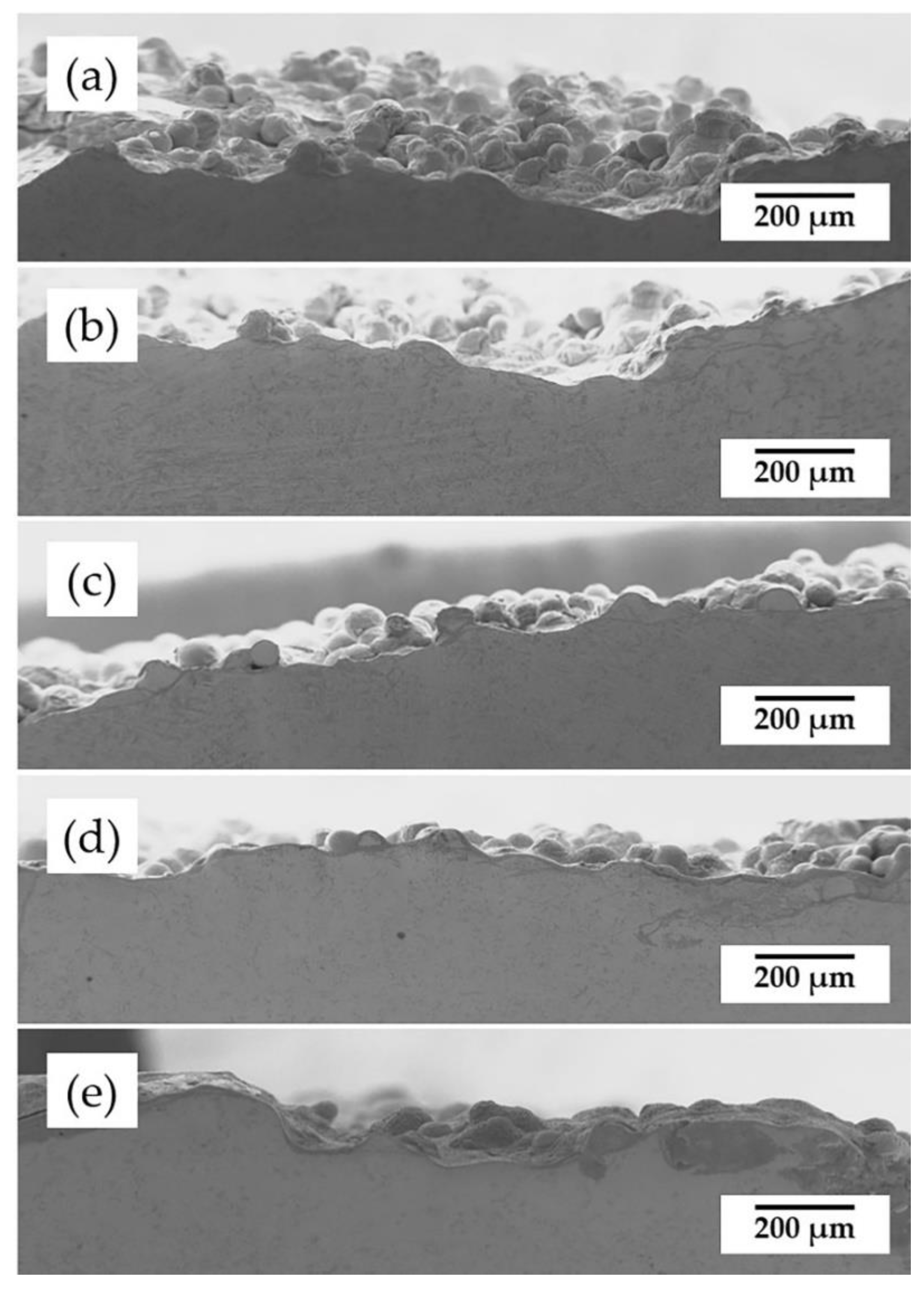
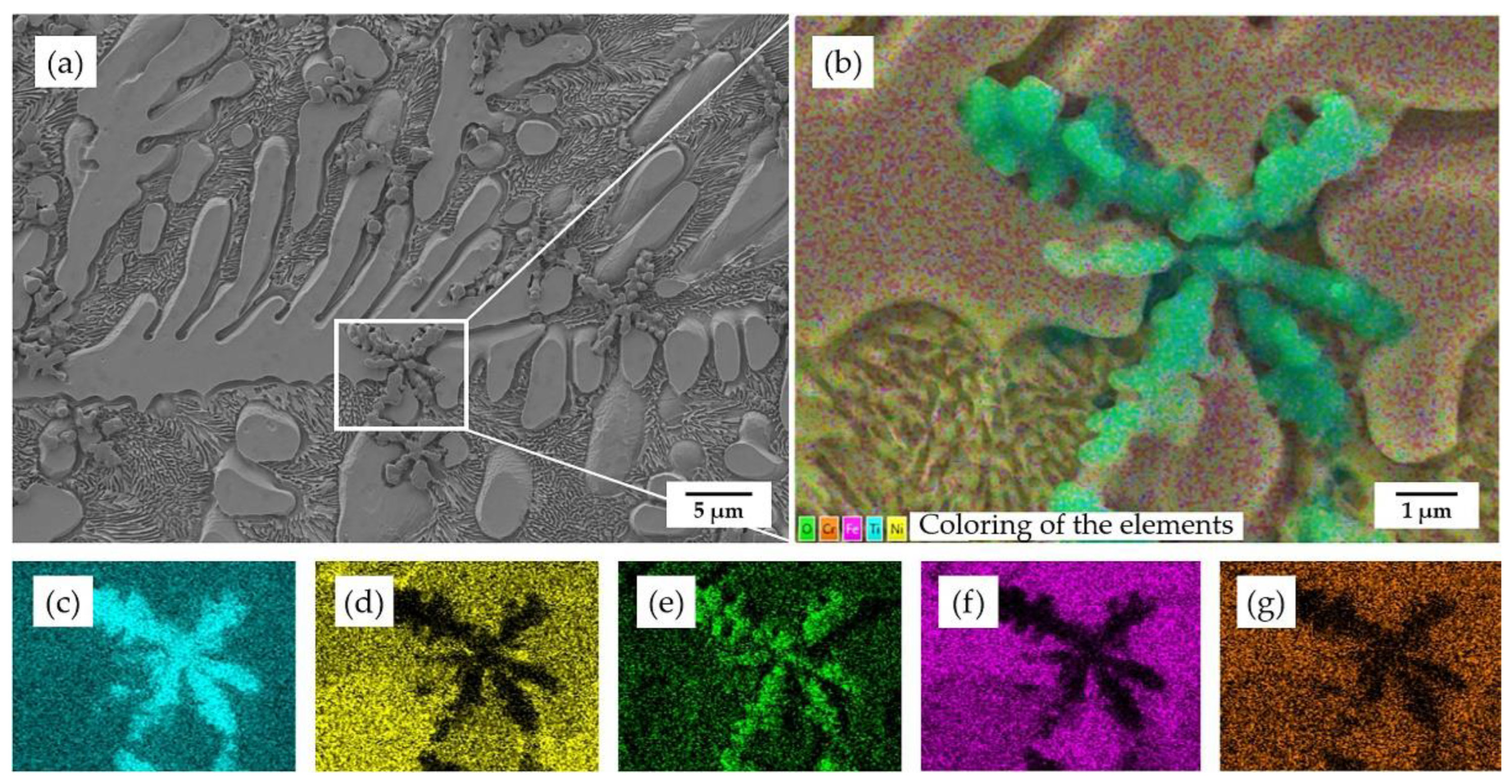
3.2. Coating Microstructural Analysis
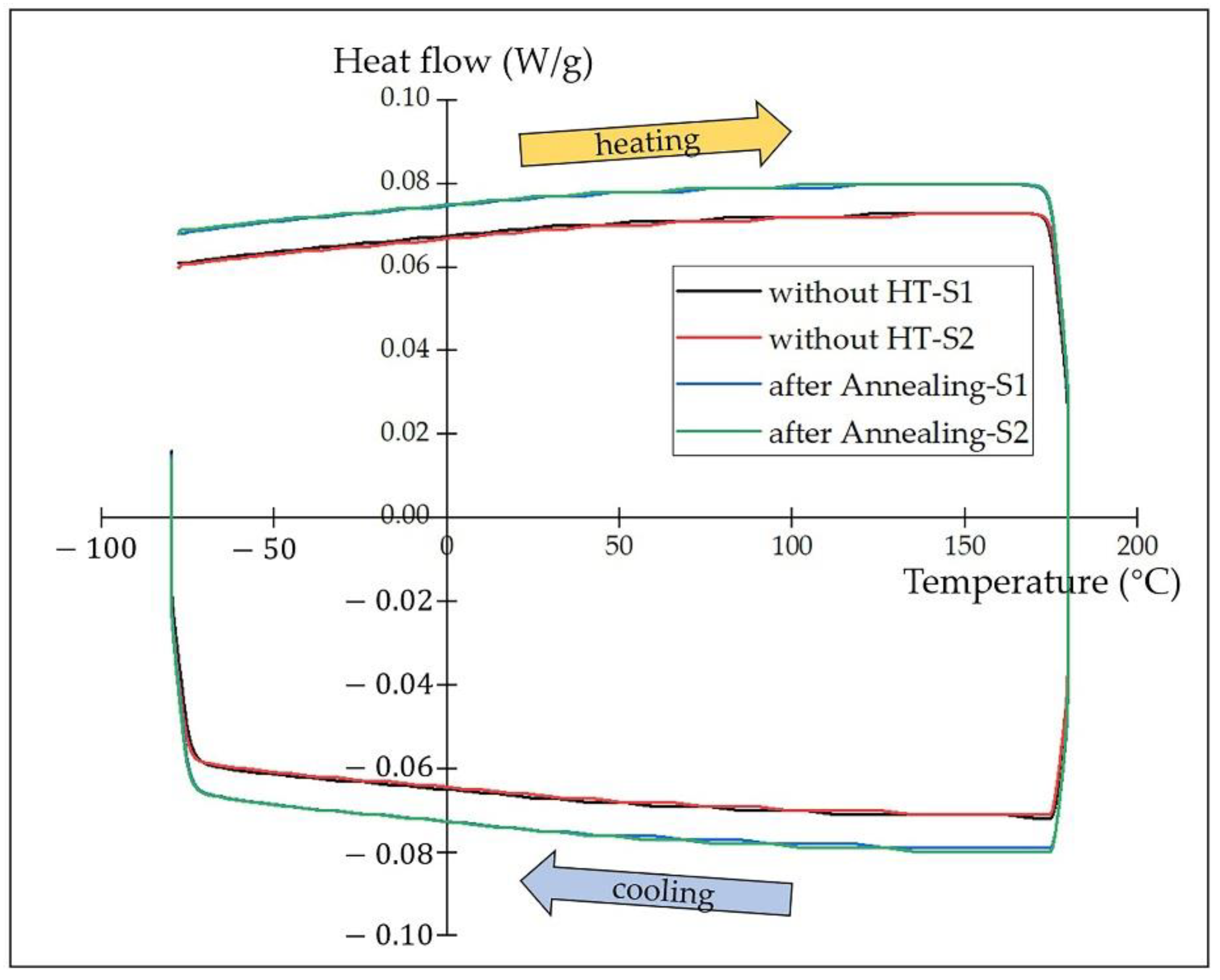
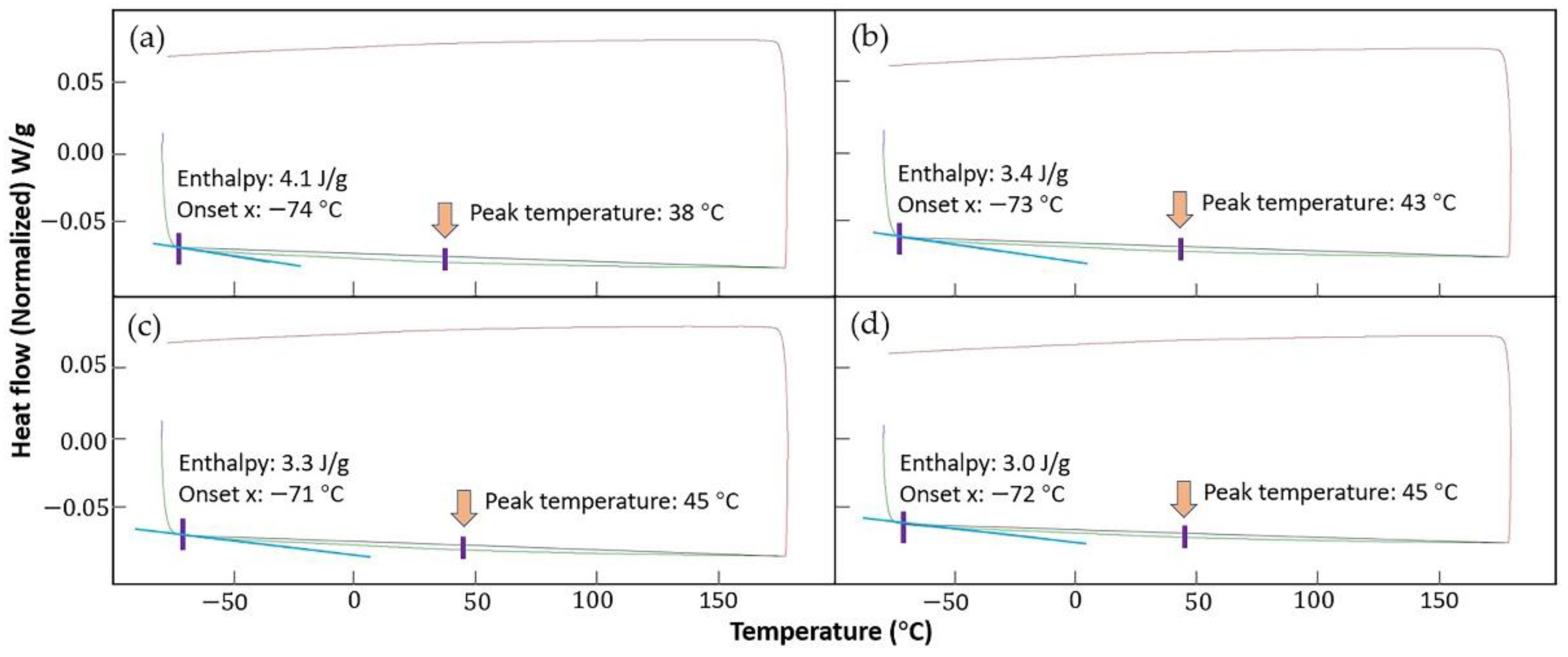
3.3. Coating Martensite Transformation Behavior
3.4. Coating Properties Analysis
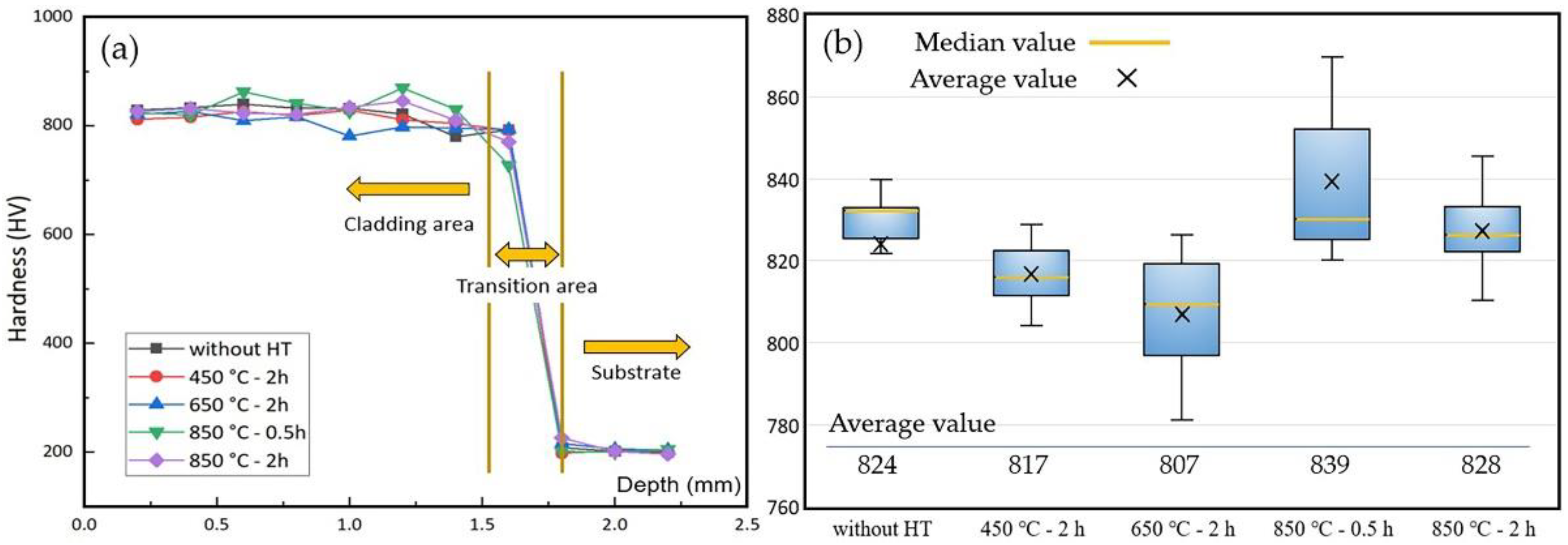
3.4.1. Coating Surface Hardness
3.4.2. Coating Wear Resistance
4. Conclusions
- The untreated coating mainly consists of NiTi and Ni3Ti phases with a typical dendrite structure. With the annealing temperature rising, the content of Ni3Ti and Ti2Ni visibly increased.
- The coating microstructure transformed from dendrite structure to single grains because of recrystallization, and the recrystallization temperature of the 55NiTi+5Ni coating is assumed to be between 650 °C and 850 °C.
- There is no martensite transformation observed in the DSC test. The martensite transformation could be suppressed by Fe atoms diffused from the substrate and probably retarded by the numerous metallic compounds after annealing as well. However, the software calculation results show that the phase-transition enthalpy decreased and the transformation temperature rose after annealing.
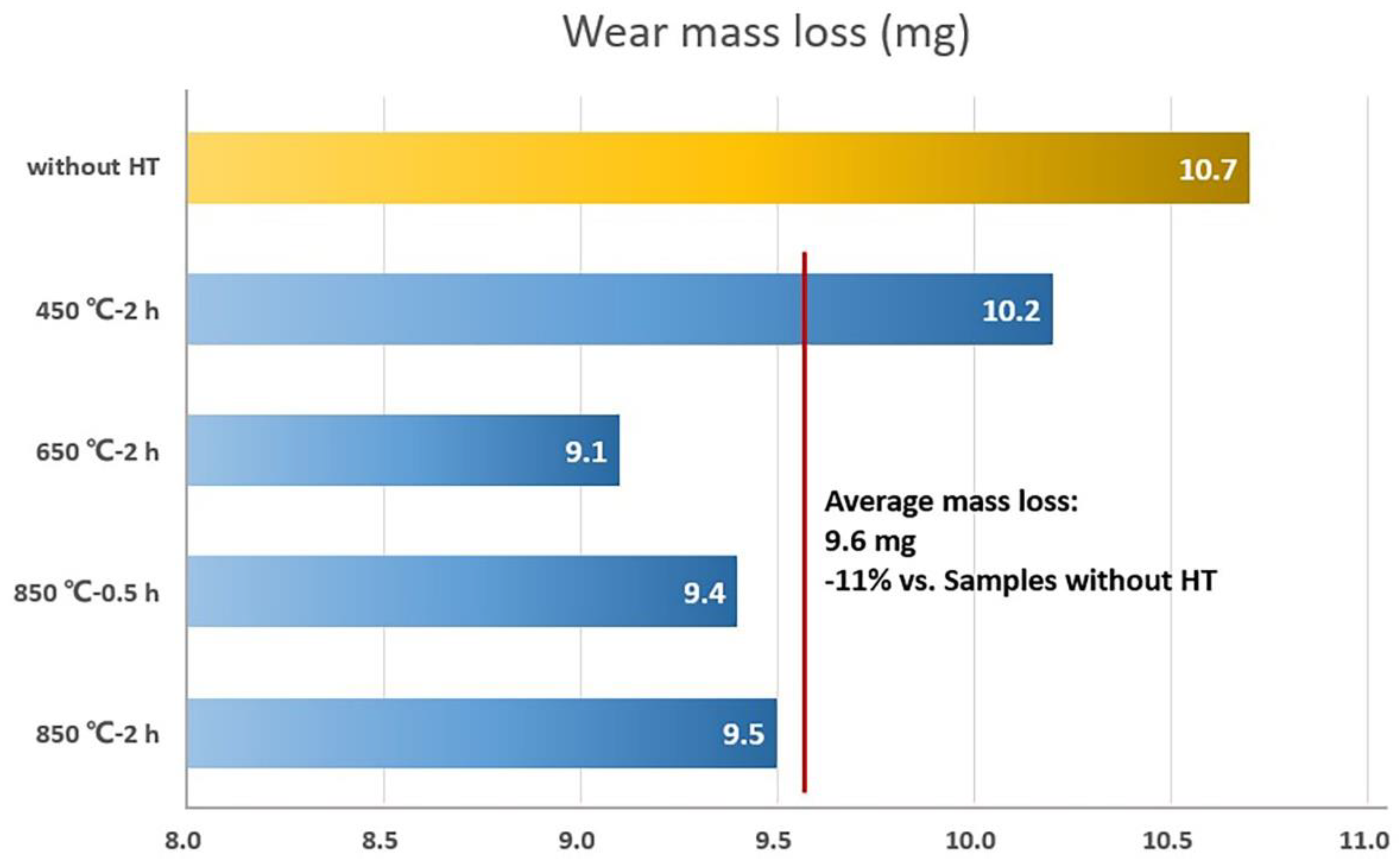
- The coating annealed under 850 °C shows the highest microhardness due to the precipitation hardening, and the wear resistance was also improved after an annealing treatment with an 11% average wear mass loss.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buehler, W.J.; Gilfrich, J.V.; Wiley, R.C. Effect of Low-Temperature Phase Changes on the Mechanical Properties of Alloys near Composition TiNi. J. Appl. Phys. 1963, 34, 1475–1477. [Google Scholar] [CrossRef]
- Jackson, C.; Wagner, H.; Wasilewski, R. 55-Nitinol-The Alloy with a Memory: It’s Physical Metallurgy Properties, and Applications. NASA SP-5110; NASA Special Publication: Washington, DC, USA, 1972; p. 5110. [Google Scholar]
- Shariat, B.S.; Liu, Y.; Rio, G. Pseudoelastic behaviour of perforated NiTi shape memory plates under tension. Intermetallics 2014, 50, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Yoneyama, T.; Miyazaki, S. Shape memory alloys for biomedical applications; Woodhead publishing limited: Cambridge, UK, 2009; p. 101. [Google Scholar]
- Otsuka, K.; Wayman, C.M. Shape memory materials; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Morgan, N. Medical shape memory alloy applications—the market and its products. Mater. Sci. Eng. A 2004, 378, 16–23. [Google Scholar] [CrossRef]
- Khanlari, K.; Ramezani, M.; Kelly, P. 60NiTi: A Review of Recent Research Findings, Potential for Structural and Mechanical Applications, and Areas of Continued Investigations. Trans. Indian Inst. Met. 2017, 71, 781–799. [Google Scholar] [CrossRef]
- Qin, Q.; Wen, Y.; Wang, G.; Zhang, L. Effects of Solution and Aging Treatments on Corrosion Resistance of As-cast 60NiTi Alloy. J. Mater. Eng. Perform. 2016, 25, 5167–5172. [Google Scholar] [CrossRef]
- Saburi, T.; Yoshida, M.; Nenno, S. Deformation behavior of shape memory Ti-Ni alloy crystals. Scr. Metall. 1984, 18, 363–366. [Google Scholar] [CrossRef]
- Wu, M.H. Fabrication of Nitinol Materials and Components. Mater. Sci. Forum 2002, 394–395, 285–292. [Google Scholar] [CrossRef]
- Kaya, E.; Kaya, İ. A review on machining of NiTi shape memory alloys: The process and post process perspective. Int. J. Adv. Manuf. Technol. 2019, 100, 2045–2087. [Google Scholar] [CrossRef]
- Ian Gibson, I.G. Additive manufacturing technologies 3D printing, rapid prototyping, and direct digital manufacturing. Johns. Matthey Technol.Rev. 2015, 59, 193–198. [Google Scholar]
- Elahinia, M.; Shayesteh Moghaddam, N.; Taheri Andani, M.; Amerinatanzi, A.; Bimber, B.A.; Hamilton, R.F. Fabrication of NiTi through additive manufacturing: A review. Prog. Mater. Sci. 2016, 83, 630–663. [Google Scholar] [CrossRef] [Green Version]
- Polozov, I.; Popovich, A. Microstructure and Mechanical Properties of NiTi-Based Eutectic Shape Memory Alloy Produced via Selective Laser Melting In-Situ Alloying by Nb. Materials 2021, 14, 2696. [Google Scholar] [CrossRef] [PubMed]
- Norhafzan, B.; Aqida, S.N.; Chikarakara, E.; Brabazon, D. Surface modification of AISI H13 tool steel by laser cladding with NiTi powder. Appl. Phys. A 2016, 122, 1–6. [Google Scholar] [CrossRef]
- Mokgalaka, M.N.; Popoola, A.P.I.; Pityana, S.L. In situ laser deposition of NiTi intermetallics for corrosion improvement of Ti–6Al–4V alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3315–3322. [Google Scholar] [CrossRef]
- Liu, F.; Mao, Y.; Lin, X.; Zhou, B.; Qian, T. Microstructure and high temperature oxidation resistance of Ti-Ni gradient coating on TA2 titanium alloy fabricated by laser cladding. Opt. Laser Technol. 2016, 83, 140–147. [Google Scholar] [CrossRef]
- Saedi, S.; Moghaddam, N.; Amerinatanzi, A.; Elahinia, M.; Karaca, H. On the effects of selective laser melting process parameters on microstructure and thermomechanical response of Ni-rich NiTi. Acta Mater. 2018, 144, 552–560. [Google Scholar] [CrossRef]
- Zhang, Q.; Hao, S.; Liu, Y.; Xiong, Z.; Guo, W.; Yang, Y.; Ren, Y.; Cui, L.; Ren, L.; Zhang, Z. The microstructure of a selective laser melting (SLM)-fabricated NiTi shape memory alloy with superior tensile property and shape memory recoverability. Appl. Mater. Today 2020, 19, 100547. [Google Scholar] [CrossRef]
- Katz-Demyanetz, A.; Koptyug, A.; Popov, V.V. In-situ Alloying as a Novel Methodology in Additive Manufacturing. In Proceedings of the 2020 IEEE 10th International Conference Nanomaterials: Applications & Properties (NAP), Sumy, Ukraine, 9–13 November 2020; pp. 02SAMA05-01–02SAMA05-04. [Google Scholar]
- Okamoto, H.; Okamoto, H. Phase diagrams for binary alloys; ASM international: Materials Park, OH, USA, 2000; Volume 44. [Google Scholar]
- Tadayyon, G.; Mazinani, M.; Guo, Y.; Zebarjad, S.M.; Tofail, S.A.M.; Biggs, M.J. The effect of annealing on the mechanical properties and microstructural evolution of Ti-rich NiTi shape memory alloy. Mater. Sci.Eng. A 2016, 662, 564–577. [Google Scholar] [CrossRef]
- Khanlari, K.; Shi, Q.; Li, K.; Xu, P.; Cao, P.; Liu, X. An investigation into the possibility to eliminate the microstructural defects of parts printed using a Ni-rich Ni-Ti elemental powder mixture. Mater. Res. Express 2020, 7, 106503. [Google Scholar] [CrossRef]
- Reddy, B.N.K.; Udayashankar, N.K. The effect of annealing temperature on the structural, morphological, mechanical and surface properties of intermetallic NiTi alloy thin films. Surfaces and Interfaces 2016, 5, 62–71. [Google Scholar] [CrossRef]
- Nishida, M.; Wayman, C.M.; Honma, T. Precipitation processes in near-equiatomic TiNi shape memory alloys. Metall. Trans. A 1986, 17, 1505–1515. [Google Scholar] [CrossRef]
- Abdul Kadir, R.A.; Shariff, N.A.; Muhammad Hussain, I. Effect of Heat Treatment on Phase Transformation of NiTi Shape Memory Alloy produced by Metal Injection Moulding. Sci. Res. J. 2021, 18, 71–82. [Google Scholar] [CrossRef]
- Sadiq, H.; Wong, M.-B.; Al-Mahaidi, R.; Zhao, X. The effects of heat treatment on the recovery stresses of shape memory alloys. Smart Mater. Struct. 2010, 19, 035021. [Google Scholar] [CrossRef]
- Yan, X.; Van Humbeeck, J. Effect of Annealing on Strain-Temperature Response under Constant Tensile Stress in Cold-Worked NiTi Thin Wire. Smart Mater. Res. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, G.; Wang, H.; Li, G.; Li, C. Effect of annealing treatment on formation of intermetallic phase in cold-sprayed Ni/Ti mechanical alloying coating. Trans. Chin. Weld Inst 2010, 31, 45–48. [Google Scholar]
- ZHOU, J.; MA, F.; LIU, P.; LIU, X. Effect of Heat Treatment on Surface Properties of NiTi Alloy. Nonferrous Met. Mater. Eng. 2019, 40, 6–13. [Google Scholar]
- Tang, W.; Sundman, B.; Sandström, R.; Qiu, C. New modelling of the B2 phase and its associated martensitic transformation in the Ti–Ni system. Acta Mater. 1999, 47, 3457–3468. [Google Scholar] [CrossRef]
- Khalil-Allafi, J.; Dlouhy, A.; Eggeler, G. Ni4Ti3-precipitation during aging of NiTi shape memory alloys and its influence on martensitic phase transformations. Acta Mater. 2002, 50, 4255–4274. [Google Scholar] [CrossRef]
- Polozov, I.; Sufiiarov, V.; Kantyukov, A.; Razumov, N.; Goncharov, I.; Makhmutov, T.; Silin, A.; Kim, A.; Starikov, K.; Shamshurin, A. Microstructure, densification, and mechanical properties of titanium intermetallic alloy manufactured by laser powder bed fusion additive manufacturing with high-temperature preheating using gas atomized and mechanically alloyed plasma spheroidized powders. Addit. Manuf. 2020, 34, 101374. [Google Scholar] [CrossRef]
- Feng, Y.; Du, Z.; Hu, Z. Study on the Effect of Ni Addition on the Microstructure and Properties of NiTi Alloy Coating on AISI 316 L Prepared by Laser Cladding. Materials 2021, 14, 4373. [Google Scholar] [CrossRef]
- Feng, Y.; Du, Z.; Hu, Z. Effect of Ni Addition on the Corrosion Resistance of NiTi Alloy Coatings on AISI 316L Substrate Prepared by Laser Cladding. Coatings 2021, 11, 1139. [Google Scholar] [CrossRef]
- Barin, I. N-NpO3*H2O. In Thermochemical Data of Pure Substances, 3rd ed.; VCH: Weinheim, Germany, 1995; pp. 1080–1237. [Google Scholar]
- Locci, A.M.; Orrù, R.; Cao, G.; Munir, Z.A. Field-activated pressure-assisted synthesis of NiTi. Intermetallics 2003, 11, 555–571. [Google Scholar] [CrossRef]
- Marattukalam, J.J.; Balla, V.K.; Das, M.; Bontha, S.; Kalpathy, S.K. Effect of heat treatment on microstructure, corrosion, and shape memory characteristics of laser deposited NiTi alloy. J. Alloy. Compd. 2018, 744, 337–346. [Google Scholar] [CrossRef]
- Hu, L.; Jiang, S.; Zhang, Y. Role of severe plastic deformation in suppressing formation of R phase and Ni4Ti3 precipitate of NiTi shape memory alloy. Metals 2017, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Salvetr, P.; Dlouhy, J.; Skolakova, A.; Prusa, F.; Novak, P.; Karlik, M.; Hausild, P. Influence of Heat Treatment on Microstructure and Properties of NiTi46 Alloy Consolidated by Spark Plasma Sintering. Materials 2019, 12, 4075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzolo, G.; Noebe, R.D.; Mosca, H.O. Site preference of ternary alloying additions to NiTi: Fe, Pt, Pd, Au, Al, Cu, Zr and Hf. J. Alloy. Compd. 2005, 389, 80–94. [Google Scholar] [CrossRef] [Green Version]
- Nagase, T.; Sasaki, A.; Yasuda, H.Y.; Terai, T.; Fukuda, T.; Kakeshita, T. In situ transmission-electron-microscopy observation of solid-state amorphization behavior in Ti50Ni44Fe6 alloy by high-voltage electron microscopy. Acta Mater. 2016, 104, 201–209. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Jiang, S.-Y.; Zhang, Y.-Q.; Liang, Y.-L. Influence of Fe Addition on Phase Transformation, Microstructure and Mechanical Property of Equiatomic NiTi Shape Memory Alloy. Acta Metall. Sin. 2017, 30, 762–770. [Google Scholar] [CrossRef]
- Frenzel, J.; George, E.P.; Dlouhy, A.; Somsen, C.; Wagner, M.-X.; Eggeler, G. Influence of Ni on martensitic phase transformations in NiTi shape memory alloys. Acta Mater. 2010, 58, 3444–3458. [Google Scholar] [CrossRef]
- Kumar, A.N.; Nair, C.S.; Kannan, M.; Jayakumar, S. TEM and nanoindentation studies on sputtered Ti40Ni60 thin films. Mater. Chem. Phys. 2006, 97, 308–314. [Google Scholar] [CrossRef]
- Lee, H.-J.; Ni, H.; Wu, D.T.; Ramirez, A.G. A microstructural map of crystallized NiTi thin film derived from in situ TEM methods. Mater. Trans. 2006, 47, 527–531. [Google Scholar] [CrossRef] [Green Version]



| Powders | Ni | Ti | Fe | Nb | Co | C | Si | O |
|---|---|---|---|---|---|---|---|---|
| Pure Ni | Bal. | - | 0.003 | - | 0.020 | 0.020 | 0.003 | 0.006 |
| 55NiTi alloy | 56.46 | Bal. | 0.005 | 0.010 | 0.005 | 0.005 | - | 0.037 |
| Fe | Cr | Ni | Mo | Mn | Si | P | C | S |
|---|---|---|---|---|---|---|---|---|
| Bal. | 16.32 | 10.12 | 2.04 | 0.92 | 0.34 | 0.026 | 0.016 | 0.015 |
| Samples | Annealing Temperature (°C) | Holding Time (min) |
|---|---|---|
| Group 1 | 450 | 120 |
| Group 2 | 650 | 120 |
| Group 3 | 850 | 30 |
| Group 4 | 850 | 120 |
| Coatings | GIC1 | GIC2 | Intercept Length (μm) | Average Grain Size (μm) |
|---|---|---|---|---|
| Figure 5a | 19 | 28.5 | L1 = 153.8 L2 = 225.1 | 8.00 |
| Figure 5b | 18 | 27 | 8.44 | |
| Figure 5c | 19 | 21 | 9.41 | |
| Figure 5d | 20.5 | 23 | 8.64 | |
| Figure 5e | 16 | 16.5 | 11.63 |
| Coating | Position | Ni (at.%) | Ti (at.%) | Fe (at.%) | Cr (at.%) | O (at.%) | Potential Phase |
|---|---|---|---|---|---|---|---|
| 850 °C for 0.5 h | 1 | 0.7 | 38.1 | 0.7 | 0.3 | 60.2 | TiO2 |
| 2 | 37.5 | 39.2 | 11.8 | 8.6 | 2.9 | NiTi | |
| 3 | 50.5 | 27.0 | 15.3 | 6.5 | 0.7 | Ni3Ti | |
| 4 | 40.1 | 26.9 | 23.5 | 7.4 | 2.1 | Ni3Ti+Fe2Ti | |
| 850 °C for 2 h | 5 | 43.3 | 21.9 | 24.7 | 6.9 | 3.2 | Ni3Ti+Fe2Ti |
| 6 | 37.0 | 34.1 | 17.8 | 7.3 | 3.8 | NiTi | |
| 7 | 0.7 | 35.2 | 0.7 | 0.4 | 63.0 | TiO2 | |
| 8 | 48.0 | 36.2 | 8.3 | 6.1 | 1.4 | NiTi+Ni3Ti |
| Coating | Onset Point (°C) | Peak Temperature (°C) | Normalized Enthalpy (J/g) |
|---|---|---|---|
| without HT-S1 | −74 | 38 | 4.1 |
| without HT-S2 | −73 | 43 | 3.4 |
| after annealing-S1 | −71 | 45 | 3.3 |
| after annealing-S2 | −72 | 45 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Gao, Z.; Hu, Z. Influence of Annealing Treatment on Microstructure and Properties of Ni-Rich NiTi Alloy Coating Prepared by Laser Cladding. Materials 2022, 15, 3298. https://doi.org/10.3390/ma15093298
Feng Y, Gao Z, Hu Z. Influence of Annealing Treatment on Microstructure and Properties of Ni-Rich NiTi Alloy Coating Prepared by Laser Cladding. Materials. 2022; 15(9):3298. https://doi.org/10.3390/ma15093298
Chicago/Turabian StyleFeng, Yuqiang, Ziyi Gao, and Zhengfei Hu. 2022. "Influence of Annealing Treatment on Microstructure and Properties of Ni-Rich NiTi Alloy Coating Prepared by Laser Cladding" Materials 15, no. 9: 3298. https://doi.org/10.3390/ma15093298







