Resolving Salt-Induced Agglomeration of Laponite Suspensions Using X-ray Photon Correlation Spectroscopy and Molecular Dynamics Simulations
Abstract
1. Introduction
2. Methodology
2.1. Sample Preparation
2.2. X-ray Photon Correlation Spectroscopy Measurements
2.3. Molecular Dynamics Simulations
3. Results and Discussion
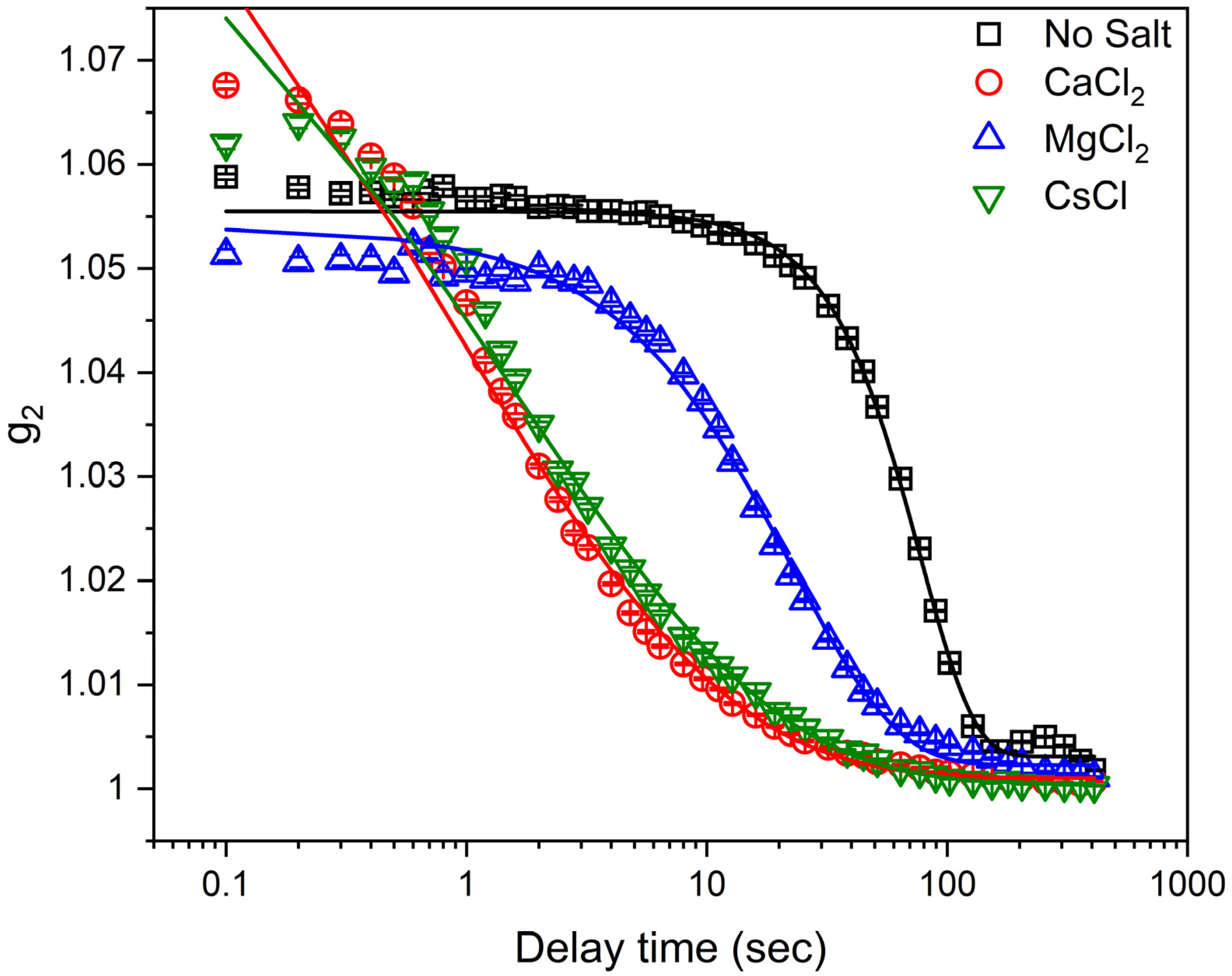
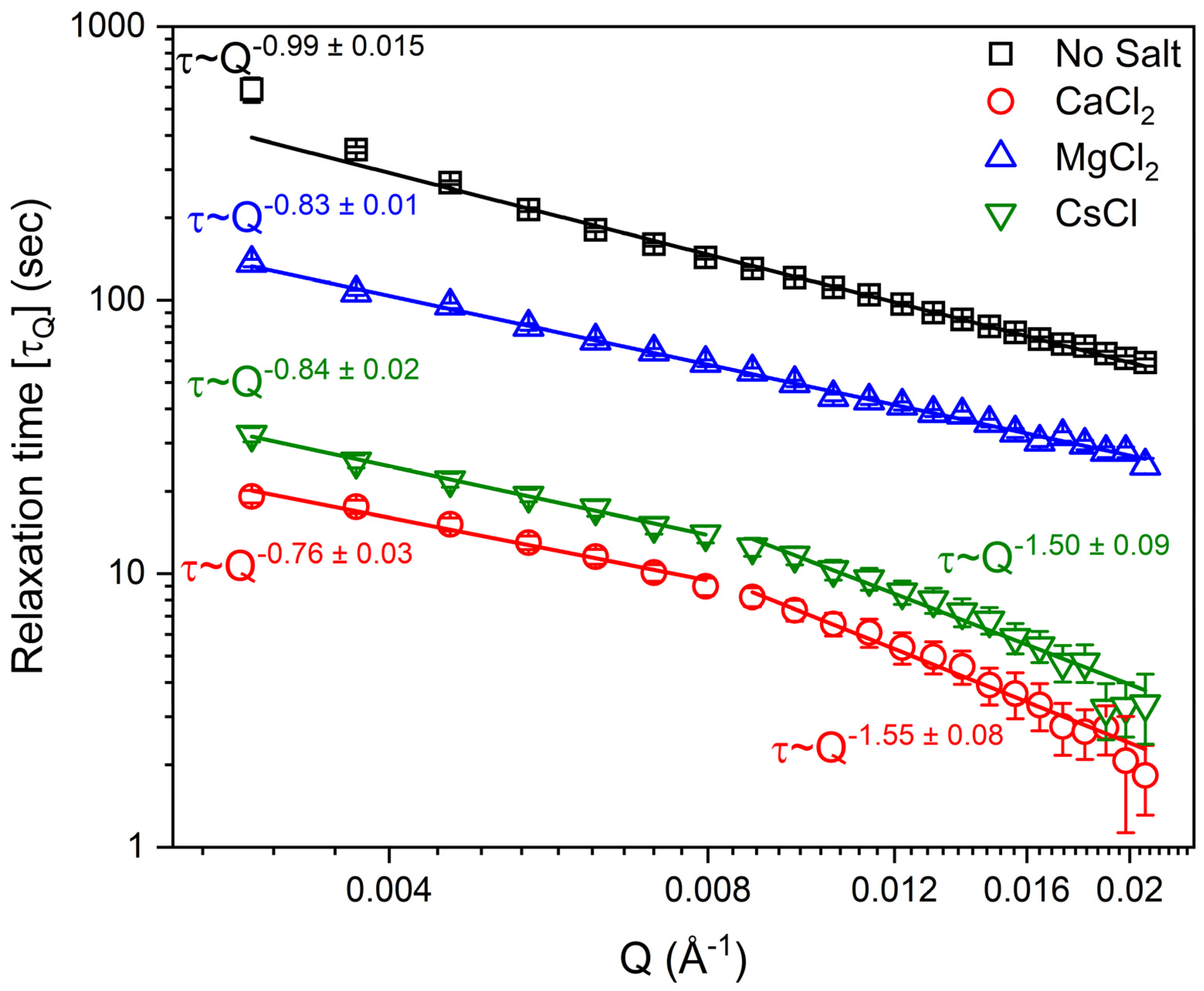
3.1. Determination of Laponite Relaxation Behavior Using XPCS Measurements
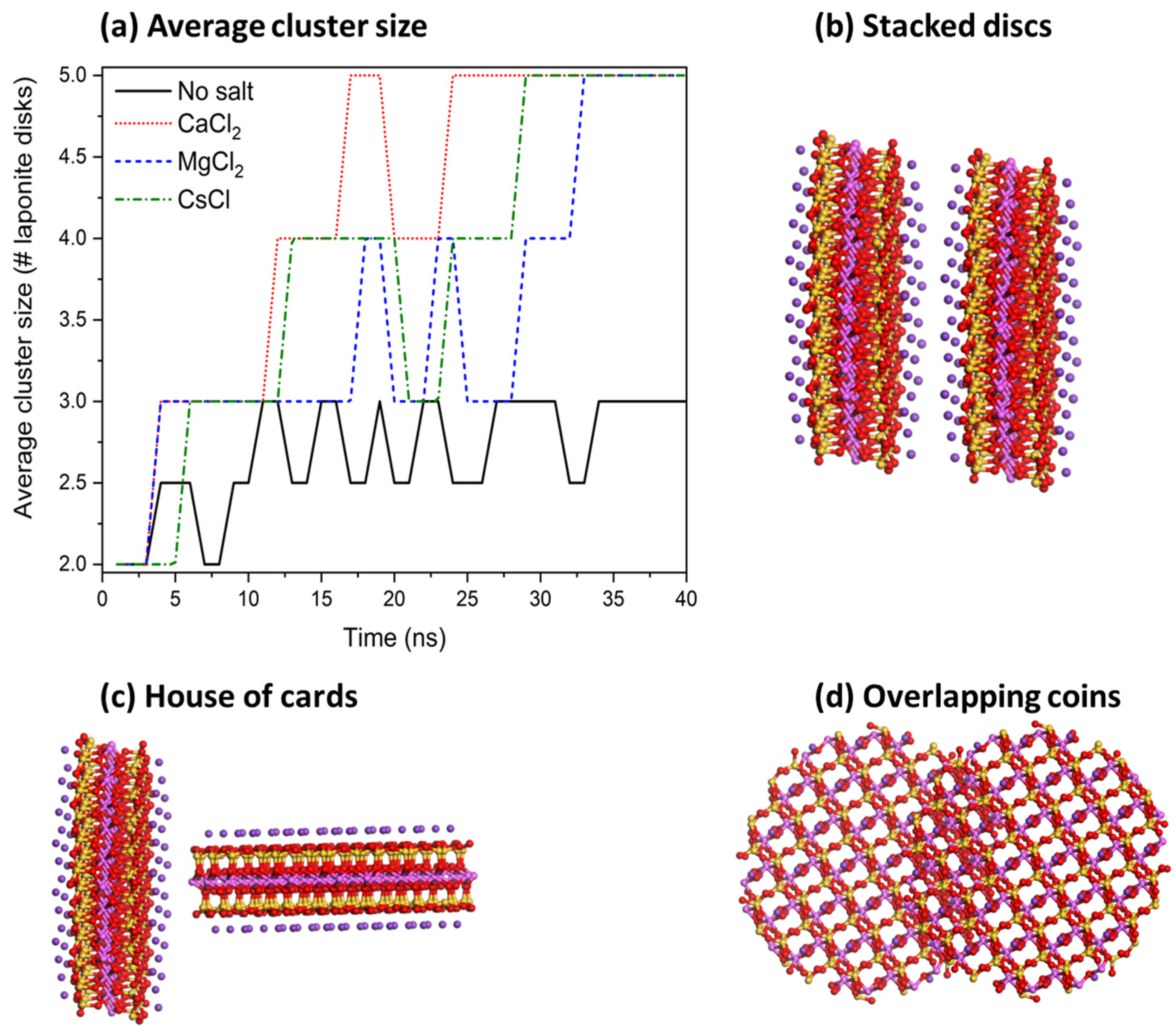
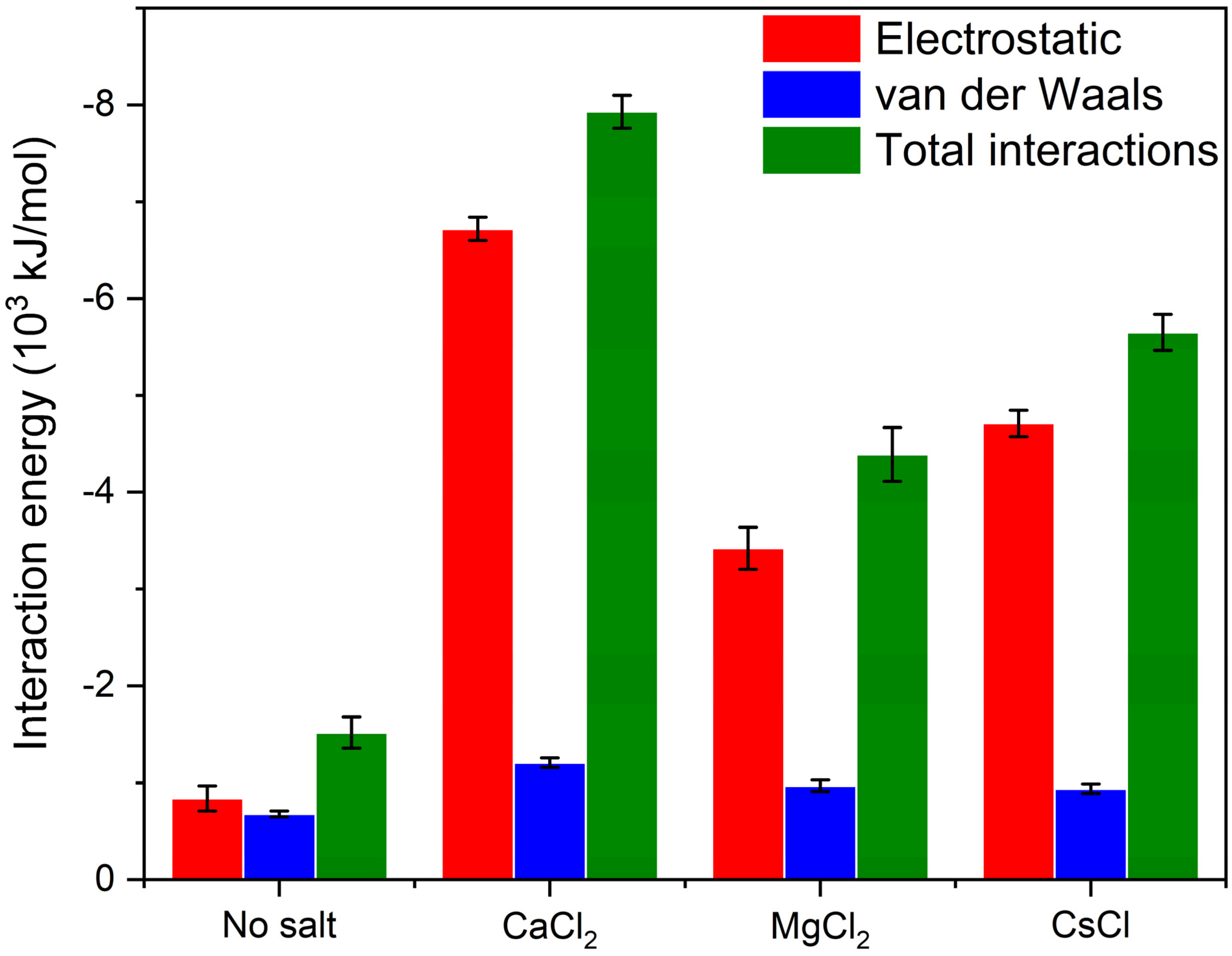
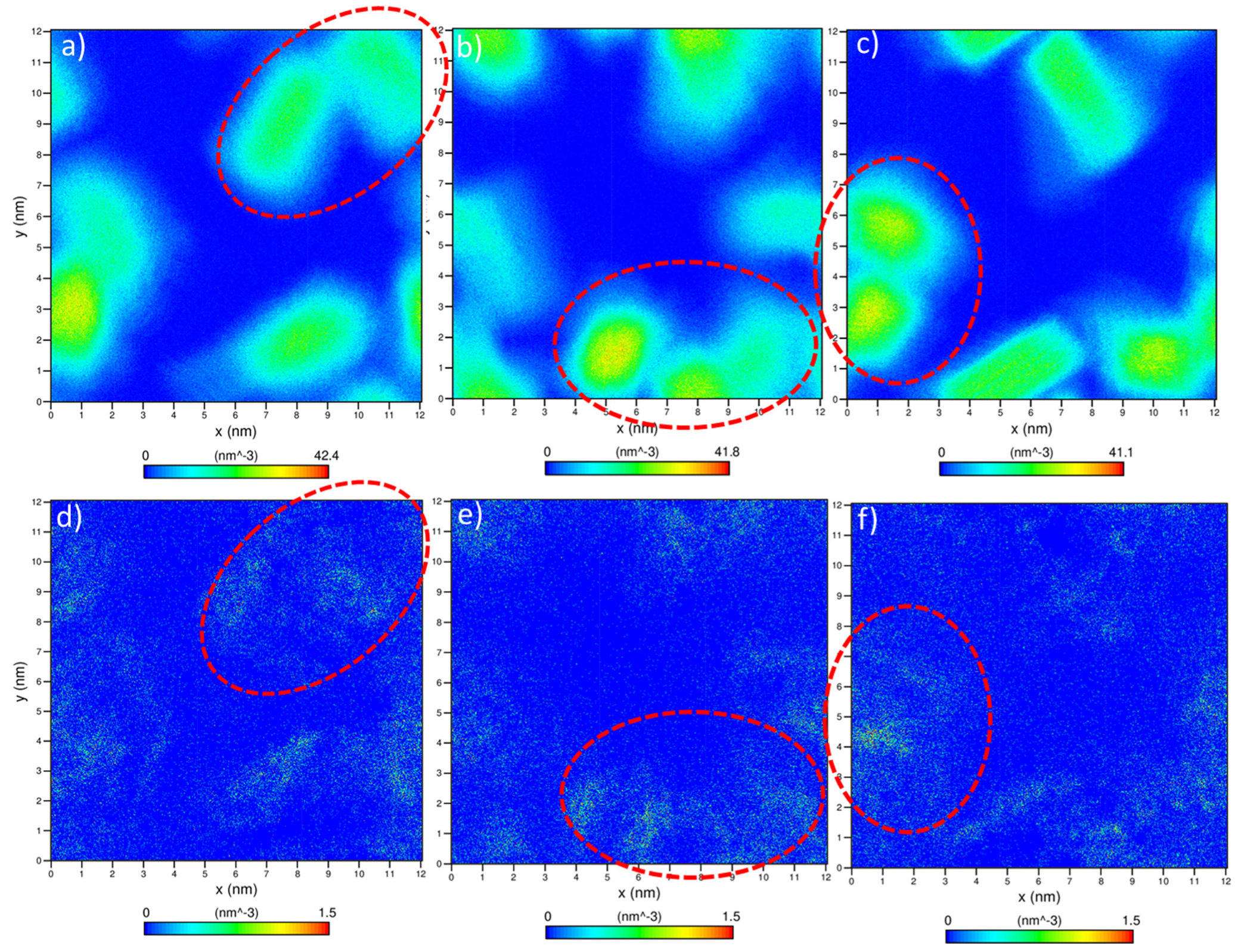
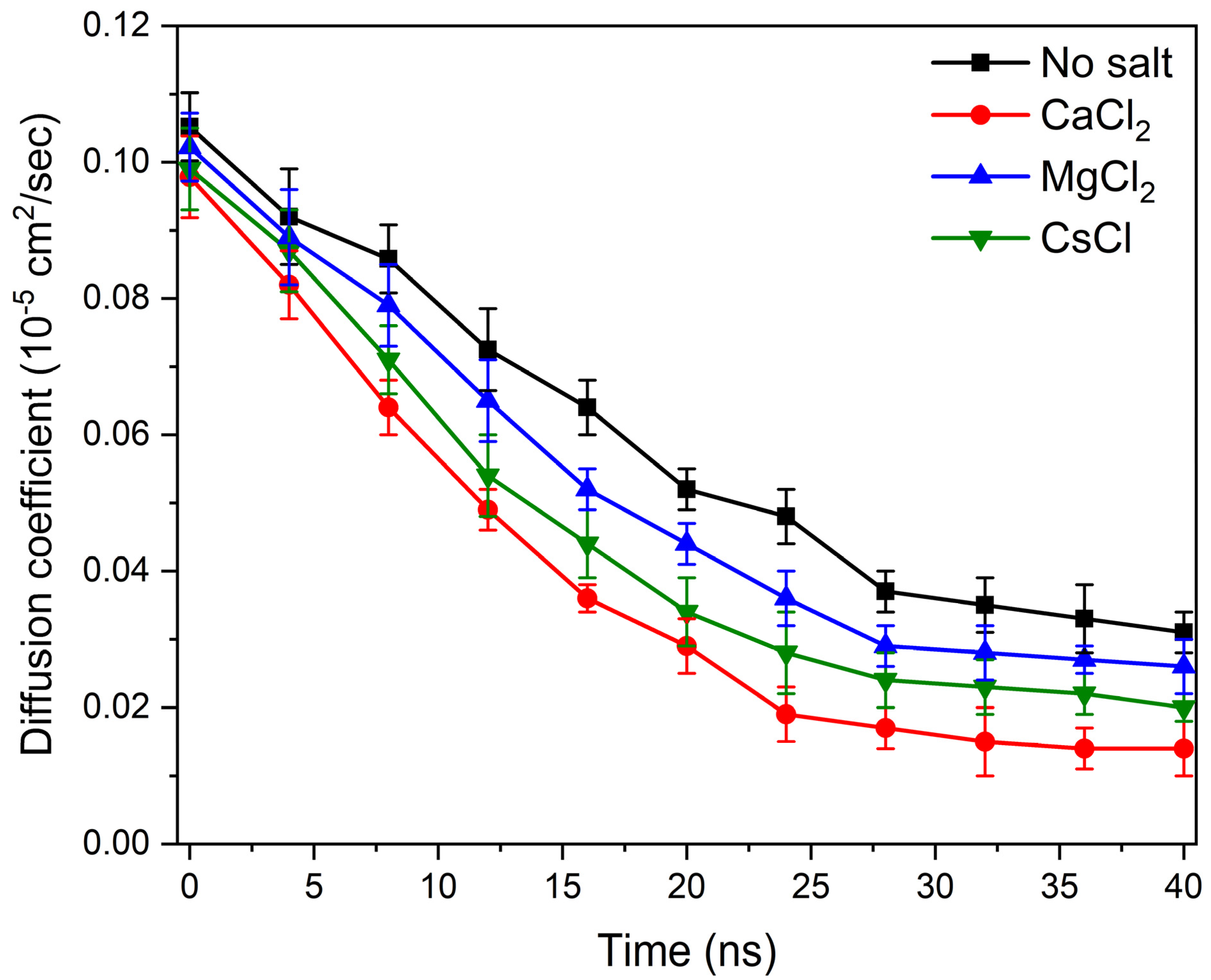
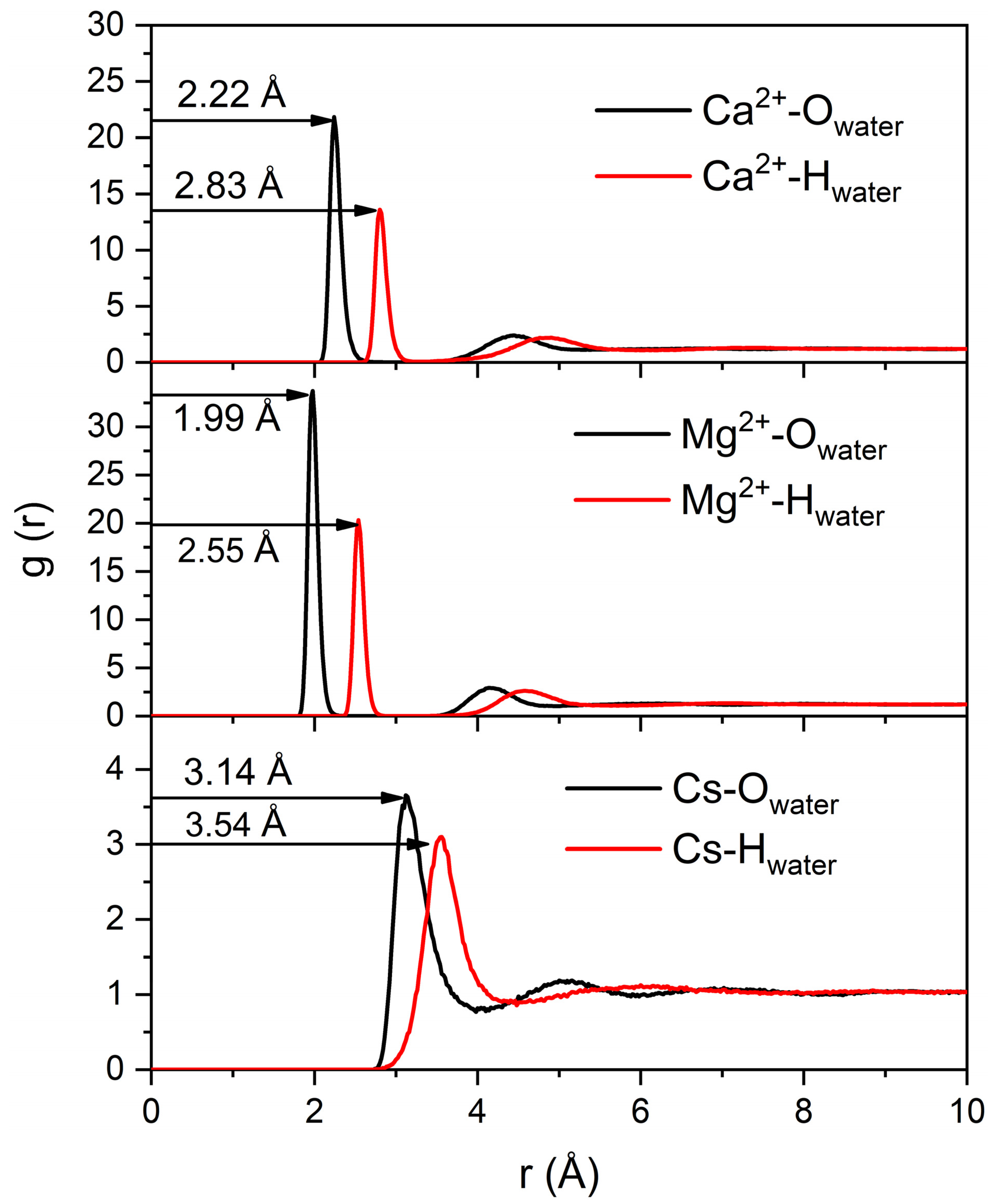
3.2. Determination of Laponite Relaxation Behavior Using MD Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luckham, P.F.; Rossi, S. The colloidal and rheological properties of bentonite suspensions. Adv. Colloid Interf. Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef]
- Tongwa, P.; Bai, B. Degradable nanocomposite preformed particle gel for chemical enhanced oil recovery applications. J. Petrol. Sci. Eng. 2014, 124, 35–45. [Google Scholar] [CrossRef]
- Li, W.; Yu, L.; Liu, G.; Tan, J.; Liu, S.; Sun, D. Oil-in-water emulsions stabilized by Laponite particles modified with short-chain aliphatic amines. Colloids Surf. A 2012, 400, 44–51. [Google Scholar] [CrossRef]
- Ashby, N.P.; Binks, B.P. Pickering emulsions stabilised by Laponite clay particles. Phys. Chem. Chem. Phys. 2000, 2, 5640–5646. [Google Scholar] [CrossRef]
- Whitby, C.P.; Fornasiero, D.; Ralston, J. Effect of oil soluble surfactant in emulsions stabilised by clay particles. J. Colloid Interf. Sci. 2008, 323, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Bon, S.A.; Colver, P.J. Pickering miniemulsion polymerization using laponite clay as a stabilizer. Langmuir 2007, 23, 8316–8322. [Google Scholar] [CrossRef] [PubMed]
- Suman, K.; Joshi, Y.M. Microstructure and soft glassy dynamics of an aqueous laponite dispersion. Langmuir 2018, 34, 13079–13103. [Google Scholar] [CrossRef]
- Huang, A.Y.; Berg, J.C. High-salt stabilization of Laponite clay particles. J. Colloid Interf. Sci. 2006, 296, 159–164. [Google Scholar] [CrossRef]
- Jatav, S.; Joshi, Y.M. Phase behavior of aqueous suspension of laponite: New insights with microscopic evidence. Langmuir 2017, 33, 2370–2377. [Google Scholar] [CrossRef]
- Nicolai, T.; Cocard, S. Light scattering study of the dispersion of laponite. Langmuir 2000, 16, 8189–8193. [Google Scholar] [CrossRef]
- Thuresson, A.; Segad, M.; Plivelic, T.S.; Skepoö, M. Flocculated Laponite-PEG/PEO Dispersions with Multivalent Salt: A SAXS, Cryo-TEM, and Computer Simulation Study. J. Phys. Chem. C 2017, 121, 7387–7396. [Google Scholar] [CrossRef]
- Thuresson, A.; Segad, M.; Turesson, M.; Skepö, M. Flocculated Laponite–PEG/PEO dispersions with monovalent salt, a SAXS and simulation study. J. Colloid Interf. Sci. 2016, 466, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Shahin, A.; Joshi, Y.M. Irreversible aging dynamics and generic phase behavior of aqueous suspensions of laponite. Langmuir 2010, 26, 4219–4225. [Google Scholar] [CrossRef] [PubMed]
- De Melo Marques, F.A.; Angelini, R.; Zaccarelli, E.; Farago, B.; Ruta, B.; Ruocco, G.; Ruzicka, B. Structural and microscopic relaxations in a colloidal glass. Soft Matter 2015, 11, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Mourchid, A.; Delville, A.; Lambard, J.; Lecolier, E.; Levitz, P. Phase diagram of colloidal dispersions of anisotropic charged particles: Equilibrium properties, structure, and rheology of laponite suspensions. Langmuir 1995, 11, 1942–1950. [Google Scholar] [CrossRef]
- Mourchid, A.; Lecolier, E.; van Damme, H.; Levitz, P. On viscoelastic, birefringent, and swelling properties of Laponite clay suspensions: Revisited phase diagram. Langmuir 1998, 14, 4718–4723. [Google Scholar] [CrossRef]
- Jabbari-Farouji, S.; Tanaka, H.; Wegdam, G.H.; Bonn, D. Multiple nonergodic disordered states in Laponite suspensions: A phase diagram. Phys. Rev. E 2008, 78, 061405. [Google Scholar] [CrossRef]
- Ruzicka, B.; Zulian, L.; Ruocco, G. More on the phase diagram of Laponite. Langmuir 2006, 22, 1106–1111. [Google Scholar] [CrossRef]
- Ruzicka, B.; Zaccarelli, E. A fresh look at the Laponite phase diagram. Soft Matter 2011, 7, 1268–1286. [Google Scholar] [CrossRef]
- Shahin, A.; Joshi, Y.M.; Ramakrishna, S.A. Interface-induced anisotropy and the nematic glass/gel state in jammed aqueous laponite suspensions. Langmuir 2011, 27, 14045–14052. [Google Scholar] [CrossRef]
- Jabbari-Farouji, S.; Wegdam, G.H.; Bonn, D. Gels and glasses in a single system: Evidence for an intricate free-energy landscape of glassy materials. Phys. Rev. Lett. 2007, 99, 065701. [Google Scholar] [CrossRef]
- Ruzicka, B.; Zulian, L.; Ruocco, G. Routes to gelation in a clay suspension. Phys. Rev. Lett. 2004, 93, 258301. [Google Scholar] [CrossRef]
- Srivastava, S.; Kishore, S.; Narayanan, S.; Sandy, A.R.; Bhatia, S.R. Multiple dynamic regimes in colloid-polymer dispersions: New insight using X-ray photon correlation spectroscopy. J. Polym. Sci. Pol. Phys. 2016, 54, 752–760. [Google Scholar] [CrossRef]
- Jabbari-Farouji, S.; Zargar, R.; Wegdam, G.H.; Bonn, D. Dynamical heterogeneity in aging colloidal glasses of Laponite. Soft Matter 2012, 8, 5507–5512. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Liang, D.; Yardimci, H.; Sessoms, D.A.; Borthwick, M.A.; Mochrie, S.G.J.; Harden, J.L.; Leheny, R.L. Evolution of particle-scale dynamics in an aging clay suspension. Phys. Rev. Lett. 2004, 93, 228302. [Google Scholar] [CrossRef]
- Leheny, R.L. XPCS: Nanoscale motion and rheology. Curr. Opin. Colloid Interf. Sci. 2012, 17, 3–12. [Google Scholar] [CrossRef]
- Lemaire, B.J.; Panine, P.; Gabriel, J.C.P.; Davidson, P. The measurement by SAXS of the nematic order parameter of laponite gels. EPL-Europhys. Lett. 2002, 59, 55. [Google Scholar] [CrossRef]
- Mossa, S.; de Michele, C.; Sciortino, F. Aging in a Laponite colloidal suspension: A Brownian dynamics simulation study. J. Chem. Phys. 2007, 126, 014905. [Google Scholar] [CrossRef]
- Odriozola, G.; Romero-Bastida, M.; Guevara-Rodriguez, F.D.J. Brownian dynamics simulations of Laponite colloid suspensions. Phys. Rev. E 2004, 70, 021405. [Google Scholar] [CrossRef]
- Jonsson, B.; Labbez, C.; Cabane, B. Interaction of nanometric clay platelets. Langmuir 2008, 24, 11406–11413. [Google Scholar] [CrossRef]
- Pujala, R.K.; Bohidar, H.B. Slow dynamics, hydration and heterogeneity in Laponite dispersions. Soft Matter 2013, 9, 2003–2010. [Google Scholar] [CrossRef]
- Gadige, P.; Saha, D.; Behera, S.K.; Bandyopadhyay, R. Study of dynamical heterogeneities in colloidal nanoclay suspensions approaching dynamical arrest. Sci. Rep. 2017, 7, 8017. [Google Scholar] [CrossRef] [PubMed]
- Shahin, A.; Joshi, Y.M. Physicochemical effects in aging aqueous Laponite suspensions. Langmuir 2012, 28, 15674–15686. [Google Scholar] [CrossRef] [PubMed]
- Eppenga, R.; Frenkel, D. Monte Carlo study of the isotropic and nematic phases of infinitely thin hard platelets. Mol. Phys. 1984, 52, 1303–1334. [Google Scholar] [CrossRef]
- Kutter, S.; Hansen, J.P.; Sprik, M.; Boek, E. Structure and phase behavior of a model clay dispersion: A molecular-dynamics investigation. J. Chem. Phys. 2000, 112, 311–322. [Google Scholar] [CrossRef]
- Delhorme, M.; Jönsson, B.; Labbez, C. Monte Carlo simulations of a clay inspired model suspension: The role of rim charge. Soft Matter 2012, 8, 9691–9704. [Google Scholar] [CrossRef]
- Madsen, A.; Leheny, R.L.; Guo, H.; Sprung, M.; Czakkel, O. Beyond simple exponential correlation functions and equilibrium dynamics in X-ray photon correlation spectroscopy. New J. Phys. 2010, 12, 055001. [Google Scholar] [CrossRef]
- Shpyrko, O.G. X-ray photon correlation spectroscopy. J. Synchrotron Radiat. 2014, 21, 1057–1064. [Google Scholar] [CrossRef]
- Zhang, Q.; Dufresne, E.M.; Narayanan, S.; Maj, P.; Koziol, A.; Szczygiel, R.; Grybos, P.; Sutton, M.; Sandy, A.R. Sub-microsecond-resolved multi-speckle X-ray photon correlation spectroscopy with a pixel array detector. J. Synchrotron Radiat. 2018, 25, 1408–1416. [Google Scholar] [CrossRef]
- Guo, H.; Bourret, G.; Lennox, R.B.; Sutton, M.; Harden, J.L.; Leheny, R.L. Entanglement-controlled subdiffusion of nanoparticles within concentrated polymer solutions. Phys. Rev. Lett. 2012, 109, 055901. [Google Scholar] [CrossRef]
- Angelini, R.; Madsen, A.; Fluerasu, A.; Ruocco, G.; Ruzicka, B. Aging behavior of the localization length in a colloidal glass. Colloids Surf. A 2014, 460, 118–122. [Google Scholar] [CrossRef]
- Cummins, H.Z. Liquid, glass, gel: The phases of colloidal Laponite. J. Non-Cryst. Solids 2007, 353, 3891–3905. [Google Scholar] [CrossRef]
- Tomás, H.; Alves, C.S.; Rodrigues, J. Laponite®: A key nanoplatform for biomedical applications? Nanomed. Nanotechnol. 2018, 14, 2407–2420. [Google Scholar] [CrossRef] [PubMed]
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Abascal, J.L.; Vega, C. A general purpose model for the condensed phases of water: TIP4P/2005. J. Chem. Phys. 2005, 123, 234505. [Google Scholar] [CrossRef] [PubMed]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Sun, W.; Yang, Y.; Wang, T.; Huang, H.; Liu, X.; Tong, Z. Effect of adsorbed poly (ethylene glycol) on the gelation evolution of Laponite suspensions: Aging time-polymer concentration superposition. J. Colloid Interf. Sci. 2012, 376, 76–82. [Google Scholar] [CrossRef]
- Chung, B.; Ramakrishnan, S.; Bandyopadhyay, R.; Liang, D.; Zukoski, C.F.; Harden, J.L.; Leheny, R.L. Microscopic dynamics of recovery in sheared depletion gels. Phys. Rev. Lett. 2006, 96, 228301. [Google Scholar] [CrossRef] [PubMed]
- Cipelletti, L.; Manley, S.; Ball, R.C.; Weitz, D.A. Universal aging features in the restructuring of fractal colloidal gels. Phys. Rev. Lett. 2000, 84, 2275. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wilking, J.N.; Liang, D.; Mason, T.G.; Harden, J.L.; Leheny, R.L. Slow, nondiffusive dynamics in concentrated nanoemulsions. Phys. Rev. E 2007, 75, 041401. [Google Scholar] [CrossRef] [PubMed]
- Cipelletti, L.; Ramos, L.; Manley, S.; Pitard, E.; Weitz, D.A.; Pashkovski, E.E.; Johansson, M. Universal non-diffusive slow dynamics in aging soft matter. Faraday Discuss. 2003, 123, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Richert, R. Heterogeneous dynamics in liquids: Fluctuations in space and time. J. Phys. Condens. Mat. 2002, 14, R703. [Google Scholar] [CrossRef]
- Berthier, L. Dynamic Heterogeneity in Amorphous Materials. Physics 2011, 4, 42. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, H.; Liu, X.; Hu, X. Molecular insight into water desalination across multilayer graphene oxide membranes. ACS Appl. Mater. Interf. 2017, 9, 22826–22836. [Google Scholar] [CrossRef]
- Yan, H.; Yuan, S.L.; Xu, G.Y.; Liu, C.B. Effect of Ca2+ and Mg2+ ions on surfactant solutions investigated by molecular dynamics simulation. Langmuir 2010, 26, 10448–10459. [Google Scholar] [CrossRef]
- Megyes, T.; Grósz, T.; Radnai, T.; Bakó, I.; Pálinkás, G. Solvation of calcium ion in polar solvents: An X-ray diffraction and ab initio study. J. Phys. Chem. A 2004, 108, 7261–7271. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, S.; Liu, M.; Zhang, Q.; Narayanan, S.; Zhang, F.; Gadikota, G. Resolving Salt-Induced Agglomeration of Laponite Suspensions Using X-ray Photon Correlation Spectroscopy and Molecular Dynamics Simulations. Materials 2023, 16, 101. https://doi.org/10.3390/ma16010101
Mohammed S, Liu M, Zhang Q, Narayanan S, Zhang F, Gadikota G. Resolving Salt-Induced Agglomeration of Laponite Suspensions Using X-ray Photon Correlation Spectroscopy and Molecular Dynamics Simulations. Materials. 2023; 16(1):101. https://doi.org/10.3390/ma16010101
Chicago/Turabian StyleMohammed, Sohaib, Meishen Liu, Qingteng Zhang, Suresh Narayanan, Fan Zhang, and Greeshma Gadikota. 2023. "Resolving Salt-Induced Agglomeration of Laponite Suspensions Using X-ray Photon Correlation Spectroscopy and Molecular Dynamics Simulations" Materials 16, no. 1: 101. https://doi.org/10.3390/ma16010101
APA StyleMohammed, S., Liu, M., Zhang, Q., Narayanan, S., Zhang, F., & Gadikota, G. (2023). Resolving Salt-Induced Agglomeration of Laponite Suspensions Using X-ray Photon Correlation Spectroscopy and Molecular Dynamics Simulations. Materials, 16(1), 101. https://doi.org/10.3390/ma16010101










