Interface Optimization of Metal Quantum Dots/Polymer Nanocomposites and their Properties: Studies of Multi-Functional Organic/Inorganic Hybrid
Abstract
:1. Introduction
2. Experimental Details
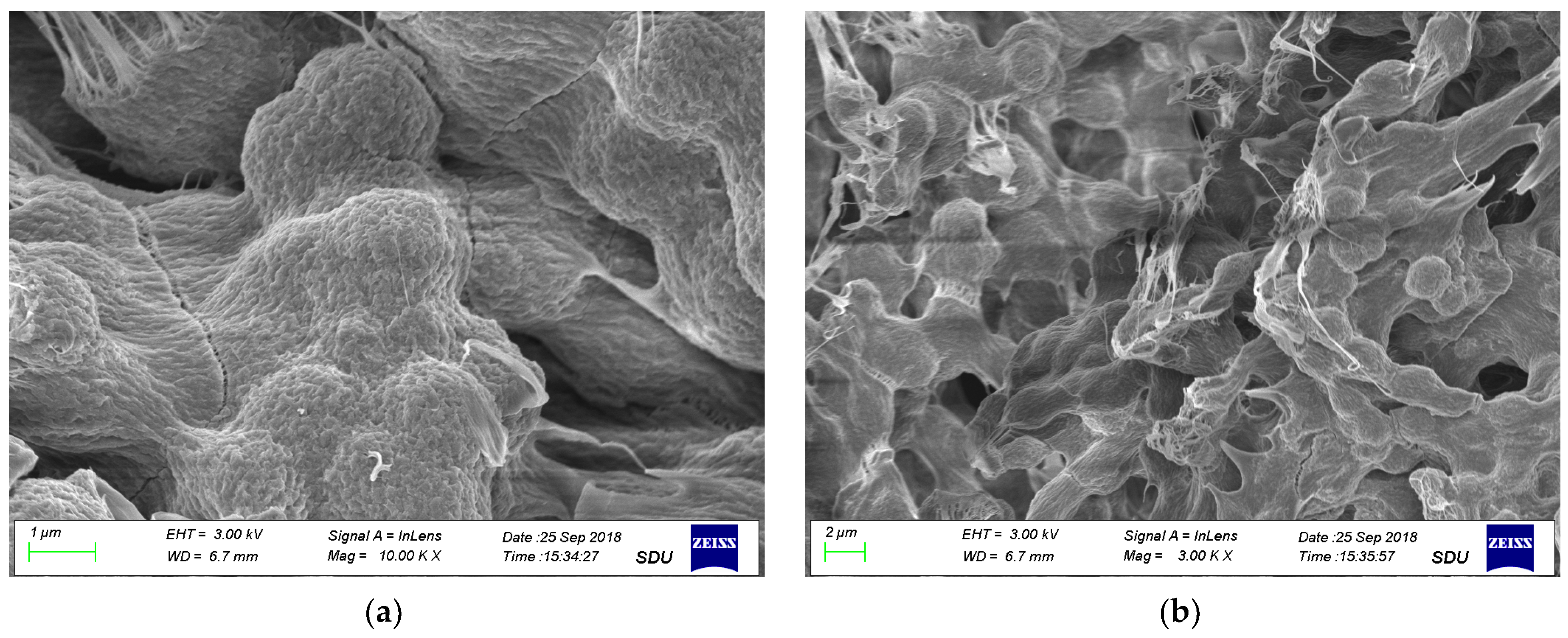
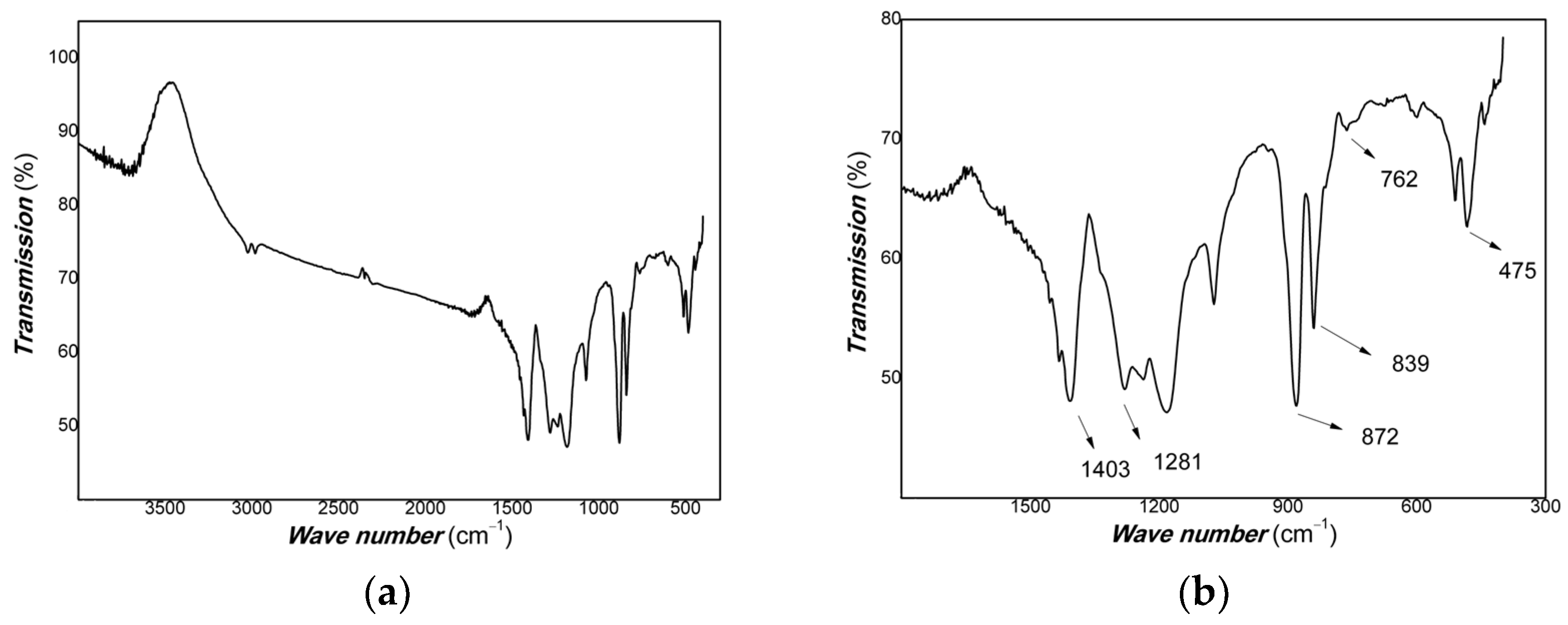
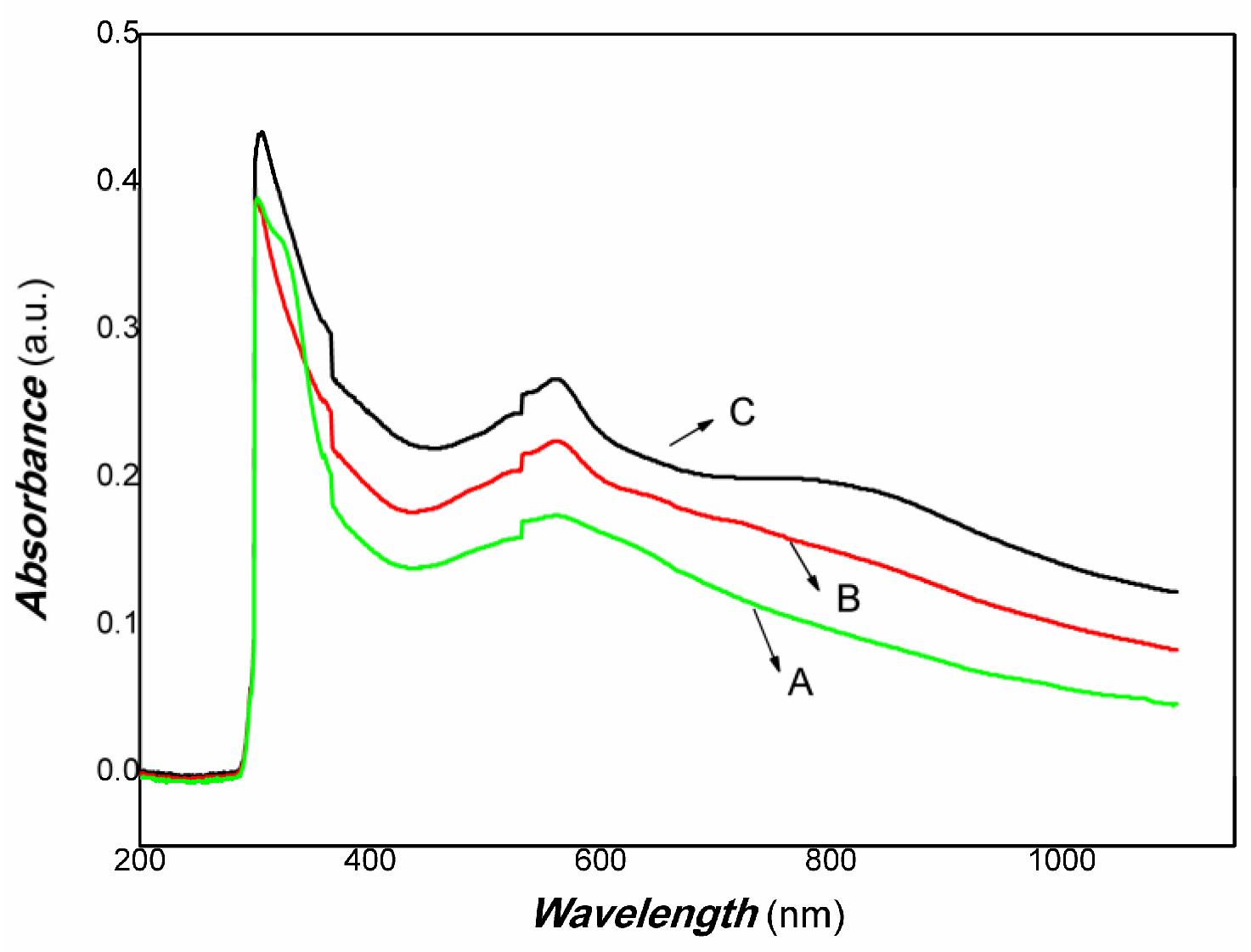
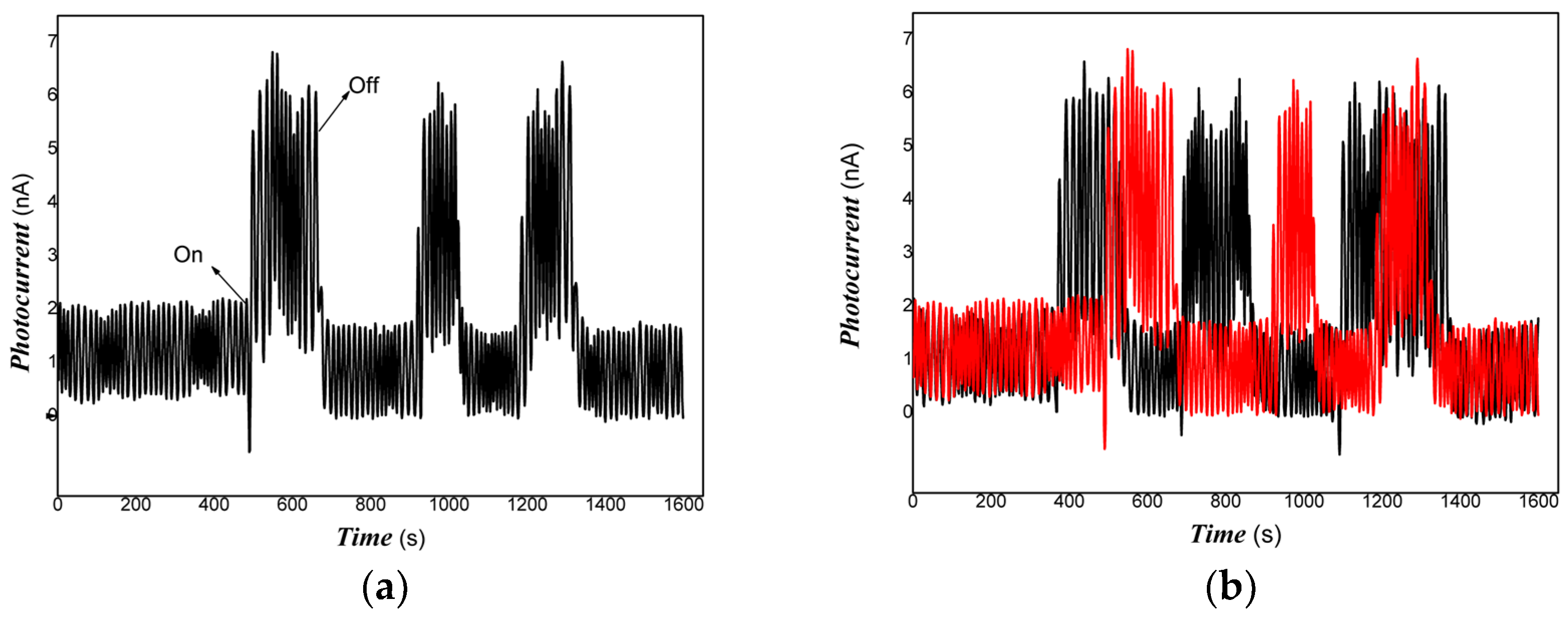
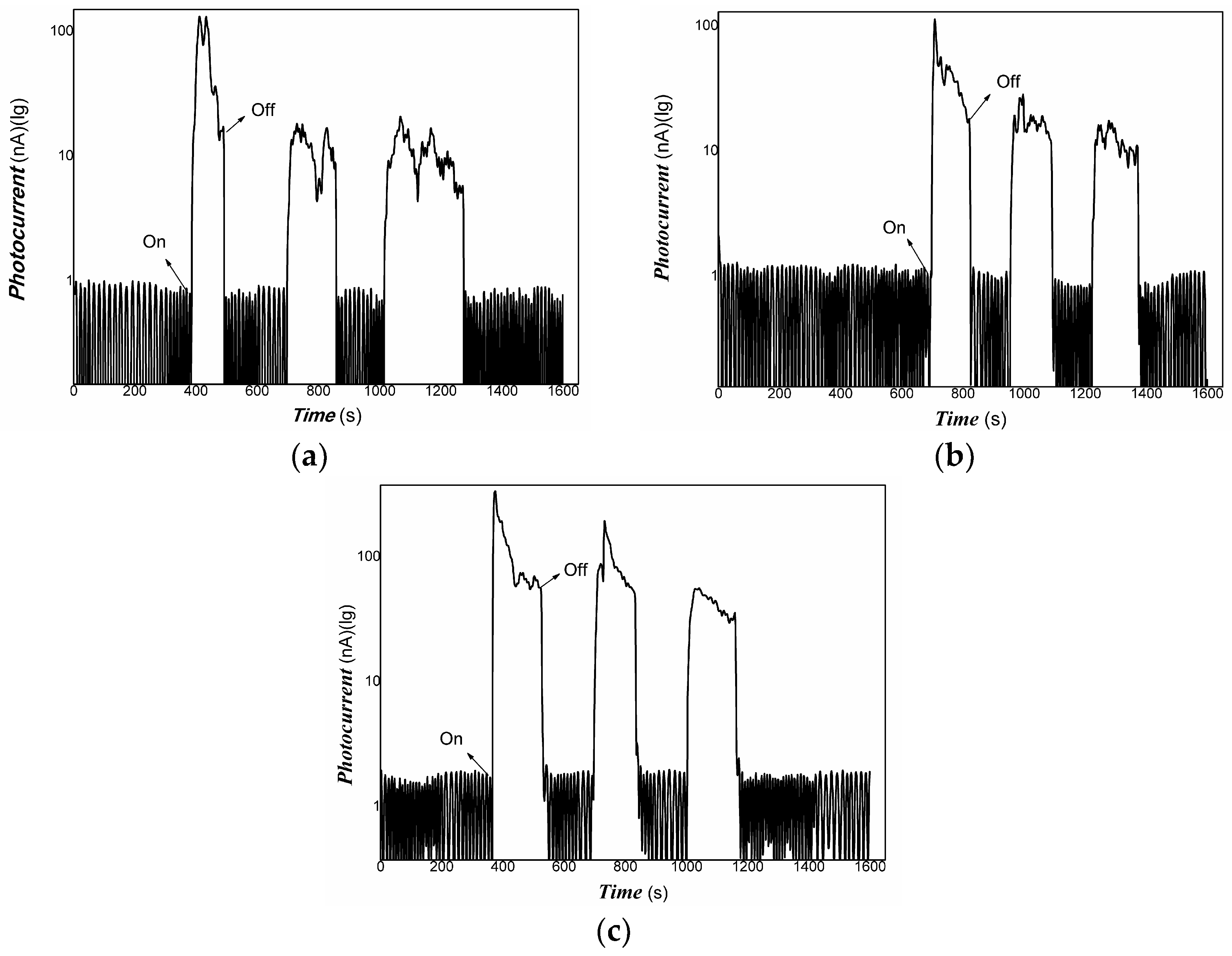
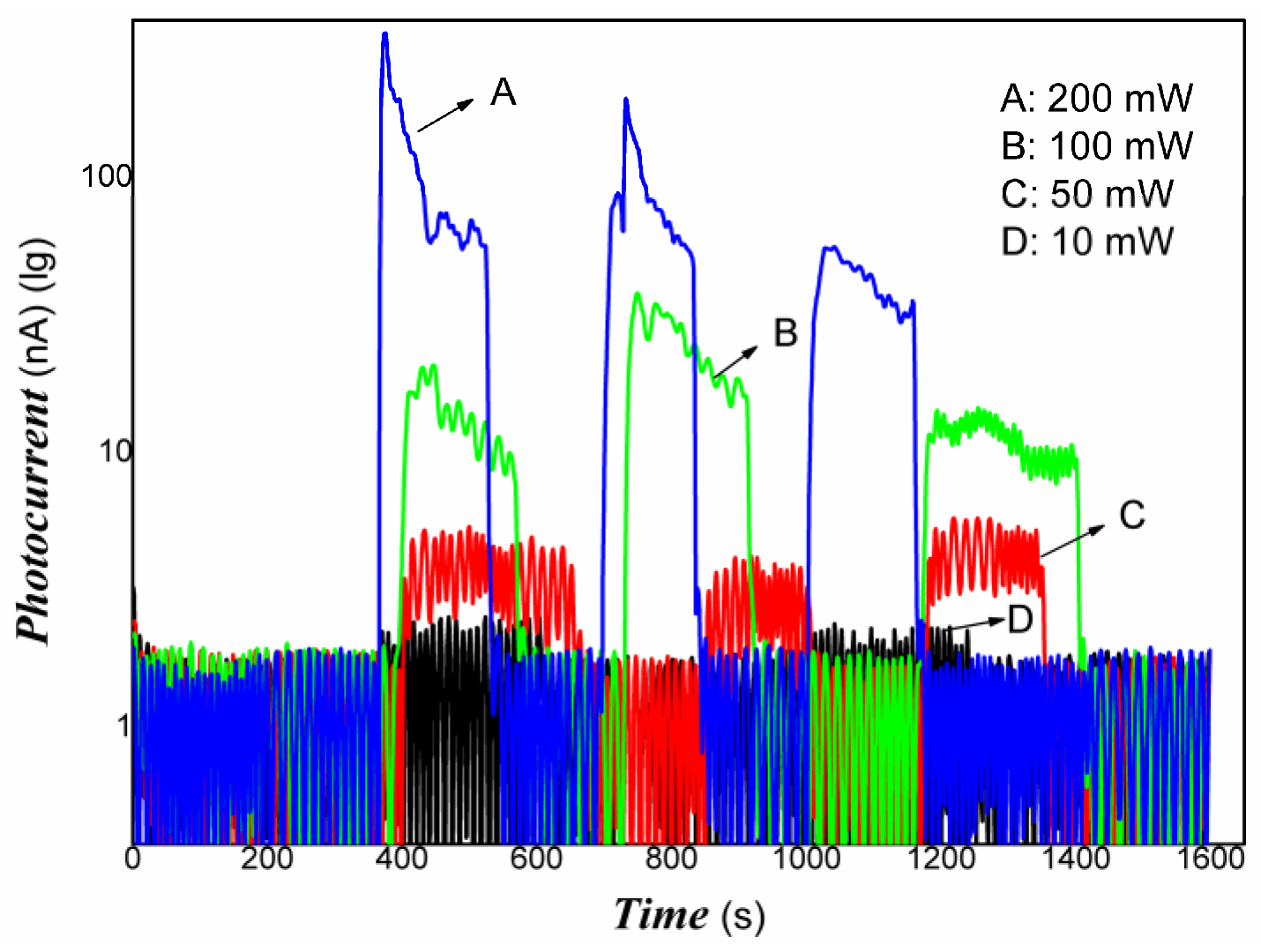
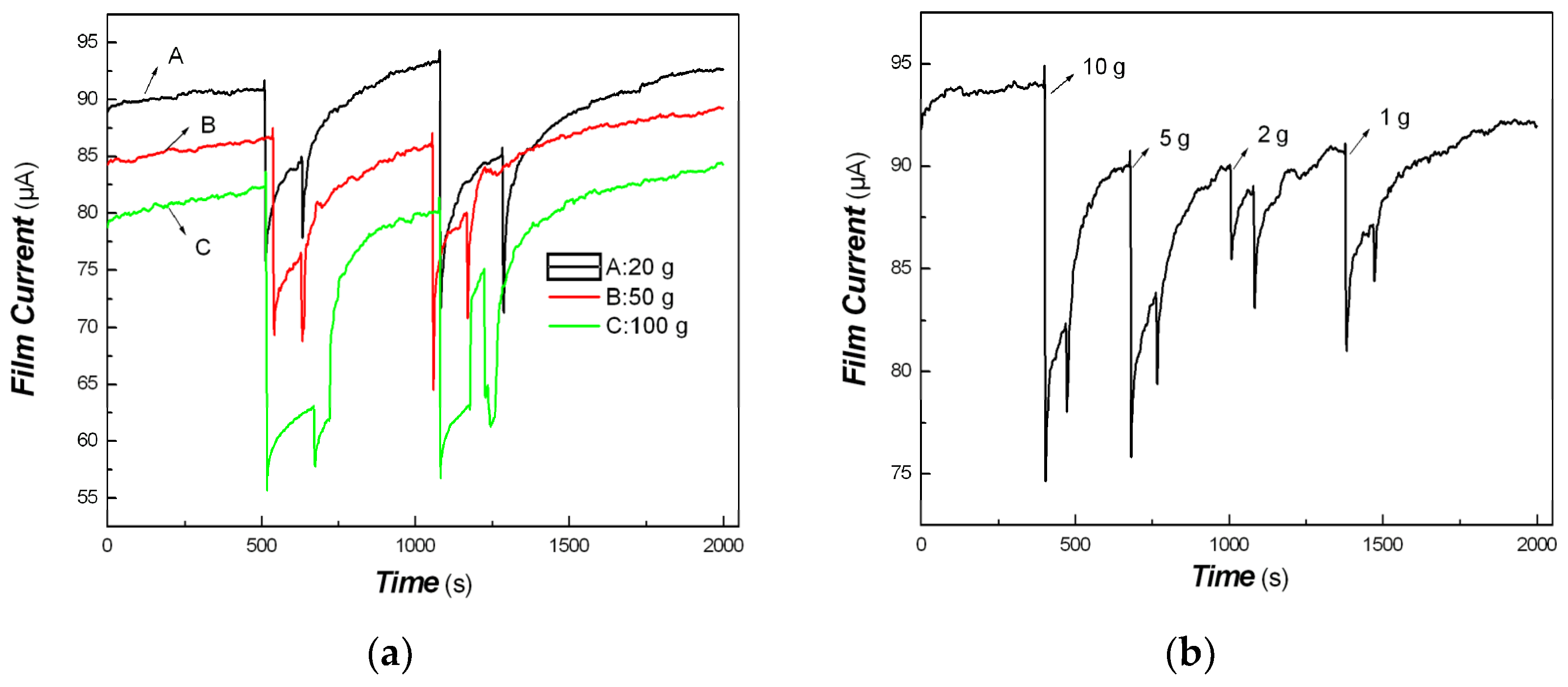
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anand, A.; Meena, D.; Bhatnagar, M. Synthesis and characterization of flexible PVDF/Bi2Al4O9/RGO based piezoelectric materials for nanogenerator application. J. Alloy Compd. 2020, 843, 156019. [Google Scholar] [CrossRef]
- Karan, S.K.; Bera, R.; Paria, S.; Das, A.K.; Maiti, S.; Maitra, A.; Khatua, B.B. An Approach to Design Highly Durable Piezoelectric Nanogenerator Based on Self-Poled PVDF/AlO-rGO Flexible Nanocomposite with High Power Density and Energy Conversion Efficiency. Adv. Energy Mater. 2016, 6, 1601016. [Google Scholar] [CrossRef]
- Zhang, K.; Cui, Z.; Xing, G.; Feng, Y.; Meng, S. Improved performance of dye-sensitized solar cells based on modified kao-lin/PVDF-HFP composite gel electrolytes. RSC Adv. 2016, 6, 100079–100089. [Google Scholar] [CrossRef]
- Solarajan, A.K.; Murugadoss, V.; Angaiah, S. High performance electrospun PVdF-HFP/SiO2 nanocomposite membrane elec-trolyte for Li-ion capacitors. J. Appl. Polym. Sci. 2017, 134, 45177. [Google Scholar] [CrossRef]
- Lin, M.; Chang, K.; Lee, C.; Wu, X. Electrospun P3HT/PVDF HFP semiconductive nanofbers for triboelectric nan-ogenerators. Sci. Rep. 2022, 12, 14842. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, T.; Zheng, Y.; Wang, E.; Wang, H.; Zhu, L.; Du, Z.; Hou, X.; Chou, K.-C. The mechanism of a PVDF/CsPbBr3 perovskite composite fiber as a self-polarization piezoelectric nanogenerator with ultra-high output voltage. J. Mater. Chem. A 2022, 10, 21893–21904. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, X.; Feng, Y.; Li, J.; Li, Y.; Zhao, H.; Yue, D.; Yin, J. Adjusting the energy gap and interface effect of titania nanosheets synergistically enhances the energy storage performance of PVDF-based composites. J. Mater. Chem. C 2022, 10, 2128–2138. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, Z.; Li, Y.; Wu, D.; Xue, Y. Improving the Energy Storage Performance of All-Polymer Composites by Blending PVDF and P(VDF−CTFE). Macromol. Rapid Commun. 2022, 2200728. [Google Scholar] [CrossRef]
- Patranabish, S.; Dhawan, S.; Haridas, V.; Sinha, A. Designer Peptide-PVDF Composite Films for High-Performance Energy Harvesting. Macromol. Rapid Commun. 2022, 43, 493. [Google Scholar] [CrossRef]
- Gao, X.; Sheng, L.; Xie, X.; Yang, L.; Bai, Y.; Dong, H.; Liu, G.; Wang, T.; Huang, X.; He, J. Morphology optimizing of polyvinylidene fluoride (PVDF) nanofiber separator for safe lithium-ion battery. J. Appl. Polym. Sci. 2022, 139, 52154. [Google Scholar] [CrossRef]
- Song, Y.; Bao, J.; Hu, Y.; Cai, H.; Xiong, C.; Yang, Q.; Tian, H.; Shi, Z. Forward polarization enhanced all-polymer based sustainable triboelectric nanogenerator from oriented electrospinning PVDF/cellulose nanofibers for energy harvesting. Sustain. Energy Fuels 2022, 6, 2377–2386. [Google Scholar] [CrossRef]
- Zabek, D.; Seunarine, K.; Spacie, C.; Bowen, C. Graphene Ink Laminate Structures on Poly(vinylidene difluoride) (PVDF) for Pyroelectric Thermal Energy Harvesting and Waste Heat Recovery. ACS Appl. Mater. Interfaces 2017, 9, 9161–9167. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Cai, K.; Wang, Y. A distinct mutual phase transition in a new PVDF based lead-free composite film with enhanced dielectric and energy storage performance and low loss. J. Mater. Chem. C 2017, 5, 2531–2541. [Google Scholar] [CrossRef]
- Chen, X.; Han, X.; Shen, Q.-D. PVDF-Based Ferroelectric Polymers in Modern Flexible Electronics. Adv. Electron. Mater. 2017, 3, 460. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Wu, X.; Ma, H.; Robertson, J.; Nathan, A. Ultrathin Multi-functional Graphene-PVDF Layers for Multidimensional Touch Interactivity for Flexible Displays. ACS Appl. Mater. Interfaces 2017, 9, 18410–18416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, P.; Yi, N.; Zhang, T.; Huang, Y.; Chang, H.; Yang, Y.; Zhou, Y.; Chen, Y. Construction of a Fish-like Robot Based on High Performance Graphene/PVDF Bimorph Actuation Materials. Adv. Sci. 2016, 3, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.-F.; Yan, X.; Yu, M.; Jia, M.-Y.; Pan, W.; He, X.-X.; Han, W.-P.; Zhang, Z.-M.; Yu, L.-M.; Long, Y.-Z. Patterned, highly stretchable and conductive nanofibrous PANI/PVDF strain sensors based on electrospinning and in situ polymerization. Nanoscale 2016, 8, 2944–2950. [Google Scholar] [CrossRef]
- Sahoo, R.; Mishra, S.; Unnikrishnan, L.; Mohanty, S.; Mahapatra, S.; Nayak, S.K.; Anwar, S.; Ramadoss, A. Enhanced dielectric and piezoelectric properties of Fe-doped ZnO/PVDF-TrFE composite films. Mater. Sci. Semicond. Process. 2020, 117, 105173. [Google Scholar] [CrossRef]
- Kadir, E.; Gayen, R.; Paul, R.; Biswas, S. Interfacial effects on ferroelectric and dielectric properties of GO reinforced free-standing and flexible PVDF/ZnO composite membranes: Bias dependent impedance spectroscopy. J. Alloy Compd. 2020, 843, 155974. [Google Scholar] [CrossRef]
- Meng, N.; Zhu, X.; Mao, R.; Reece, M.J.; Bilotti, E. Nanoscale interfacial electroactivity in PVDF/PVDF-TrFE blended films with enhanced dielectric and ferroelectric properties. J. Mater. Chem. C 2017, 5, 3296–3305. [Google Scholar] [CrossRef]
- Chae, I.; Ahmed, S.; Ben Atitallah, H.; Luo, J.; Wang, Q.; Ounaies, Z.; Kim, S.H. Vibrational Sum Frequency Generation (SFG) Analysis of Ferroelectric Response of PVDF-Based Copolymer and Terpolymer. Macromolecules 2017, 50, 2838–2844. [Google Scholar] [CrossRef]
- Bhagavathula, S.D.; Varaprasad, K.; Acuna, D.Q.; Koduri, R.; Veluri, S.; Reddy, V. Insight of electrical behavior in ferroelectric-semiconductor polymer nanocomposite films of PVDF/ZnSe and PVDF/Cu:ZnSe. J. Appl. Polym. Sci. 2017, 134, 44983. [Google Scholar] [CrossRef]
- Ma, J.; Nan, X.; Liu, J. Investigation of the dielectric, mechanical, and thermal properties of noncovalent functionalized MWCNTs/polyvinylidene fluoride (PVDF) composites. Polym. Adv. Technol. 2016, 28, 166–173. [Google Scholar] [CrossRef]
- Issa, A.A.; Al-Maadeed, M.; Luyt, A.S.; Mrlik, M.; Hassan, M.K. Investigation of the physico-mechanical properties of electrospun PVDF/cellulose (nano)fibers. J. Appl. Polym. Sci. 2016, 133, 43594. [Google Scholar] [CrossRef]
- Motamedi, A.S.; Mirzadeh, H.; Hajiesmaeilbaigi, F.; Khoulenjani, S.B.; Shokrgozar, M.A. Piezoelectric electrospun nanocomposite comprising Au NPs/PVDF for nerve tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 1984–1993. [Google Scholar] [CrossRef]
- Sanchez, F.A.; González-Benito, J. PVDF/BaTiO3/Carbon Nanotubes Ternary Nanocomposites: Effect of Nanofillers and Processing. Polym. Compos. 2017, 38, 227–235. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Bu, D.; Feng, Y.; Chi, M. Influence of spinel ZnMn2O4 particles on the dielectric performance of PVDF-based composites. Polym. Compos. 2022, 48, 8715–8724. [Google Scholar] [CrossRef]
- Kim, M.; Fan, J. Piezoelectric Properties of Three Types of PVDF and ZnO Nanofbrous Composites. Adv. Fiber Mater. 2021, 3, 160–171. [Google Scholar] [CrossRef]
- Zhao, Y.; Da, X.; Weng, X.; Gao, Y.; Gao, G.; Su, Y.; Ding, S. High lithium salt content PVDF-based solid-state composite polymer electrolyte enhanced by h-BN nanosheets. ChemSusChem 2022, 15, e202201554. [Google Scholar] [CrossRef]
- Panda, M. Filler dependent microstructural and optical properties of PVDF/PEG blends versus PVDF/GO nanocomposites. Opt. Quantum Electron. 2022, 54, 765. [Google Scholar] [CrossRef]
- Ramanujam, B.T.S.; Adhyapak, P.V.; Radhakrishnan, S.; Marimuthu, R. Effect of casting solvent on the structure development, electrical, thermal behavior of polyvinylidene fuoride (PVDF)–carbon nanofber (CNF) conducting binary and hybrid nanocomposites. Polym. Bull. 2021, 78, 1735–1751. [Google Scholar] [CrossRef]
- Verma, S.; Sharma, M.; Vaish, R. Photo-piezocatalysis in electrospun PVDF + WS2 membrane. Environ. Sci. Nano 2022, 9, 3885–3899. [Google Scholar] [CrossRef]
- Su, Y.; Li, W.; Cheng, X.; Zhou, Y.; Yang, S.; Zhang, X.; Chen, C.; Yang, T.; Pan, H.; Xie, G.; et al. High-performance piezoelectric composites via β phase programming. Nat. Commun. 2022, 13, 4867. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.; Koch, M.M.; Rata, A.D.; Dörr, K. Mechanical Nanoscale Polarization Control in Ferroelectric PVDF-TrFE Films. Adv. Electron. Mater. 2022, 8, 2101416. [Google Scholar] [CrossRef]
- Pusty, M.; Lichchhavi; Shirag, P.M. Defect-Induced Self-Poling in a W18O49/PVDF Piezoelectric Energy Harvester. Langmuir 2022, 38, 11787–11800. [Google Scholar] [CrossRef]
- Sharma, S.; Mishra, S.S.; Kumar, R.P.; Yadav, R.M. Recent progress on polyvinylidene difluoride based nanocomposite: Applications in energy harvesting and sensing. New J. Chem. 2022, 46, 18613–18646. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. A comprehensive review on fundamental properties and applications of poly(vinylidene fuoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Sellami, F.; Kebiche-Senhadji, O.; Marais, S.; Colasse, L.; Fatyeyeva, K. Enhanced removal of Cr(VI) by polymer inclusion membrane based on poly (vinylidene flfluoride) and Aliquat 336. Sep. Purif. Technol. 2020, 248, 117038. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Naddeo, V.; Banat, F.; Hasan, S.W. Preparation of novel polyvinylidene fluoride (PVDF)-Tin(IV) oxide (SnO2) ion exchange mixed matrix membranes for the removal of heavy metals from aqueous solutions. Sep. Purif. Technol. 2020, 250, 117250. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, Z.; Jiang, Y.; Yu, G.; Wang, Q.; Liu, W. One-step in situ preparation of flexible CuS/TiO2/polyvinylidene fluoride fibers with controlled surface morphology for visible light-driven photocatalysis. J. Phys. Chem. Solids 2020, 144, 109512. [Google Scholar] [CrossRef]
- Kolesnyk, I.; Kujawa, J.; Bubela, H.; Konovalova, V.; Burban, A.; Cyganiuk, A.; Kujawski, W. Photocatalytic properties of PVDF membranes modified with g-C3N4 in the process of Rhodamines decomposition. Sep. Purif. Technol. 2020, 250, 117231. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, L.; Huang, L.; Wu, L.; Li, C.; Cai, J. Photo-induced antifouling polyvinylidene fluoride ultrafiltration mem-brane driven by weak visible Light. J. Ind. Eng. Chem. 2020, 89, 476–484. [Google Scholar] [CrossRef]
- Ahsani, M.; Hazrati, H.; Javadi, M.; Ulbricht, M.; Yegani, R. Preparation of antibiofouling nanocomposite PVDF/Ag-SiO2 membrane and long-term performance evaluation in the MBR system fed by real pharmaceutical wastewater. Sep. Purif. Technol. 2020, 249, 116938. [Google Scholar] [CrossRef]
- Sun, J.; Liu, L.; Yang, F. A composite cathode membrane with CoFe2O4–rGO/PVDF on carbon fiber cloth: Synthesis and performance in a photocatalysis-assisted MFCMBR system. Environ. Sci. Nano. 2017, 4, 335–345. [Google Scholar]
- Nassrullah, H.; Makanjuola, O.; Janajreh, I.; AlMarzooqi, F.A.; Hashaikeh, R. Incorporation of nanosized LTL zeolites in dual-layered PVDF-HFP/cellulose membrane for enhanced membrane distillation performance. J. Membr. Sci. 2020, 611, 118298. [Google Scholar] [CrossRef]
- Radzi, N.H.M.; Ahmad, A.L. Double layer PVDF blends PVDF-HFP membrane with modified ZnO nanoparticles for direct contact membrane distillation (DCMD). Asia-Pacific J. Chem. Eng. 2022, 17, e2753. [Google Scholar] [CrossRef]
- Mahdavi, H.; Zeinalipour, N.A.; Heidari, A. Fabrication of PVDF mixed matrix nanofiltration membranes incorporated with TiO2 nanoparticles and an amphiphilic PVDF-g-PMMA copolymer. J. Appl. Polym. 2022, 139, e52740. [Google Scholar] [CrossRef]
- Thamizhlarasan, A.; Vignesh, R.; Anbarasan, R.; Tung, K. Synthesis and characterization of functionalized polyvinylidene fluoride (PVDF) and the high temperature catalytic activity of PVDF-g-MAH/V2O5 nanocomposite toward transesterification reaction. Polym. Eng. Sci. 2022, 62, 3010–3025. [Google Scholar] [CrossRef]
- Van Goethem, C.; Magboo, M.M.; Mertens, M.; Thijs, M.; Koeckelberghs, G.; Vankelecom, I.F. A scalable crosslinking method for PVDF-based nanofiltration membranes for use under extreme pH conditions. J. Membr. Sci. 2020, 611, 118274. [Google Scholar] [CrossRef]
- Pan, T.; Liu, Y.; Li, Z.; Fan, J.; Wang, L.; Liu, J.; Shou, W. A Sm-doped Egeria-densa-like ZnO nanowires@PVDF nanofiber membrane for high-effificiency water clean. Sci. Total Environ. 2020, 737, 139818. [Google Scholar] [CrossRef]
- Park, H.M.; Oh, H.; Jee, K.Y.; Lee, Y.T. Synthesis of PVDF/MWCNT nanocomplex microfiltration membrane via atom transfer radical addition (ATRA) with enhanced fouling performance. Sep. Purif. Technol. 2020, 246, 116860. [Google Scholar] [CrossRef]
- Ji, K.; Ma, X.; Xu, H.; Yin, J.; Jiang, X. Hyperbranched Poly (ether amine)@Poly (vinylidene fluoride) Porous Membrane (hPEA@PVDF) for Selective Adsorption and Molecular Filtration of Hydrophilic Dyes. J. Mater. Chem. A 2017, 5, 10470–10479. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Y.; Xuan, H.; He, C. Investigation of one-dimensional multi-functional zwitterionic Ag nanowires as a novel modifier for PVDF ultrafiltration membranes. New J. Chem. 2015, 40, 441–446. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, Q.; Gong, H.; Du, M. Preparation of a novel dual-layer polyvinylidene fluoride hollow fiber composite membrane with hydrophobic inner layer for carbon dioxide absorption in a membrane contactor. Sep. Purif. Technol. 2020, 248, 117045. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Wang, L.; Zhao, B.; Li, J.; Zhang, H. Wetting mechanism of a PVDF hollow fiber membrane in immersed membrane contactors for CO2 capture in the presence of monoethanolamine. RSC Adv. 2017, 7, 13451–13457. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Liu, Q. Oxidant-Induced High-Efficient Mussel-Inspired Modification on PVDF Membrane with Superhy-drophilicity and Underwater Superoleophobicity Characteristics for Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 8297–8307. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, W.; Zhang, F.; Xing, T.; Jin, J. Superhydrophilic In-Situ-Cross-Linked Zwitterionic Polyelectrolyte/PVDF-Blend Membrane for Highly Efficient Oil/Water Emulsion Separation. ACS Appl. Mater. Interfaces 2017, 9, 9603–9613. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Lei, W.; Yu, P.; Luo, Y. Polyvinylidene fluoride (PVDF)/hydrophobic nanosilica (H-SiO2) coated superhydro-phobic porous materials for water/oil separation. RSC Adv. 2016, 6, 10365–10371. [Google Scholar] [CrossRef]
- Venkatesh, K.; Arthanareeswaran, G.; Bose, A.C. PVDF mixed matrix nano-filtration membranes integrated with 1D-PANI/TiO2 NFs for oil–water emulsion separation. RSC Adv. 2016, 6, 18899–18908. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Sun, H.; Jia, F.; Kumar, P.; Liu, B. Synthesis and room-temperature H2S sensing of Pt nanoparticle func-tionalized SnO2 mesoporous nanoflflowers. J. Alloy. Compd. 2020, 842, 155813. [Google Scholar] [CrossRef]
- Chang, F.; Huang, J.; Chen, C. Au/Pt/Pd spherically self-assembled nano-sieves as SERS sensors. J. Alloy. Compd. 2020, 843, 155885. [Google Scholar] [CrossRef]
- Xu, S.; Gao, T.; Feng, X.; Fan, X.; Liu, G.; Mao, Y.; Yu, X.; Lin, J.; Luo, X. Near infrared fluorescent dual ligand functionalized Au NCs based multidimensional sensor array for pattern recognition of multiple proteins and serum discrimination. Biosens. Bioelectron. 2017, 97, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Lin, X.; Zhu, J.-J.; Tong, Q.-X. Pd-Au@carbon dots nanocomposite: Facile synthesis and application as an ultrasensitive electrochemical biosensor for determination of colitoxin DNA in human serum. Biosens. Bioelectron. 2017, 94, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, X.; Sun, X.; Ma, H.; Zhang, Y.; Wei, Q. Dual-responsive electrochemical immunosensor for prostate specific antigen detection based on Au-CoS/graphene and CeO2/ionic liquids doped with carboxymethyl chitosan complex. Biosens. Bioelectron. 2017, 94, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tai, H.; Zhang, P.; Ye, Z.; Su, Y.; Jiang, Y. Enhanced ammonia-sensing properties of PANI-TiO2-Au ter-naryself-assembly nanocomposite thin film at room temperature. Sens. Actuators B 2017, 246, 85–95. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A. Novel Cu2ZnSnS4/Pt/g-C3N4 heterojunction photocatalyst with straddling band con-figuration for enhanced solar to fuel conversion. Appl. Catal. B: Environ. 2020, 277, 119239. [Google Scholar] [CrossRef]
- Barba-Nieto, I.; Caudillo-Flores, U.; Gómez-Cerezo, M.N.; Kubacka, A.; Fernández-García, M. Boosting Pt/TiO2 hydrogen photoproduction through Zr doping of the anatase structure: A spectroscopic and mechanistic study. Chem. Eng. J. 2020, 398, 125665. [Google Scholar] [CrossRef]
- Barba-Nieto, I.; Christoforidis, K.C.; Fernández-García, M.; Kubacka, A. Promoting H2 photopro-duction of TiO2-based materials by surface decoration with Pt nanoparticles and SnS2 nanoplatelets. Appl. Catal. B Environ. 2020, 277, 119246. [Google Scholar] [CrossRef]
- da Silva, A.G.; Fernandes, C.G.; Hood, Z.D.; Peng, R.; Wu, Z.; Dourado, A.H.; Parreira, L.S.; de Oliveira, D.C.; Camargo, P.H.; de Torresi, S.I.C. Pd-Pt-TiO2 nanowires: Correlating composition, electronic effffects and O vacancies with activities towards water splitting and oxygen reduction. Appl. Catal. B Environ. 2020, 277, 119177. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Han, Y.; Yu, Y.; Xu, M.; Zhang, X.; Huang, L.; Dong, S. RGO/Au NPs/N-doped CNTs supported on nickel foam as an anode for enzymatic biofuel cells. Biosens. Bioelectron. 2017, 97, 34–40. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Wang, X.; Hu, J.; Liu, Y.; Fu, G.; Tang, Y. Concave PtCo nanocrosses for methanol oxidation reaction. Appl. Catal. B: Environ. 2020, 277, 119135. [Google Scholar] [CrossRef]
- Alvear, M.; Aho, A.; Simakova, I.L.; Grénman, H.; Salmi, T.; Murzin, D.Y. Aqueous phase reforming of alcohols over a bimetallic Pt-Pd catalyst in the presence of formic acid. Chem. Eng. J. 2020, 398, 125541. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, L.; Hao, H.; Cai, H.; Gao, P.; Liu, F.; Yu, X.; Wu, C.; Yang, S. The “electric-dipole” effect of Pt/Ni for enhanced catalytic dehydrogenation of ammonia borane. J. Alloy. Compd. 2020, 844, 156253. [Google Scholar] [CrossRef]
- Clauser, A.L.; Giulian, R.; McClure, Z.D.; Sarfo, K.O.; Ophus, C.; Ciston, J.; Árnadóttir, L.; Santala, M.K. Orientation and morphology of Pt nanoparticles in γ -alumina processed via ion implantation and thermal annealing. Scr. Mater. 2020, 188, 44–49. [Google Scholar] [CrossRef]
- Amoli-Diva, M.; Sadighi-Bonabi, R.; Pourghazi, K. Switchable on/off drug release from gold nanoparticles-grafted dual light- and temperature-responsive hydrogel for controlled drug delivery. Mater. Sci. Eng. C 2017, 76, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Wang, J.; Zhang, X.-D. Near-infrared II emissive metal clusters: From atom physics to biomedicine. Coord. Chem. Rev. 2021, 448, 214184. [Google Scholar] [CrossRef]
- Schmidt, I.; Gad, A.; Scholz, G.; Boht, H.; Martens, M.; Schilling, M.; Wasisto, H.S.; Waag, A.; Schröder, U. Gold-modified indium tin oxide as a transparent window in optoelectronic diagnostics of electrochemically active biofilms. Biosens. Bioelectron. 2017, 94, 74–80. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Liu, Y.; Zhang, L.; Wang, F.; Lu, Y. High performance surface-enhanced Raman scattering sensing based on Au nanoparticle-monolayer graphene-Ag nanostar array hybrid system. Sensors Actuators B Chem. 2017, 247, 850–857. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, B.; Cong, Q.; He, X.; Gao, M.; Li, G. Organic/inorganic nanocomposites of ZnO/CuO/chitosan with improved properties. Mater. Chem. Phys. 2016, 178, 88–97. [Google Scholar] [CrossRef]
- Cong, Q.; He, X.; Gao, M.; Ma, X.; Li, G. ZnO/CuS heterostructured nanocomposite and its organic functionalization. Mater. Res. Innov. 2014, 18, 740–746. [Google Scholar] [CrossRef]
- Cong, Q.; Geng, H.; He, X.; Gao, M.; Ma, X.; Li, G. Surface modification of ZnO nanosheets with Au/polyaniline and their properties. Mater. Res. Innov. 2014, 18, 30–36. [Google Scholar]
- Ma, X.; Wang, M.; Li, G.; Chen, H.; Bai, R. Preparation of Polyaniline-TiO2 Composite Film with in-situ Polymerization Approach and Its Gas-sensitivity at Room Temperature. Mater. Chem. Phys. 2006, 98, 241–247. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, B.; Cong, Q.; He, X.; Gao, M.; Li, G. Highly-Enhanced Performance of TiO2 Nanotube Attached CdS Quantum Dots. Curr. Nanosci. 2016, 12, 500–507. [Google Scholar] [CrossRef]
- Ma, X.; Liu, A.; Xu, H.; Li, G.; Hu, M.; Wu, G. A large-scale-oriented ZnO rod array grown on a glass substrate via an in situ deposition method and its photoconductivity. Mater. Res. Bull. 2008, 43, 2272–2277. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Qiu, J.; Du, W.; He, X.; Gao, M.; Li, X.; Ma, X.; Li, G. Dispersion of Graphene Oxide in Polyvinylidene Difluoride and Its Improvement of Photoresponse Properties of Nanocomposite, Advanced Functional Materials. In Proceedings of the Chinese Materials Conference 2017 (CMC 2017), Yinchuan City, China, 6–12 July 2017; pp. 805–815. [Google Scholar]
- Ma, X.; Li, C.; Zhang, X.; Gao, M.; Li, G. Broadband Spectrum Light-Driven PANI/Au/Beta-Cyclodextrin Nanocomposite and Its Light-Triggered Interfacial Carrier Transfer. Coatings 2022, 12, 1401. [Google Scholar] [CrossRef]
- Fazal, S.; Jayasree, A.; Sasidharan, S.; Koyakutty, M.; Nair, S.V.; Menon, D. Green Synthesis of Anisotropic Gold Nanoparticles for Photothermal Therapy of Cancer. ACS Appl. Mater. Interfaces 2014, 6, 8080–8089. [Google Scholar] [CrossRef]
- Toker, D.; Azulay, D.; Shimoni, N.; Balberg, I.; Millo, O. Tunneling and percolation in metal-insulator composite materials. Phys. Rev. B 2003, 68, 041403(R). [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Li, W.; Wang, T.; Jiang, L.; Luo, L.; Hua, D.; Zhu, Y. The influence of self-assembly behavior of nanoparticles on the dielectric polymer composites. AIP Adv. 2013, 3, 112106. [Google Scholar] [CrossRef]
- Panda, M.; Srinivas, V.; Thakur, A.K. Surface and interfacial effect of filler particle on electrical properties of polyvi-nyledene fluoride/nickel composites. Appl. Phys. Lett. 2008, 93, 242908. [Google Scholar] [CrossRef]
- Panda, M.; Srinivas, V.; Thakur, A.K. Role of polymer matrix in large enhancement of dielectric constant in polymermetal composites. Appl. Phys. Lett. 2011, 99, 042905. [Google Scholar] [CrossRef]
- Deepa, K.S.; Sebastian, M.T.; James, J. Effect of interparticle distance and interfacial area on the properties of insu-latorconductor composites. Appl. Phys. Lett. 2007, 91, 202904. [Google Scholar] [CrossRef]
- Lee, G.-H.; Yu, Y.-J.; Lee, C.; Dean, C.; Shepard, K.L.; Kim, P.; Hone, J. Electron tunneling through atomically flat and ultrathin hexagonal boron nitride. Appl. Phys. Lett. 2011, 99, 243114. [Google Scholar] [CrossRef]
- Perea-López, N.; Elías, A.L.; Berkdemir, A.; Castro-Beltran, A.; Gutiérrez, H.R.; Feng, S.; Lv, R.; Hayashi, T.; Lopez-Urias, F.; Gosh, S.; et al. Photosensor Device Based on Few-Layered WS2 Films. Adv. Funct. Mater. 2013, 23, 5511–5517. [Google Scholar] [CrossRef]
- Kim, M.; Wang, Y.; Kim, D.; Shao, Q.; Lee, H.; Park, H. Resistive switching properties for fluorine doped titania fab-ricated using atomic layer deposition. APL Mater. 2022, 10, 031105. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Meng, F.; Wang, P.; Wang, S.; Liang, S.; Miao, F. 2D Layered Materials for Memristive and Neu-romorphic Applications. Adv. Electron. Mater. 2019, 6, 1901107. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Xiong, L.; Yuan, X.; Li, P.; Wu, J.; Bi, H.; Zhang, J. Resistive switching behavior of the memristor based on WS2 nanosheets and polyvinylpyrrolidone nanocomposites. Appl. Phys. Lett. 2022, 120, 232105. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, D.; Yang, Y.; Du, C.; Zhang, B. A fast self-healing multifunctional polyvinyl alcohol nano-organic composite hydrogel as a building block for highly sensitive strain/pressure sensors. J. Mater. Chem. A 2021, 9, 22082–22094. [Google Scholar] [CrossRef]
- Huang, X.; Qin, Q.; Wang, X.; Xiang, H.; Zheng, J.; Lu, Y.; Lv, C.; Wu, K.; Yan, L.; Wang, N.; et al. Piezoelectric Nanogenerator for Highly Sensitive and Synchronous Multi-Stimuli Sensing. ACS Nano 2021, 15, 19783–19792. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Li, C.; Gao, M.; Zhang, X.; Wang, Y.; Li, G. Interface Optimization of Metal Quantum Dots/Polymer Nanocomposites and their Properties: Studies of Multi-Functional Organic/Inorganic Hybrid. Materials 2023, 16, 150. https://doi.org/10.3390/ma16010150
Ma X, Li C, Gao M, Zhang X, Wang Y, Li G. Interface Optimization of Metal Quantum Dots/Polymer Nanocomposites and their Properties: Studies of Multi-Functional Organic/Inorganic Hybrid. Materials. 2023; 16(1):150. https://doi.org/10.3390/ma16010150
Chicago/Turabian StyleMa, Xingfa, Caiwei Li, Mingjun Gao, Xintao Zhang, You Wang, and Guang Li. 2023. "Interface Optimization of Metal Quantum Dots/Polymer Nanocomposites and their Properties: Studies of Multi-Functional Organic/Inorganic Hybrid" Materials 16, no. 1: 150. https://doi.org/10.3390/ma16010150





