Electrical and Structural Properties of Semi-Polar-ZnO/a-Al2O3 and Polar-ZnO/c-Al2O3 Films: A Comparative Study
Abstract
1. Introduction
2. Growth Details and Experimental Techniques
3. Experimental Results
3.1. AFM
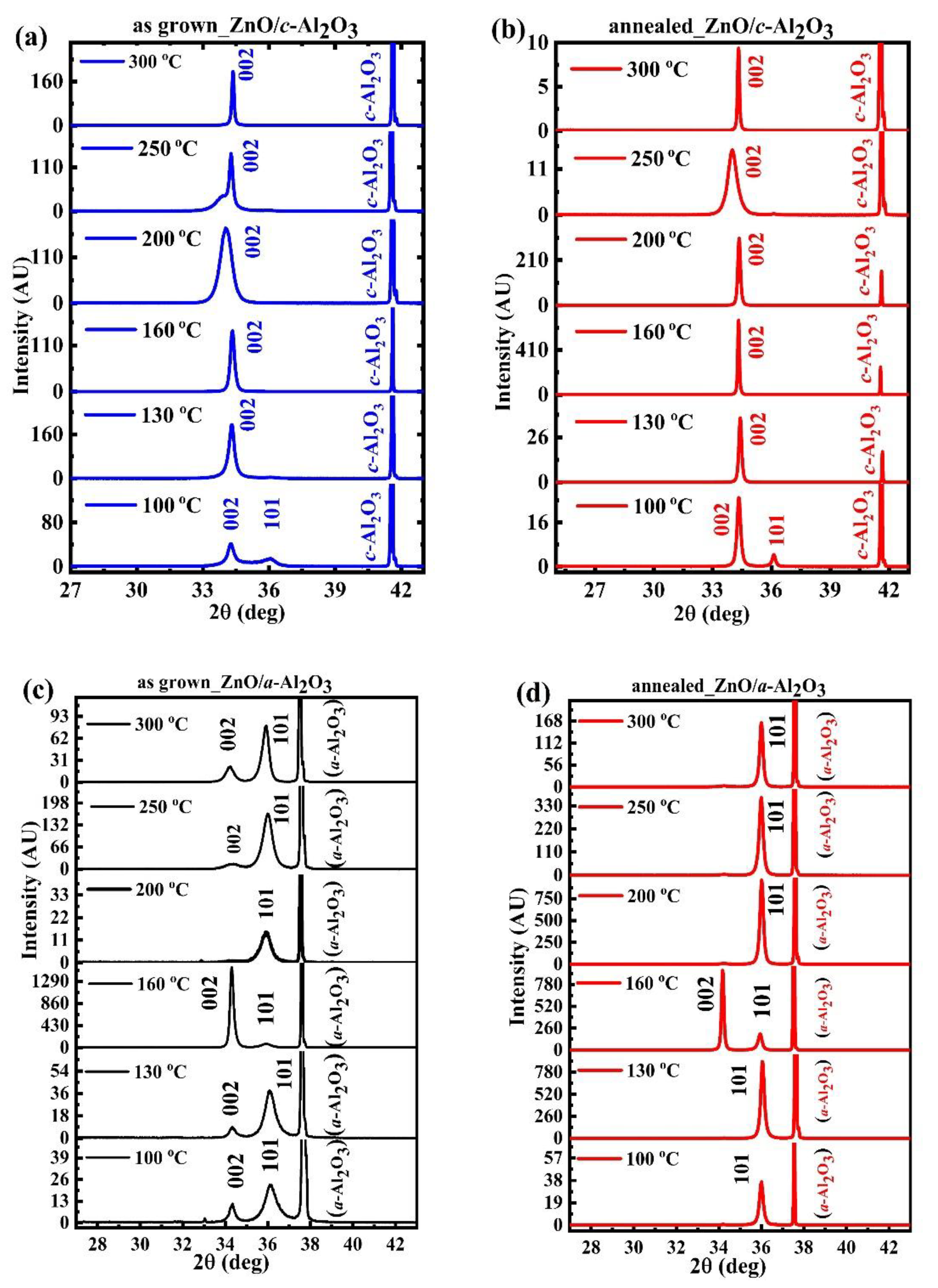
3.2. XRD
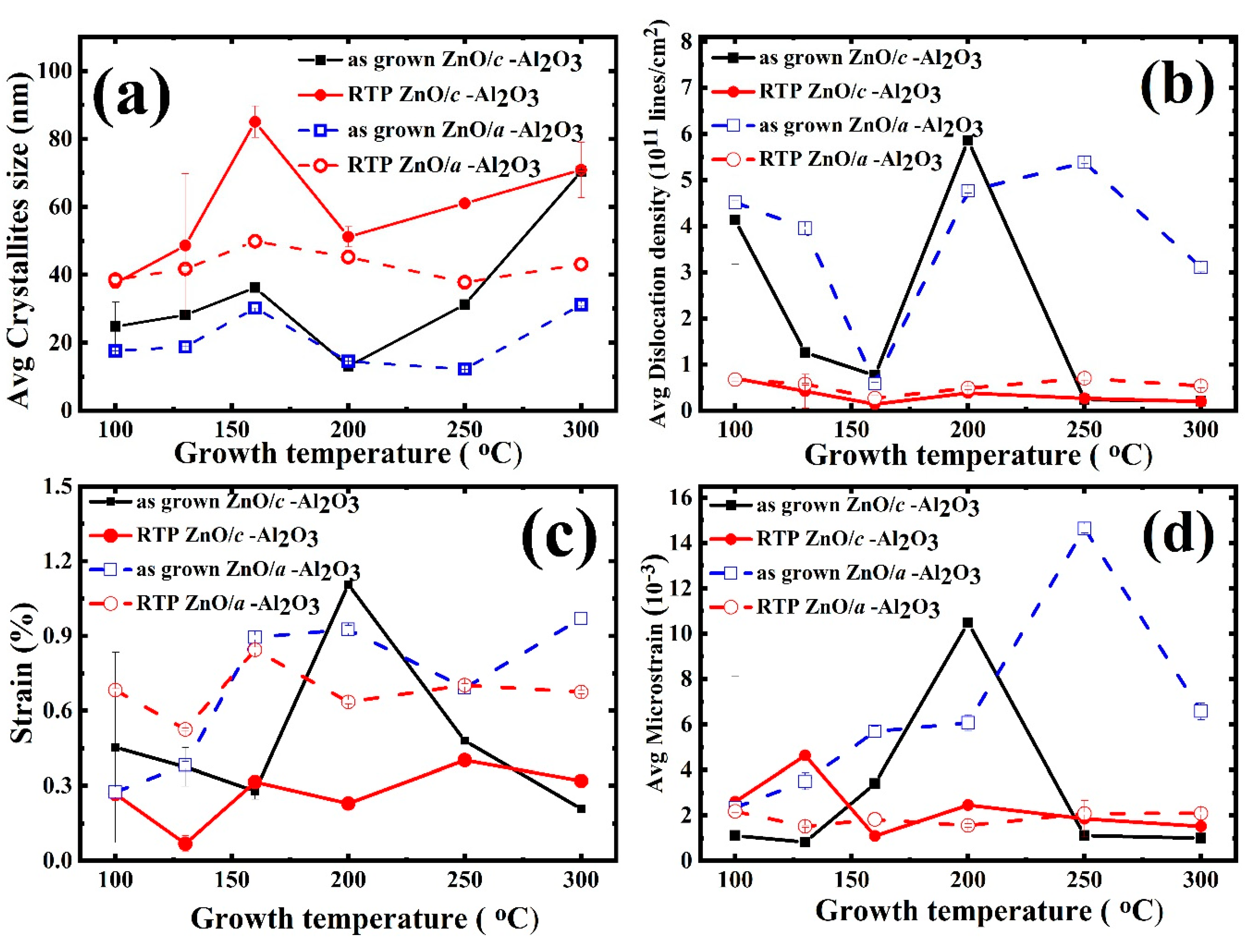
3.2.1. Crystallite Size
3.2.2. Dislocation Density
3.2.3. Strain and Micro-Strain
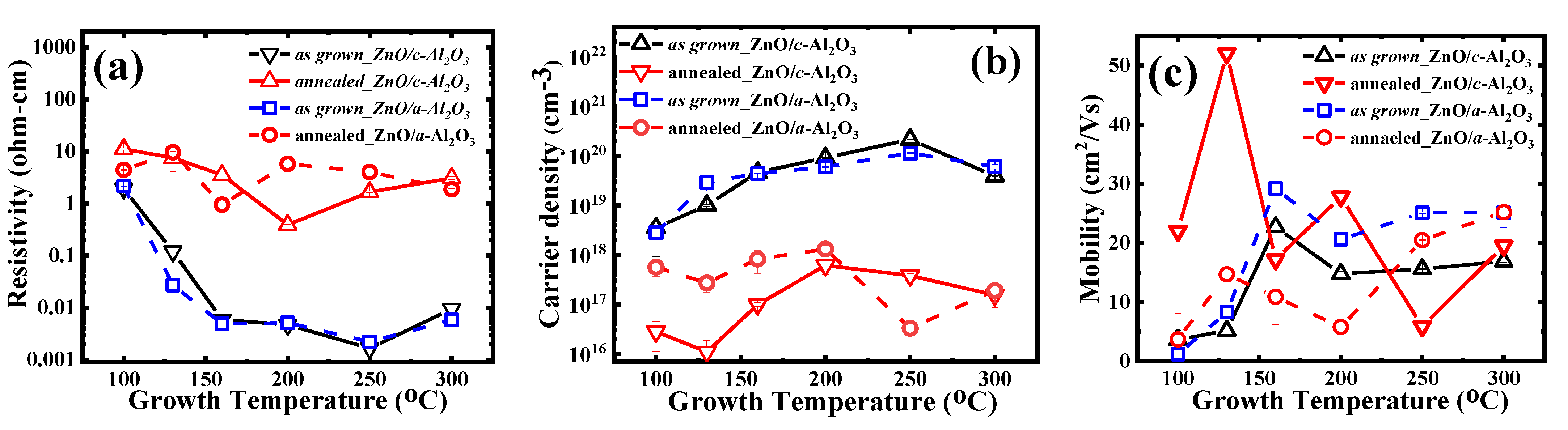
3.3. Electrical Properties
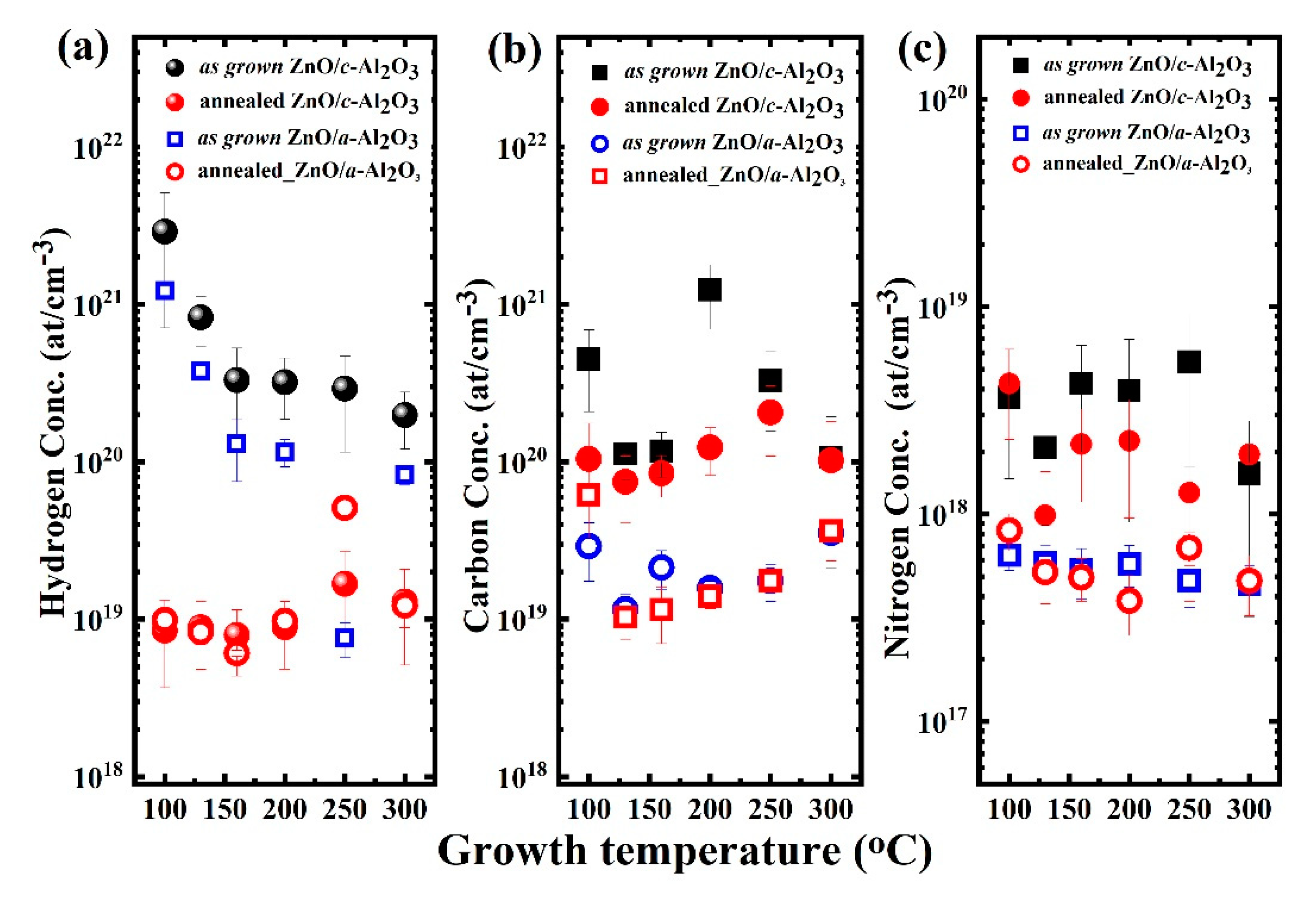
3.4. SIMS
4. Summary and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Shaikh, S.M.F.; Rahman, G.; Mane, R.S.; Joo, O.S. Bismuth oxide nanoplates-based efficient DSSCs: Influence of ZnO surface passivation layer. Electrochim. Acta 2013, 111, 593–600. [Google Scholar] [CrossRef]
- Ayoub, I.; Kumar, V.; Abolhassani, R.; Sehgal, R.; Sharma, V.; Sehgal, R.; Swart, H.C.; Mishra, Y.K. Advances in ZnO: Manipulation of defects for enhancing their technological potentials. Nanotechnol. Rev. 2022, 11, 575–619. [Google Scholar] [CrossRef]
- Frankenstein, H.; Leng, C.Z.; Losego, M.D.; Frey, G.L. Atomic layer deposition of ZnO electron transporting layers directly onto the active layer of organic solar cells. Org. Electron. 2019, 64, 37–46. [Google Scholar] [CrossRef]
- Shimizu, H.; Sato, W. Interactions of intrinsic defects formed in ZnO and their contribution to electric conductivity. J. Appl. Phys. 2019, 126, 125704. [Google Scholar] [CrossRef]
- Lv, J.; Li, C.; Choi, Z. Defect luminescence and its mediated physical properties in ZnO. J. Lumin. 2019, 208, 225–237. [Google Scholar] [CrossRef]
- Sato, W.; Takata, M.; Shimizu, H.; Komatsuda, S.; Yoshida, Y.; Moriyama, A.; Shimamura, K.; Ohkubo, Y. Atomic level control of association-dissociation behavior of In impurities in polycrystalline ZnO. Phys. Rev. Mat. 2022, 6, 063801. [Google Scholar] [CrossRef]
- Cossuet, T.; Donatini, T.; Lord, A.M.; Appert, E.; Pernot, J.; Consonni, V. Polarity-dependent high electrical conductivity of ZnO nanorods and its relation to hydrogen. J. Phys. Chem. C 2018, 122, 22767–22775. [Google Scholar] [CrossRef]
- Przezdziecka, E.; Guziewicz, E.; Jarosz, D.; Snigurenko, D.; Sulich, A.; Sybilski, P.; Jakiela, R.; Paszkowicz, W. Influence of oxygen-rich and zinc-rich conditions on donor and acceptor states and conductivity mechanism of ZnO films grown by ALD—Experimental studies. J. Appl. Phys. 2020, 127, 075104. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, K.M.; Kim, M.; Park, H.H. Low temperature method to passivate oxygen vacancies in un-doped ZnO films using atomic layer deposition. Thin Solid Film. 2018, 660, 852–858. [Google Scholar] [CrossRef]
- Saini, S.; Mele, P.; Honda, H.; Suzuki, T.; Matsumoto, K.; Miyazaki, K.; Tiwari, A. Effect of self-grown seed layer on thermoelectric properties of ZnO thin films. Thin Solid Films 2016, 605, 289–294. [Google Scholar] [CrossRef]
- Mishra, S.; Przezdziecka, E.; Wozniak, W.; Adhikari, A.; Jakiela, R.; Paszkowicz, W.; Guziewicz, E. Structural properties of thin ZnO films deposited by ALD under O-rich and Zn-Rich conditions and their relationship with electrical parameters. Materials 2021, 14, 4048. [Google Scholar] [CrossRef] [PubMed]
- Kalusniak, S.; Sadofev, S.; Puls, J.; Wünsche, H.J.; Henneberger, F. Polarization fields in (Zn, Cd) O∕ZnO quantum well structures. Phys. Rev. B 2008, 77, 113312. [Google Scholar] [CrossRef]
- Pung, S.Y.; Choy, K.L.; Hou, X.; Shan, C. Preferential growth of ZnO thin films by the atomic layer deposition technique. Nanotechnology 2008, 19, 435609. [Google Scholar] [CrossRef]
- Speck, J.S.; Chichibu, S.F. Nonpolar and semipolar group III nitride-based materials. MRS Bull. 2009, 34, 304–312. [Google Scholar] [CrossRef]
- Baxter, J.B.; Aydil, E.S. Epitaxial growth of ZnO nanowires on a- and c-plane sapphire. J. Cryst. Growth 2005, 274, 407. [Google Scholar] [CrossRef]
- Tynell, T.; Karppinen, M. Atomic layer deposition of ZnO: A review. Semicond. Sci. Technol. 2014, 29, 043001. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Liu, J.; Adair, K.; Zhao, F.; Sun, Y.; Wu, T.; Bi, X.; Amine, K.; Liu, J.; et al. Atomic/molecular layer deposition for energy storage and conversion. Chem. Soc. Rev. 2021, 50, 3889. [Google Scholar]
- Crivello, C.; Sevim, S.; Graniel, O.; Franco, C.; Pane, S.; Puigmarti-Luis, J.; Munoz-Rojas, D. Advanced technologies for the fabrication of MOF thin films. Mat. Horizons 2021, 8, 168. [Google Scholar] [CrossRef]
- Guziewicz, E.; Godlewski, M.; Wachnicki, L.; Krajewski, T.A.; Luka, G.; Gieraltowska, S.; Jakiela, R.; Stonert, A.; Lisowski, W.; Krawczyk, M.; et al. ALD grown zinc oxide with controllable electrical properties. Semicond. Sci. Technol. 2012, 27, 074011. [Google Scholar] [CrossRef]
- Jakiela, R. The role of atmospheric elements in wide band gap semiconductors. Acta Phys. Pol. A 2019, 136, 916. [Google Scholar] [CrossRef]
- McMurdie, H.F.; Morris, M.C.; Evans, E.H.; Paretzkin, B.; Wonh-Ng, W.; Ettinger, L.; Hubbard, C.R. Standart X-ray diffraction powder pattern from the JCPDS research associateship. Powder Diffr. 1986, 1, 64–77. [Google Scholar] [CrossRef]
- Kondoh, J. Origin of the hump on the left shoulder of the X-ray diffraction peaks observed in Y2O3-fully and partially stabilized ZrO2. J. Alloys Compd. 2004, 375, 270–282. [Google Scholar] [CrossRef]
- De Keijser, T.H.; Langford, J.I.; Mittemeijer, E.J.; Vogels, A.B.P. Use of the Voigt function in a single-line method for the analysis of X-ray diffraction line broadening. J. Appl. Crystallogr. 1982, 15, 308–314. [Google Scholar] [CrossRef]
- De Keijser, T.H.; Mittemeijer, E.J.; Rozendaal, H.C.F. The determination of crystallite-size and lattice-strain parameters in conjunction with the profile-refinement method for the determination of crystal structures. J. Appl. Crystallogr. 1983, 16, 309–316. [Google Scholar] [CrossRef]
- Krajewski, T.A.; Luka, G.; Wachnicki, L.; Zakrzewski, A.J.; Witkowski, B.S.; Lukasiewicz, M.I.; Kruszewski, P.; Lusakowska, E.; Jakiela, R.; Godlewski, M.; et al. Electrical parameters of ZnO films and ZnO-based junctions obtained by atomic layer deposition. Semicond. Sci. Technol. 2011, 26, 085013. [Google Scholar] [CrossRef]
- Mendil, D.; Challali, F.; Touam, T.; Chelouche, A.; Souici, A.H.; Ouhenia, S.; Djouadi, D. Influence of growth time and substrate type on the microstructure and luminescence properties of ZnO thin films deposited by RF sputtering. J. Lumin. 2019, 215, 116631. [Google Scholar] [CrossRef]
- Kaźmierczak-Bałata, A.; Grządziel, L.; Guziewicz, M.; Venkatachalapathy, V.; Kuznietsov, A.; Krzywiecki, M. Correlations of thermal properties with grain structure, morphology, and defect balance in nanoscale polycrystalline ZnO films. Appl. Surf. Sci. 2021, 546, 149095. [Google Scholar] [CrossRef]
- Williamson, G.K.; Smallman, R.E. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray Debye-Scherrer spectrum. Philos. Mag. 1956, 1, 34. [Google Scholar] [CrossRef]
- Macherauch, E.; Wohlfahrt, H.; Wolfstieg, U. Zur Zweckmaessigen Definition Von Eigenspannungen. HTM-Haerterei-Tech. Mitt. 1973, 28, 201. [Google Scholar]
- Hauk, V. Structural and Residual Stress Analysis by Nondestructive Methods; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]

| Tg (°C) | As-Grown Samples | Annealed Samples | ||||||
|---|---|---|---|---|---|---|---|---|
| ZnO/c-Al2O3 | ZnO/c-Al2O3 | |||||||
| (Thick ± RMS) (nm) | nc (cm−3) | µ (cm2/Vs) | ρ (Ωcm) | (Thick ± RMS) (nm) | nc (cm−3) | µ (cm2/Vs) | ρ (Ωcm) | |
| 100 | 150 | 3.3 × 1018 | 3.65 | 1.92 | 151.1 | 3.0 × 1016 | 22 | 11.1 |
| 130 | 162.5 | 1.0 × 1019 | 5.2 | 1.2 × 10−1 | 165 | 1.1 × 1016 | 52 | 7.53 |
| 160 | 158.5 | 4.8 × 1019 | 22.7 | 5.87 × 10−3 | 161.7 | 1.3 × 1017 | 17.2 | 3.53 |
| 200 | 135.7 | 9.4 × 1019 | 14.8 | 4.69 × 10−3 | 137.4 | 5.9 × 1017 | 27.8 | 3.89 × 10−1 |
| 250 | 114.4 | 2.4 × 1020 | 15.6 | 1.65 × 10−3 | 116 | 4.5 × 1017 | 5.88 | 1.66 |
| 300 | 123 | 3.9 × 1019 | 16.9 | 9.4 × 10−3 | 128.4 | 1.54 × 1017 | 19.5 | 3.07 |
| Tg (°C) | As-Grown Samples | Annealed Samples | ||||||
|---|---|---|---|---|---|---|---|---|
| ZnO/a-Al2O3 | ZnO/a-Al2O3 | |||||||
| (Thick ± RMS) (nm) | nc (cm−3) | µ (cm2/Vs) | ρ (Ωcm) | (Thick ± RMS) (nm) | nc (cm−3) | µ (cm2/Vs) | ρ (Ωcm) | |
| 100 | 135 | 2.8 × 1018 | 1.2 | 2.15 | 139.5 | 5.61 × 1017 | 3.7 | 4.39 |
| 130 | 160 | 2.9 × 1019 | 8.3 | 2.7 × 10−2 | 161 | 2.7 × 1017 | 14.7 | 9.62 |
| 160 | 140 | 4.4 × 1019 | 29.2 | 4.86 × 10−3 | 151 | 8.2 × 1017 | 10.9 | 0.95 |
| 200 | 123 | 5.9 × 1019 | 20.6 | 5.1 × 10−3 | 123 | 1.3 × 1018 | 5.81 | 5.75 |
| 250 | 110 | 1.1 × 1020 | 25.1 | 2.2 × 10−3 | 111.5 | 3.3 × 1016 | 20.5 | 4.02 |
| 300 | 97.2 | 6.0 × 1019 | 25 | 5.78 × 10−3 | 100 | 1.91× 1017 | 25.2 | 1.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, S.; Paszkowicz, W.; Sulich, A.; Jakiela, R.; Ożga, M.; Guziewicz, E. Electrical and Structural Properties of Semi-Polar-ZnO/a-Al2O3 and Polar-ZnO/c-Al2O3 Films: A Comparative Study. Materials 2023, 16, 151. https://doi.org/10.3390/ma16010151
Mishra S, Paszkowicz W, Sulich A, Jakiela R, Ożga M, Guziewicz E. Electrical and Structural Properties of Semi-Polar-ZnO/a-Al2O3 and Polar-ZnO/c-Al2O3 Films: A Comparative Study. Materials. 2023; 16(1):151. https://doi.org/10.3390/ma16010151
Chicago/Turabian StyleMishra, Sushma, Wojciech Paszkowicz, Adrian Sulich, Rafal Jakiela, Monika Ożga, and Elżbieta Guziewicz. 2023. "Electrical and Structural Properties of Semi-Polar-ZnO/a-Al2O3 and Polar-ZnO/c-Al2O3 Films: A Comparative Study" Materials 16, no. 1: 151. https://doi.org/10.3390/ma16010151
APA StyleMishra, S., Paszkowicz, W., Sulich, A., Jakiela, R., Ożga, M., & Guziewicz, E. (2023). Electrical and Structural Properties of Semi-Polar-ZnO/a-Al2O3 and Polar-ZnO/c-Al2O3 Films: A Comparative Study. Materials, 16(1), 151. https://doi.org/10.3390/ma16010151






