Efficient Propylene/Ethylene Separation in Highly Porous Metal–Organic Frameworks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis
2.3. Single-Crystal X-ray Diffraction
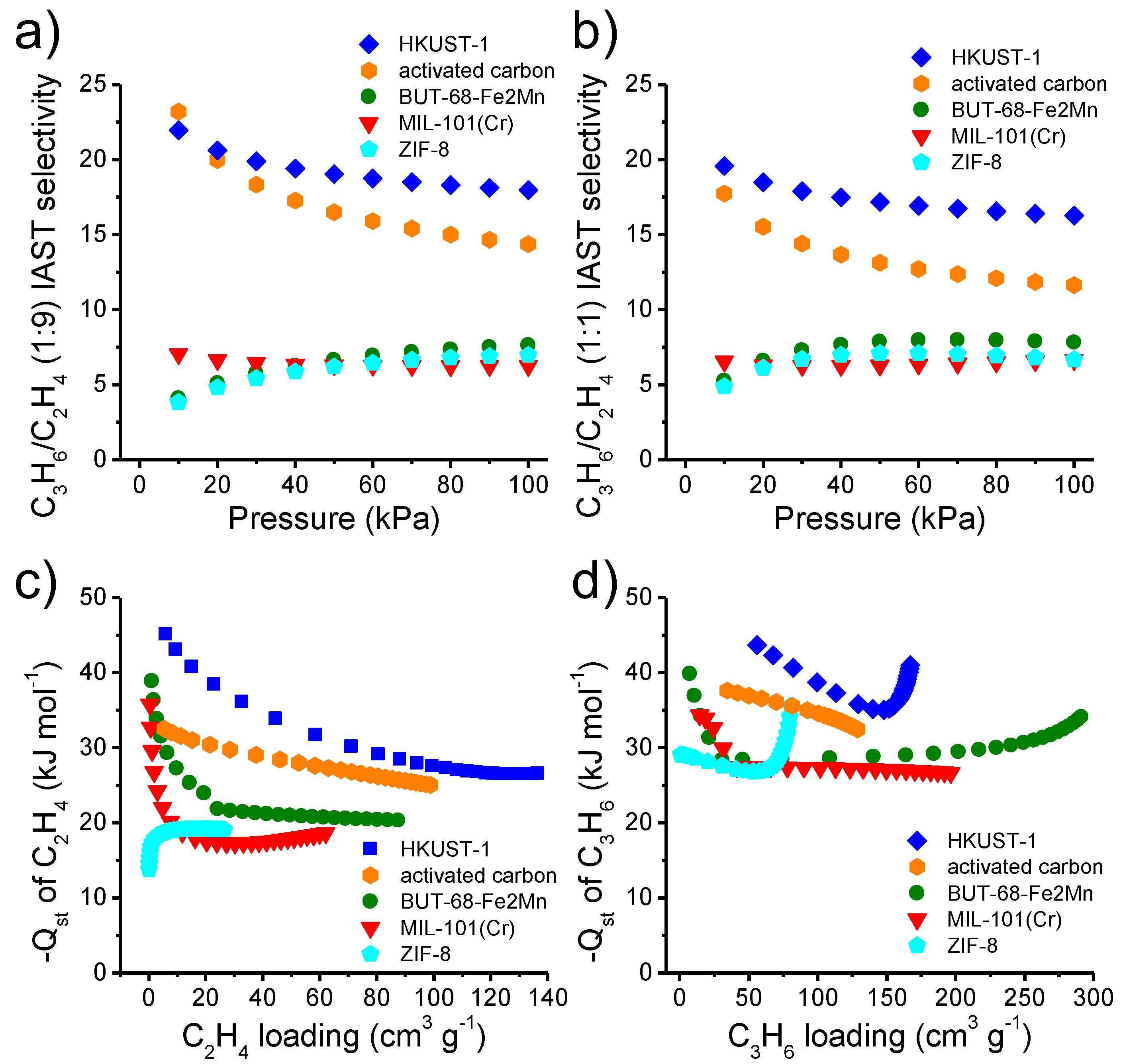
2.4. Estimation of C3H6 and C2H4 Adsorption Heat
2.5. Prediction of IAST C3H6/C2H4 Selectivity
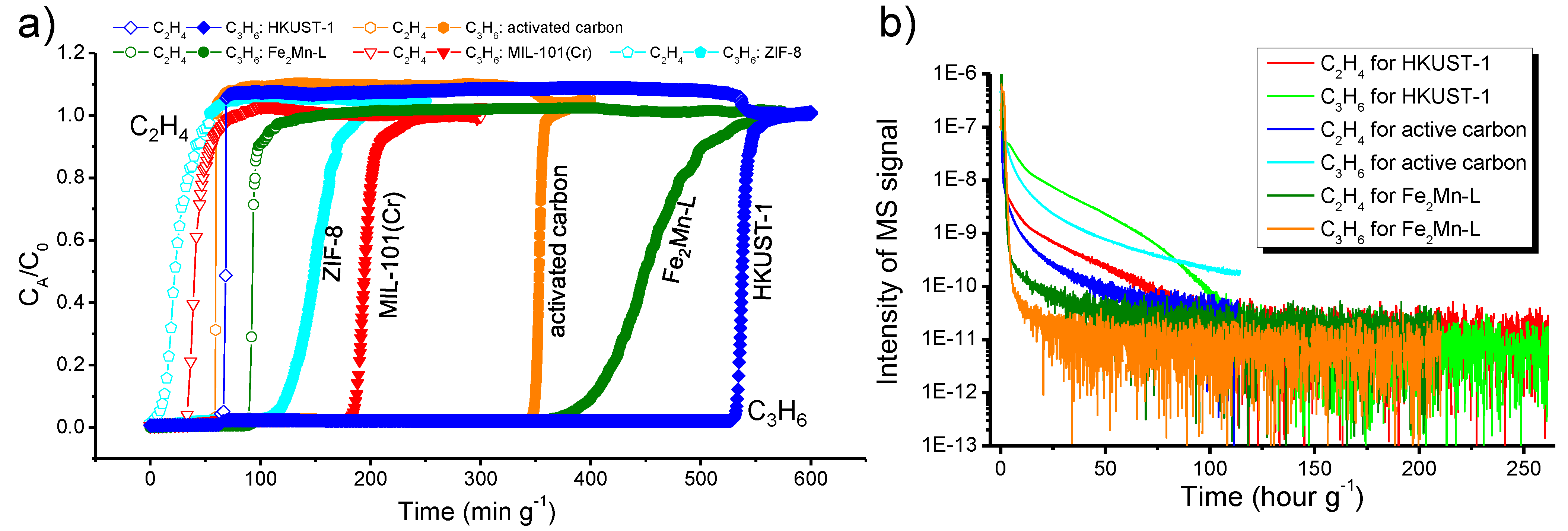
2.6. Gas Mixture Breakthrough
3. Results and Discussion
3.1. Crystal Structure and Porosity
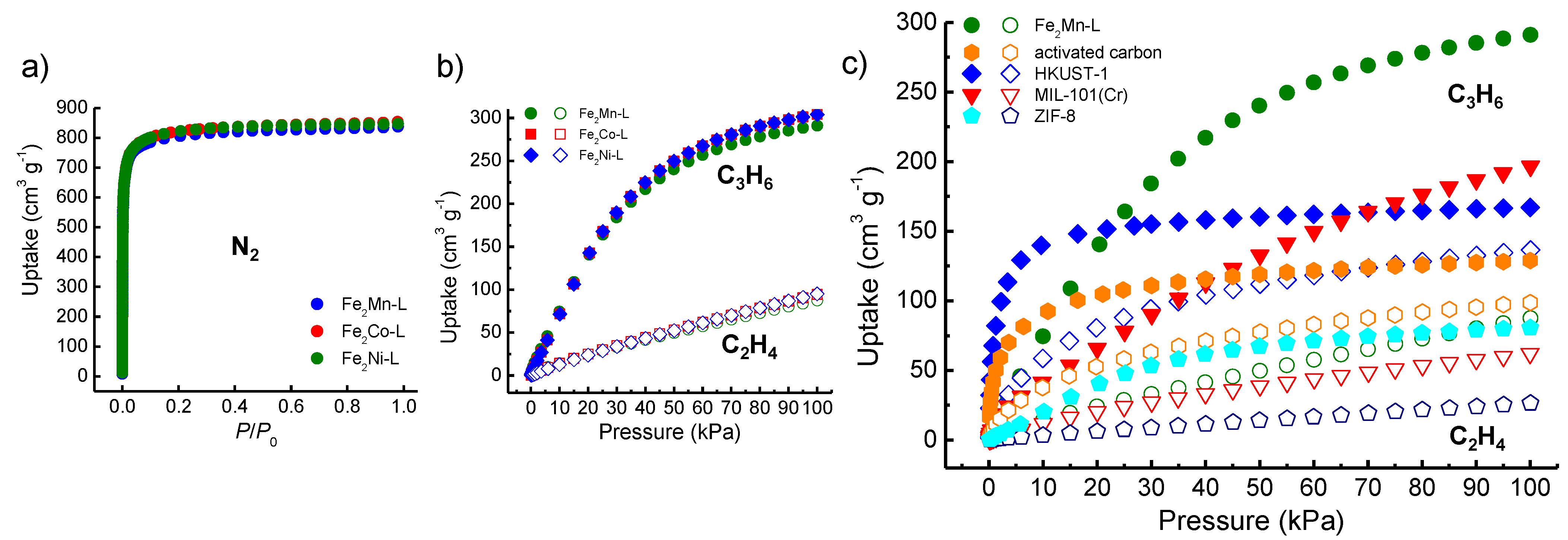
3.2. Adsorption Study for C2H4 and C3H6
3.3. Dynamic Breakthrough of Binary C3H6/C2H4 Gas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galvis, H.M.T.; Bitter, J.H.; Khare, C.B.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Supported Iron Nanoparticles as Catalysts for Sustainable Production of Lower Olefins. Science 2012, 335, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Wezendonk, T.A.; Jaén, J.J.D.; Dugulan, A.I.; Nasalevich, M.A.; Islam, H.-U.; Chojecki, A.; Sartipi, S.; Sun, X.; Hakeem, A.A.; et al. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Commun. 2015, 6, 6451. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M.; et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef]
- Zhong, L.; Yu, F.; An, Y.; Zhao, Y.; Sun, Y.; Li, Z.; Lin, T.; Lin, Y.; Qi, X.; Dai, Y.; et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas. Nature 2016, 538, 84–87. [Google Scholar] [CrossRef]
- Han, X.-H.; Gong, K.; Huang, X.; Yang, J.-W.; Feng, X.; Xie, J.; Wang, B. Syntheses of Covalent Organic Frameworks via a One-Pot Suzuki Coupling and Schiff’s Base Reaction for C2H4/C3H6 Separation. Angew. Chem. Int. Ed. 2022, 61, e202202912. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Huang, W. Separation of a C3H6/C2H4 mixture using Pebax® 2533/PEG600 blend membranes. Chin. J. Chem. Eng. 2022, in press. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, M.; Feng, X.; Wang, X.; Huang, W. Ethylene/propylene separation using mixed matrix membranes of poly (ether block amide)/nano-zeolite (NaY or NaA). Korean J. Chem. Eng. 2021, 38, 576–586. [Google Scholar] [CrossRef]
- Liu, L.; Chakma, A.; Feng, X. Sorption, diffusion, and permeation of light olefins in poly(ether block amide) membranes. Chem. Eng. Sci. 2006, 61, 6142–6153. [Google Scholar] [CrossRef]
- Choi, S.-H.; Kim, J.-H.; Lee, S.-B. Sorption and permeation behaviors of a series of olefins and nitrogen through PDMS membranes. J. Membr. Sci. 2007, 299, 54–62. [Google Scholar] [CrossRef]
- Ye, P.; Fang, Z.; Su, B.; Xing, H.; Yang, Y.; Su, Y.; Ren, Q. Adsorption of Propylene and Ethylene on 15 Activated Carbons. J. Chem. Eng. Data 2010, 55, 5669–5672. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Kitagawa, S. Metal-Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef]
- Kaskel, S. The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Xu, Q.; Kitagawa, H. MOFs: New Useful Materials—A Special Issue in Honor of Prof. Susumu Kitagawa. Adv. Mater. 2018, 30, 1803613. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, H.-F.; Li, C.; Xu, Q. Bimetallic metal–organic frameworks and their derivatives. Chem. Sci. 2020, 11, 5369–5403. [Google Scholar] [CrossRef] [PubMed]
- Abednatanzi, S.; Gohari Derakhshandeh, P.; Depauw, H.; Coudert, F.-X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef]
- Feng, D.; Wang, K.; Wei, Z.; Chen, Y.-P.; Simon, C.M.; Arvapally, R.K.; Martin, R.L.; Bosch, M.; Liu, T.-F.; Fordham, S.; et al. Kinetically tuned dimensional augmentation as a versatile synthetic route towards robust metal–organic frameworks. Nat. Commun. 2014, 5, 5723. [Google Scholar] [CrossRef]
- Dong, C.; Yang, J.-J.; Xie, L.-H.; Cui, G.; Fang, W.-H.; Li, J.-R. Catalytic ozone decomposition and adsorptive VOCs removal in bimetallic metal-organic frameworks. Nat. Commun. 2022, 13, 4991. [Google Scholar] [CrossRef]
- Liao, P.-Q.; Zhang, W.-X.; Zhang, J.-P.; Chen, X.-M. Efficient purification of ethene by an ethane-trapping metal-organic framework. Nat. Commun. 2015, 6, 8697. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Krishna, R.; Wu, Z.; Ma, D.; Shi, Z.; Pham, T.; Forrest, K.; Space, B.; Ma, S. Highly selective adsorption of ethylene over ethane in a MOF featuring the combination of open metal site and π-complexation. Chem. Commun. 2015, 51, 2714–2717. [Google Scholar] [CrossRef]
- Gücüyener, C.; van den Bergh, J.; Gascon, J.; Kapteijn, F. Ethane/Ethene Separation Turned on Its Head: Selective Ethane Adsorption on the Metal−Organic Framework ZIF-7 through a Gate-Opening Mechanism. J. Am. Chem. Soc. 2010, 132, 17704–17706. [Google Scholar] [CrossRef]
- Li, L.; Lin, R.-B.; Krishna, R.; Li, H.; Xiang, S.; Wu, H.; Li, J.; Zhou, W.; Chen, B. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 2018, 362, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-J.; Madden, D.G.; Mukherjee, S.; Pham, T.; Forrest, K.A.; Kumar, A.; Space, B.; Kong, J.; Zhang, Q.-Y.; Zaworotko, M.J. Synergistic sorbent separation for one-step ethylene purification from a four-component mixture. Science 2019, 366, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-B.; Li, L.; Zhou, H.-L.; Wu, H.; He, C.; Li, S.; Krishna, R.; Li, J.; Zhou, W.; Chen, B. Molecular sieving of ethylene from ethane using a rigid metal–organic framework. Nat. Mater. 2018, 17, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.D.; Queen, W.L.; Krishna, R.; Zadrozny, J.M.; Brown, C.M.; Long, J.R. Hydrocarbon Separations in a Metal-Organic Framework with Open Iron(II) Coordination Sites. Science 2012, 335, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Cadiau, A.; Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Eddaoudi, M. A metal-organic framework–based splitter for separating propylene from propane. Science 2016, 353, 137–140. [Google Scholar] [CrossRef]
- Zeng, H.; Xie, M.; Wang, T.; Wei, R.-J.; Xie, X.-J.; Zhao, Y.; Lu, W.; Li, D. Orthogonal-array dynamic molecular sieving of propylene/propane mixtures. Nature 2021, 595, 542–548. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, X.; Xie, Y.; Lin, R.-B.; Krishna, R.; Cui, H.; Li, Z.; Shi, Y.; Wu, H.; Zhou, W.; et al. An Ultramicroporous Metal–Organic Framework for High Sieving Separation of Propylene from Propane. J. Am. Chem. Soc. 2020, 142, 17795–17801. [Google Scholar] [CrossRef]
- Wang, H.; Dong, X.; Colombo, V.; Wang, Q.; Liu, Y.; Liu, W.; Wang, X.-L.; Huang, X.-Y.; Proserpio, D.M.; Sironi, A.; et al. Tailor-Made Microporous Metal–Organic Frameworks for the Full Separation of Propane from Propylene Through Selective Size Exclusion. Adv. Mater. 2018, 30, e1805088. [Google Scholar] [CrossRef]
- Yu, L.; Han, X.; Wang, H.; Ullah, S.; Xia, Q.; Li, W.; Li, J.; da Silva, I.; Manuel, P.; Rudić, S.; et al. Pore Distortion in a Metal–Organic Framework for Regulated Separation of Propane and Propylene. J. Am. Chem. Soc. 2021, 143, 19300–19305. [Google Scholar] [CrossRef]
- Wang, G.-D.; Li, Y.-Z.; Shi, W.-J.; Hou, L.; Wang, Y.-Y.; Zhu, Z. One-Step C2H4 Purification from Ternary C2H6/C2H4/C2H2 Mixtures by a Robust Metal–Organic Framework with Customized Pore Environment. Angew. Chem. Int. Ed. 2022, e202205427. [Google Scholar] [CrossRef]
- Shen, J.; He, X.; Ke, T.; Krishna, R.; van Baten, J.M.; Chen, R.; Bao, Z.; Xing, H.; Dincǎ, M.; Zhang, Z.; et al. Simultaneous interlayer and intralayer space control in two-dimensional metal−organic frameworks for acetylene/ethylene separation. Nat. Commun. 2020, 11, 6259. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Shao, K.; Wang, J.-X.; Wen, H.-M.; Yang, Y.; Cui, Y.; Krishna, R.; Li, B.; Qian, G. A Chemically Stable Hofmann-Type Metal−Organic Framework with Sandwich-Like Binding Sites for Benchmark Acetylene Capture. Adv. Mater. 2020, 32, e1908275. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-Q.; Huang, N.-Y.; Zhang, W.-X.; Zhang, J.-P.; Chen, X.-M. Controlling guest conformation for efficient purification of butadiene. Science 2017, 356, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, K.; Yang, L.; Li, L.; Hu, E.; Yang, L.; Shao, K.; Xing, H.; Cui, Y.; Yang, Y.; et al. Benchmark C2H2/CO2 Separation in an Ultra-Microporous Metal-Organic Framework via Copper(I)-Alkynyl Chemistry. Angew. Chem. Int. Ed. 2021, 60, 15995–16002. [Google Scholar] [CrossRef]
- Pei, J.; Wen, H.-M.; Gu, X.-W.; Qian, Q.-L.; Yang, Y.; Cui, Y.; Li, B.; Chen, B.; Qian, G. Dense Packing of Acetylene in a Stable and Low-Cost Metal-Organic Framework for Efficient C2H2/CO2 Separation. Angew. Chem. Int. Ed. 2021, 60, 25068–25074. [Google Scholar] [CrossRef]
- Gu, X.-W.; Wang, J.-X.; Wu, E.; Wu, H.; Zhou, W.; Qian, G.; Chen, B.; Li, B. Immobilization of Lewis Basic Sites into a Stable Ethane-Selective MOF Enabling One-Step Separation of Ethylene from a Ternary Mixture. J. Am. Chem. Soc. 2022, 144, 2614–2623. [Google Scholar] [CrossRef]
- Lamia, N.; Jorge, M.; Granato, M.A.; Almeida Paz, F.A.; Chevreau, H.; Rodrigues, A.E. Adsorption of propane, propylene and isobutane on a metal–organic framework: Molecular simulation and experiment. Chem. Eng. Sci. 2009, 64, 3246–3259. [Google Scholar] [CrossRef]
- Böhme, U.; Barth, B.; Paula, C.; Kuhnt, A.; Schwieger, W.; Mundstock, A.; Caro, J.; Hartmann, M. Ethene/Ethane and Pro-pene/Propane Separation via the Olefin and Paraffin Selective Metal–Organic Framework Adsorbents CPO-27 and ZIF-8. Langmuir 2013, 29, 8592–8600. [Google Scholar] [CrossRef]
- Su, W.; Zhang, A.; Sun, Y.; Ran, M.; Wang, X. Adsorption Properties of C2H4 and C3H6 on 11 Adsorbents. J. Chem. Eng. Data 2017, 62, 417–421. [Google Scholar] [CrossRef]
- Huang, X.-C.; Lin, Y.-Y.; Zhang, J.-P.; Chen, X.-M. Ligand-Directed Strategy for Zeolite-Type Metal–Organic Frameworks: Zinc(II) Imidazolates with Unusual Zeolitic Topologies. Angew. Chem. Int. Ed. 2006, 45, 1557–1559. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.D.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Cravillon, J.; Münzer, S.; Lohmeier, S.-J.; Feldhoff, A.; Huber, K.; Wiebcke, M. Rapid Room-Temperature Synthesis and Characterization of Nanocrystals of a Prototypical Zeolitic Imidazolate Framework. Chem. Mater. 2009, 21, 1410–1412. [Google Scholar] [CrossRef]
- Zhao, T.; Jeremias, F.; Boldog, I.; Nguyen, B.; Henninger, S.K.; Janiak, C. High-yield, fluoride-free and large-scale synthesis of MIL-101(Cr). Dalton Trans. 2015, 44, 16791–16801. [Google Scholar] [CrossRef]
- Huo, J.; Brightwell, M.; El Hankari, S.; Garai, A.; Bradshaw, D. A versatile, industrially relevant, aqueous room temperature synthesis of HKUST-1 with high space-time yield. J. Mater. Chem. A 2013, 1, 15220–15223. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond, Version 3.1d. Copyright 1997–2006. Crystal Impact GbR: Bonn, Germany, 2006.
- Tóth, J. Uniform interpretation of gas/solid adsorption. Adv. Colloid Interface Sci. 1995, 55, 1–239. [Google Scholar] [CrossRef]
- Pan, H.; Ritter, A.J.A.; Balbuena, P.B. Examination of the Approximations Used in Determining the Isosteric Heat of Adsorption from the Clausius−Clapeyron Equation. Langmuir 1998, 14, 6323–6327. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated Synthesis of Zr-Based Metal-Organic Frameworks: From Nano to Single Crystals. Chem. A Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-M.; Dong, L.-Z.; Qin, J.-S.; Guan, W.; Liu, J.; Li, S.-L.; Lu, M.; Lan, Y.-Q.; Su, Z.-M.; Zhou, H.-C. Effect of Imidazole Arrangements on Proton-Conductivity in Metal–Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 6183–6189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Ji, W.-J.; Hu, M.-C.; Li, S.-N.; Jiang, Y.-C.; Zhang, X.-M.; Qu, P.; Zhai, Q.-G. A superstable 3p-block metal–organic framework platform towards prominent CO2 and C1/C2-hydrocarbon uptake and separation performance and strong Lewis acid catalysis for CO2 fixation. Inorg. Chem. Front. 2019, 6, 813–819. [Google Scholar] [CrossRef]
- Fan, W.; Yuan, S.; Wang, W.; Feng, L.; Liu, X.; Zhang, X.; Wang, X.; Kang, Z.; Dai, F.; Yuan, D.; et al. Optimizing Multivariate Metal–Organic Frameworks for Efficient C2H2/CO2 Separation. J. Am. Chem. Soc. 2020, 142, 8728–8737. [Google Scholar] [CrossRef]
- Fang, M.; Zhang, G.; Liu, Y.; Xiong, R.; Wu, W.; Yang, F.; Liu, L.; Chen, J.; Li, J. Exploiting Giant-Pore Systems of Nanosized MIL-101 in PDMS Matrix for Facilitated Reverse-Selective Hydrocarbon Transport. ACS Appl. Mater. Interfaces 2020, 12, 1511–1522. [Google Scholar] [CrossRef]
- Ma, Q.; Mo, K.; Gao, S.; Xie, Y.; Wang, J.; Jin, H.; Feldhoff, A.; Xu, S.; Lin, J.Y.S.; Li, Y. Ultrafast Semi-Solid Processing of Highly Durable ZIF-8 Membranes for Propylene/Propane Separation. Angew. Chem. Int. Ed. 2020, 59, 21909–21914. [Google Scholar] [CrossRef]
- James, J.B.; Wang, J.; Meng, L.; Lin, Y.S. ZIF-8 Membrane Ethylene/Ethane Transport Characteristics in Single and Binary Gas Mixtures. Ind. Eng. Chem. Res. 2017, 56, 7567–7575. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, H.; Lin, R.-B.; Krishna, R.; Zhang, Z.-Y.; Liu, T.; Liang, B.; Chen, B. Realization of Ethylene Production from Its Quaternary Mixture through Metal–Organic Framework Materials. ACS Appl. Mater. Interfaces 2021, 13, 22514–22520. [Google Scholar] [CrossRef]

| Adsorbent | Pore Volume (cm3 g−1) | SBET (m2 g−1) | SLangmuir (m2 g−1) |
|---|---|---|---|
| HKUST-1 | 0.63 | 1513.6 | 1759.3 |
| MIL-101(Cr) | 1.47 | 2691.8 | 4702.1 |
| ZIF-8 | 0.85 | 1510.8 | 2007.9 |
| activated carbon | 0.46 | 1086.4 | 1262.6 |
| Fe2Mn-L | 1.29 | 3105.2 | 3599.6 |
| Fe2Co-L | 1.32 | 3168.1 | 3674.5 |
| Fe2Ni-L | 1.31 | 3168.9 | 3674.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.-M.; Xie, L.-H.; Wu, Y. Efficient Propylene/Ethylene Separation in Highly Porous Metal–Organic Frameworks. Materials 2023, 16, 154. https://doi.org/10.3390/ma16010154
Liu X-M, Xie L-H, Wu Y. Efficient Propylene/Ethylene Separation in Highly Porous Metal–Organic Frameworks. Materials. 2023; 16(1):154. https://doi.org/10.3390/ma16010154
Chicago/Turabian StyleLiu, Xiao-Min, Lin-Hua Xie, and Yufeng Wu. 2023. "Efficient Propylene/Ethylene Separation in Highly Porous Metal–Organic Frameworks" Materials 16, no. 1: 154. https://doi.org/10.3390/ma16010154
APA StyleLiu, X.-M., Xie, L.-H., & Wu, Y. (2023). Efficient Propylene/Ethylene Separation in Highly Porous Metal–Organic Frameworks. Materials, 16(1), 154. https://doi.org/10.3390/ma16010154








