Metallic Dental Implants Wear Mechanisms, Materials, and Manufacturing Processes: A Literature Review
Abstract
:1. Introduction
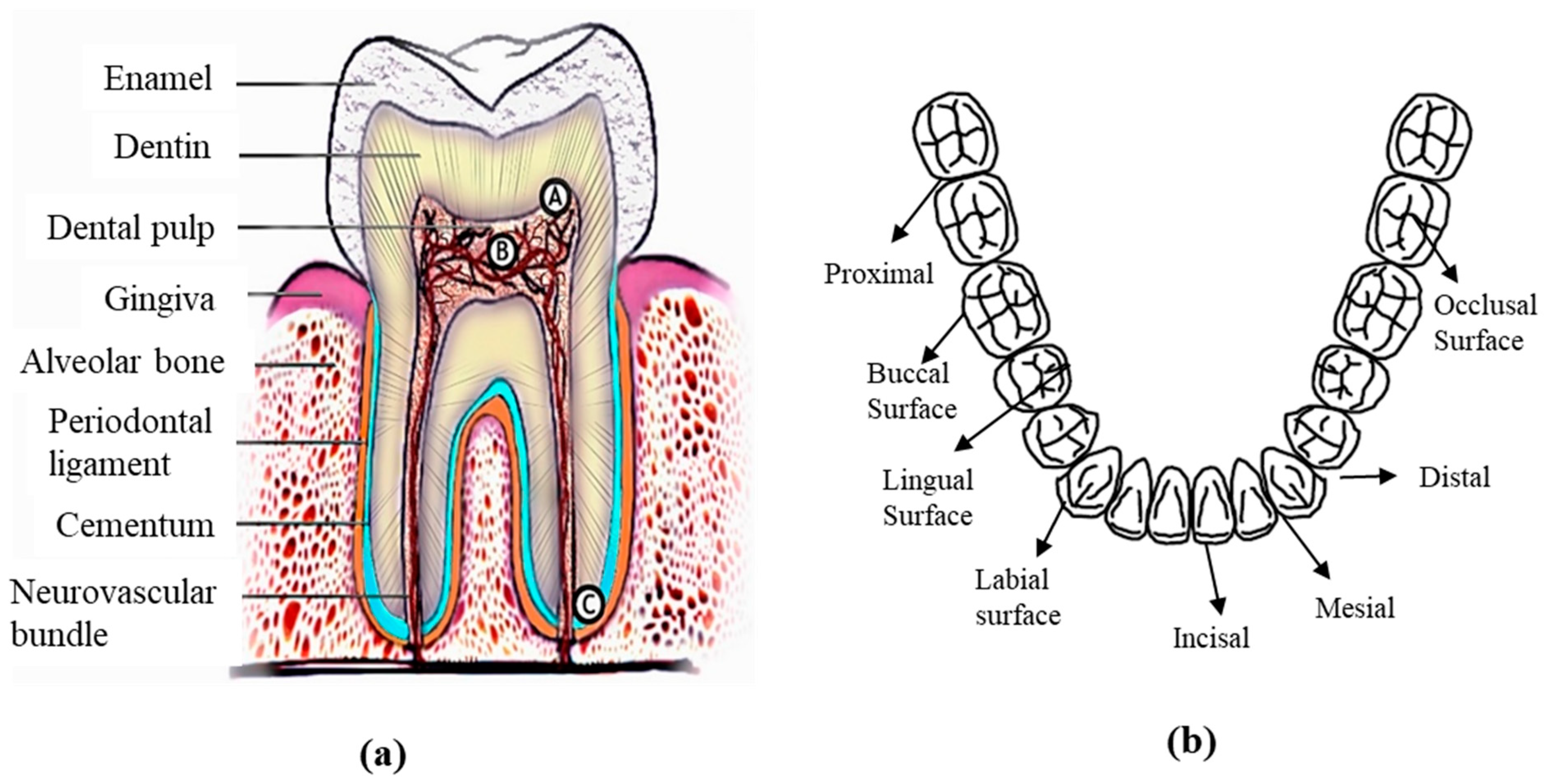
2. Human Mouth Anatomy
2.1. Teeth
2.2. Root
- Occlusal: The chewing surface of the posterior teeth.
- Distal: The surface facing away from the face’s midline.
- Mesial: The surface closest to the face’s midline.
- Incisal: The tooth’s biting edge.
- Facial: The surface that faces the cheeks or lips. This surface is called labial when it faces the lips and buccal when it faces the cheeks.
- Lingual: The surface facing the tongue.
- Proximal: The surface between adjacent teeth.
2.3. Saliva and Its Composition
3. Major Dental Wear Types, Locations, and Corresponding Mechanisms
3.1. Dental Wear Mode Types
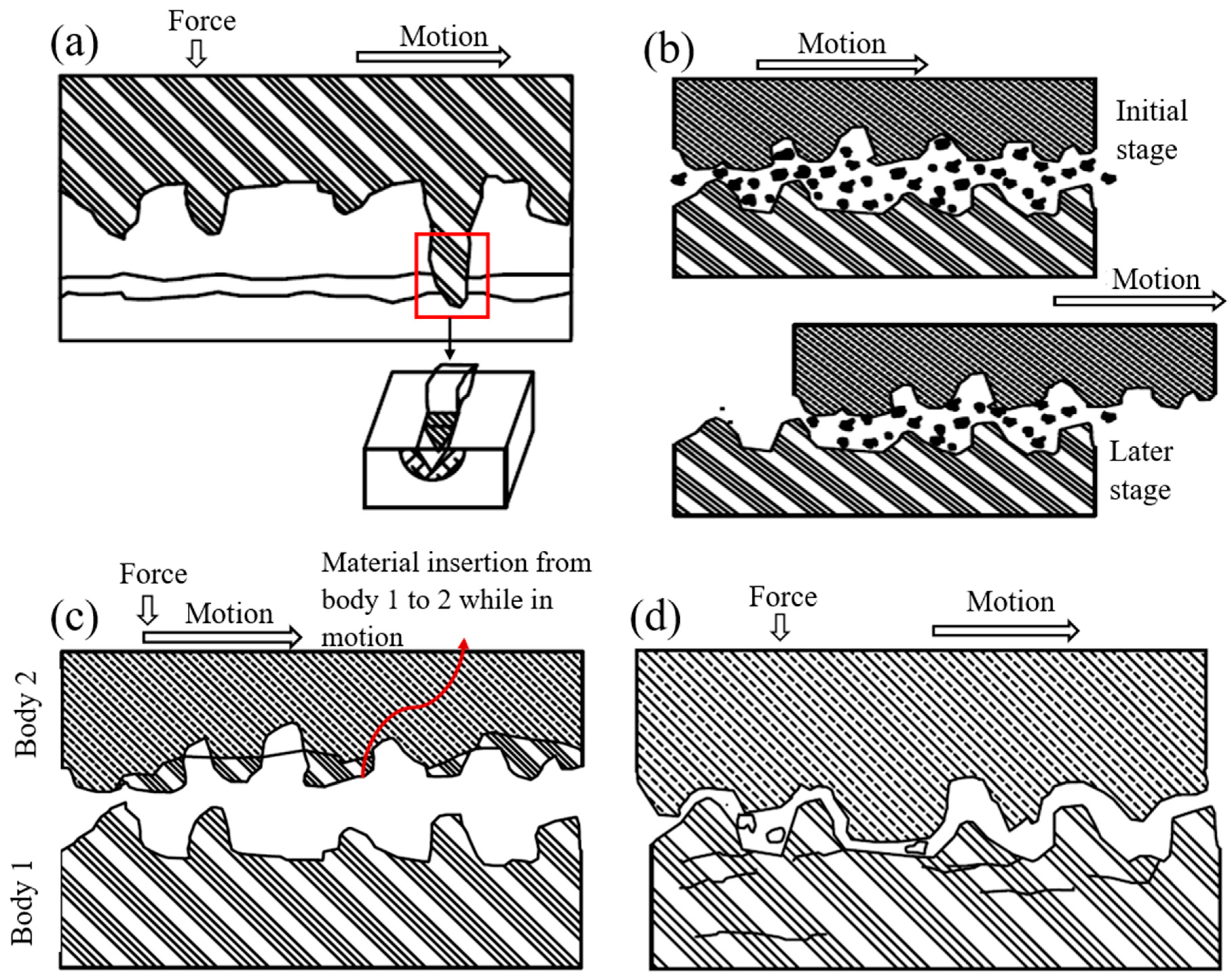
3.2. Abrasive Wear
3.2.1. Two-Body Abrasion
3.2.2. Three-Body Abrasion
3.3. Adhesive Wear
3.4. Fatigue Wear
3.5. Corrosive Wear
4. Dental Prosthetics or Implants
4.1. Endosteal or Endosseous Dental Implants
4.1.1. Root Implants
4.1.2. Blade Implants
4.2. Subperiosteal Dental Implants
- i.
- CLASS I: Bilateral Posterior Edentulous Area;
- ii.
- CLASS II: Unilateral Posterior Edentulous Area;
- iii.
- CLASS III: Unilateral or Bilateral Edentulous Area(s) Bounded by Remaining Tooth/Teeth;
- iv.
- CLASS IV: Single Edentulous Area Anterior to Remaining Teeth and Crossing the Midline.
4.3. Transosteal Implants
4.4. Other Dental Implant Types
4.4.1. Dental Crown
4.4.2. Dental Braces
4.4.3. Dental Veneers
5. Key Metallic Materials Used in Dental Prosthetics
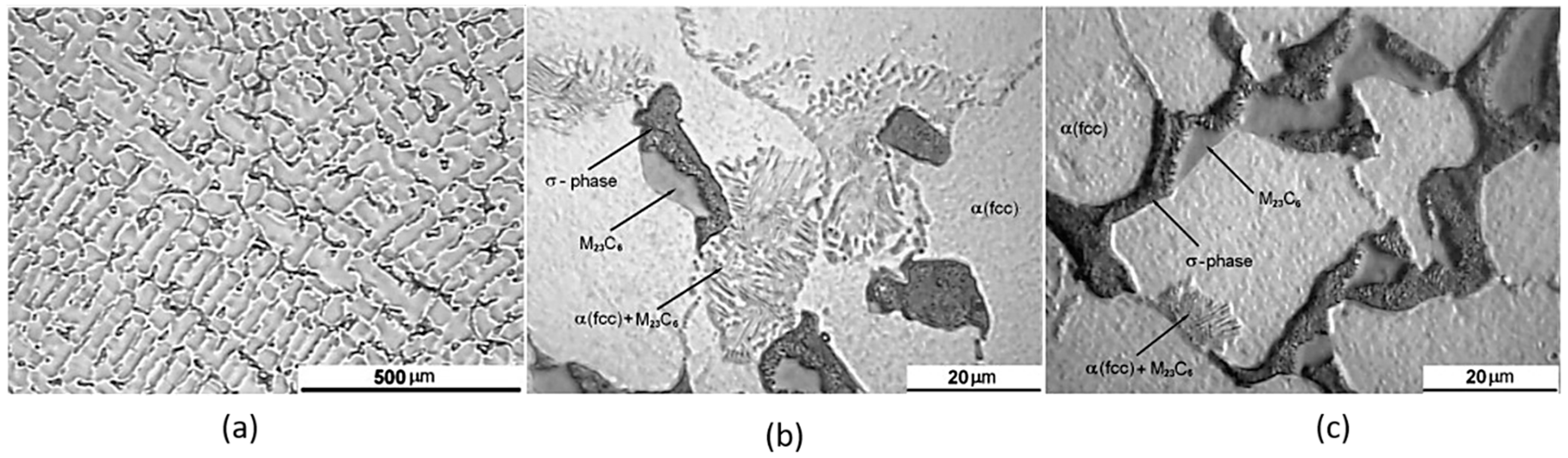
5.1. Cobalt–Chromium Alloys
5.2. Stainless Steels
5.3. Titanium-Based Alloys and Comparison to Zirconia-Based Dental Implants
5.4. Noble Metals
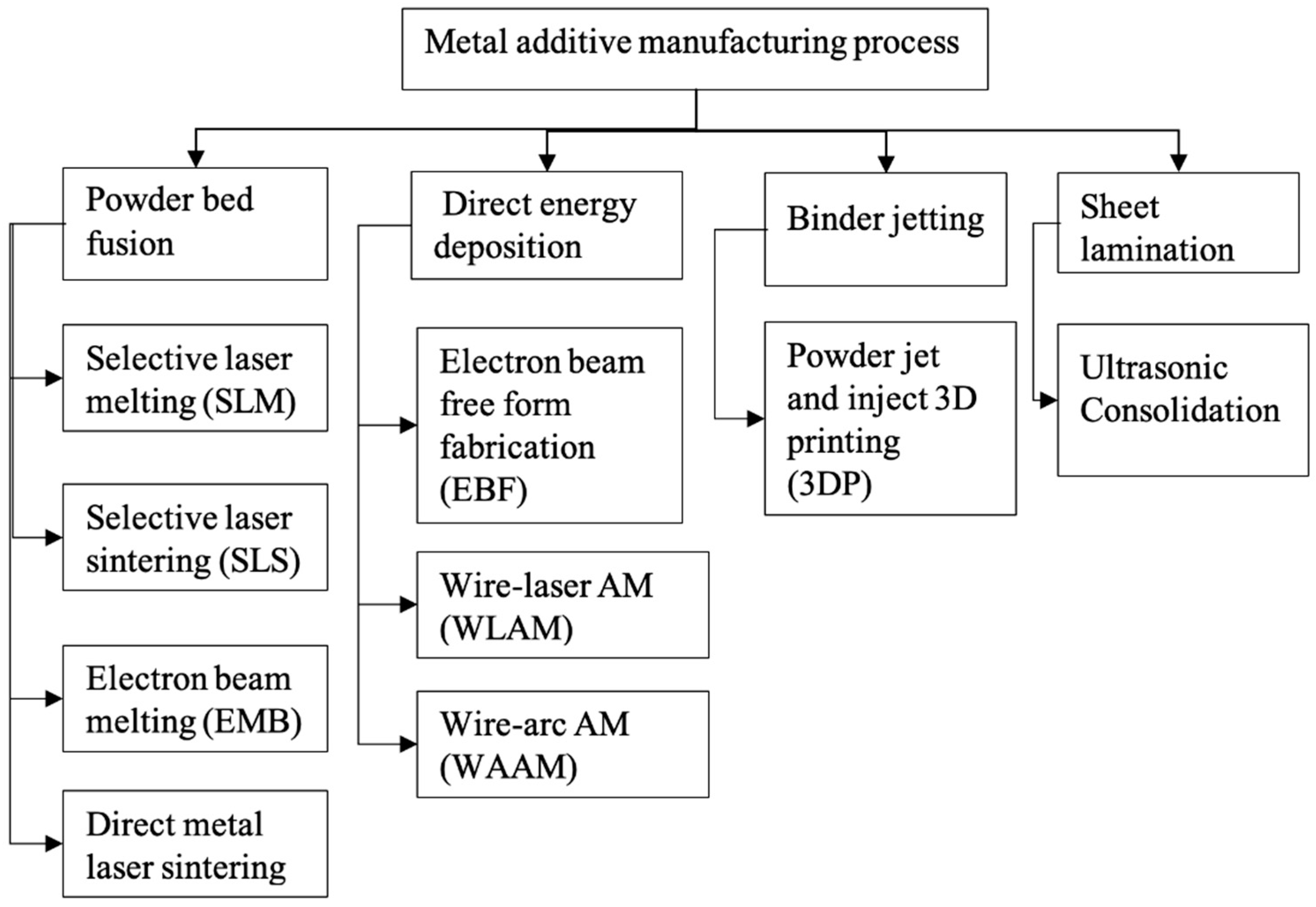
6. Fabrication Techniques of Dental Prosthetics
6.1. Traditional Manufacturing Techniques
Lost Wax Method
6.2. Additive Manufacturing
6.2.1. Selective Laser Melting (SLM)
6.2.2. Directed Energy Deposition
6.3. Comparison between Different Manufacturing Methods
7. Conclusions and Future Research Directions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Boehm, M.W.; Yakubov, G.E.; Stokes, J.R.; Baier, S.K. The role of saliva in oral processing: Reconsidering the breakdown path paradigm. J. Texture Stud. 2020, 51, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mair, A.; Mair, L.H. Wear in dentistry-current terminology. J. Dent. 1992, 20, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Z.R. Study of in vitro wear of human tooth enamel. Tribol. Lett. 2007, 26, 181–189. [Google Scholar] [CrossRef]
- Lanza, A.; Ruggiero, A.; Sbordone, L. Tribology and dentistry: A commentary. Lubricants 2019, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50. [Google Scholar] [CrossRef] [PubMed]
- Ankur, G.; Dhanraj, M.S.G. Status of surface treatment in endosseous implant: A literary overview Controlling the Bone Implant Interface by Biomaterial Selection and Modification Physicochemical Method. Indian J. Dent. Res. 2021, 21, 431–438. Available online: https://pubmed.ncbi.nlm.nih.gov/20930358/ (accessed on 4 April 2021).
- Guillaume, B. Dental implants: A review [Les implants dentaires: Revue]. Morphologie 2016, 100, 189–198. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84961167982&doi=10.1016%2Fj.morpho.2016.02.002&partnerID=40&md5=f2ea3544c09ab02315535918e4331ad5 (accessed on 8 April 2021). [CrossRef]
- Osman, R.B.; Swain, M.V. A critical review of dental implant materials with an emphasis on titanium versus zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [Green Version]
- Saini, M. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52. [Google Scholar] [CrossRef]
- Edelmann, A.; Riedel, L.; Hellmann, R. Realization of a dental framework by 3D printing in material cobalt-chromium with superior precision and fitting accuracy. Materials 2020, 13, 5390. [Google Scholar] [CrossRef]
- Michelle Grandin, H.; Berner, S.; Dard, M. A review of Titanium Zirconium (TiZr) alloys for use in endosseous dental implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef]
- Mitsuo, N. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. Available online: http://www.sciencedirect.com/science/article/pii/S092150939700806X (accessed on 20 April 2021).
- Adell, R.; Eriksson, B.; Lekholm, U.; Brånemark, P.I.; Jemt, T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int. J. Oral Maxillofac. Implant. 1990, 5, 347–359. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2094653 (accessed on 23 April 2021).
- Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Florido-Suarez, N.; Saceleanu, M.V.; Mirza-Rosca, J.C. New titanium alloys, promising materials for medical devices. Materials 2021, 14, 5934. [Google Scholar] [CrossRef]
- Savin, A.; Vizureanu, P.; Prevorovsky, Z.; Chlada, M.; Krofta, J.; Baltatu, M.S.; Istrate, B.; Steigmann, R. Noninvasive Evaluation of Special Alloys for Prostheses Using Complementary Methods. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012030. [Google Scholar] [CrossRef]
- Yokoyama, K.; Ichikawa, T.; Murakami, H.; Miyamoto, Y.; Asaoka, K. Fracture mechanisms of retrieved titanium screw thread in dental implant. Biomaterials 2002, 23, 2459–2465. [Google Scholar] [CrossRef]
- Patterson, E.A.; Johns, R.B. Theoretical analysis of the fatigue life of fixture screws in osseointegrated dental implants. Int. J. Oral Maxillofac. Implant. 1992, 7, 26–33. [Google Scholar]
- Morgan, M.J.; James, D.F.; Pilliar, R.M. Fractures of the fixture component of an osseointegrated implant. Int. J. Oral Maxillofac. Implant. 1993, 8, 409–414. Available online: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L24856507 (accessed on 18 November 2022).
- Ahmadi Toussi, C.; Ezatpour, H.R.; Haddadnia, J.; Gholami Shiri, J. Effect of using different metal and ceramic materials as restorations on stress distribution around dental implants: A comparative finite element study. Mater. Res. Express 2018, 5, 115403. [Google Scholar] [CrossRef]
- Barrère, F.; Van Der Valk, C.M.; Meijer, G.; Dalmeijer, R.A.J.; De Groot, K.; Layrolle, P. Osteointegration of Biomimetic Apatite Coating Applied onto Dense and Porous Metal Implants in Femurs of Goats. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2003, 67, 655–665. [Google Scholar] [CrossRef]
- Gahlert, M.; Burtscher, D.; Grunert, I.; Kniha, H.; Steinhauser, E. Failure analysis of fractured dental zirconia implants. Clin. Oral Implant. Res. 2012, 23, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.B.; Ma, S.; Duncan, W.; De Silva, R.K.; Siddiqi, A.; Swain, M.V. Fractured zirconia implants and related implant designs: Scanning electron microscopy analysis. Clin. Oral Implant. Res. 2013, 24, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Spiller, M.S. Castable Metal Alloys in Dentistry. Dmd 2017, 2–33. Available online: https://www.dentallearning.org/course/DentalAlloys/CastableMetalAlloys.pdf (accessed on 1 July 2022).
- Jevremovic, D.; Puskar, T.; Kosec, B.; Vukelic, D.; Budak, I.; Aleksandrovic, S.; Egbeer, D.; Williams, R. The analysis of the mechanical properties of F75 Co-Cr alloy for use in selective laser melting (SLM) manufacturing of removable partial dentures (RPD). Metalurgija 2012, 51, 171–174. [Google Scholar]
- Strub, J.R. Computer-aided design and fabrication of dental restorations: Current systems and future possibilities. J. Am. Dent. Assoc. 2006, 137, 1289–1296. [Google Scholar] [CrossRef]
- Bae, E.J.; Do Jeong, I.; Kim, W.C.; Kim, J.H. A comparative study of additive and subtractive manufacturing for dental restorations. J. Prosthet. Dent. 2017, 118, 187–193. [Google Scholar] [CrossRef]
- Lima, J.M.C.; Anami, L.C.; Araujo, R.M.; Pavanelli, C.A. Removable partial dentures: Use of rapid prototyping. J. Prosthodont. 2014, 23, 588–591. [Google Scholar] [CrossRef]
- Augustyn-Pieniazek, J.; Kurtyka, P.; Sulima, I.; Stopka, J. Selected properties and tribological wear alloys Co-Cr-Mo and Co-Cr-Mo-W used in dental prosthetics. Arch. Metall. Mater. 2015, 60, 1569–1574. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wu, S.; Fu, F.; Ye, G.; Mai, S.; Qian, Z.; Song, C.; Yang, Y. Effect of Pretreatment Process on the Metal-Porcelain Bonding Mechanism and Properties of CoCr Alloy Dental Crown and Bridge Manufactured by SLM. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2018, 47, 2328–2334. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Lou, D.; Xia, W.; Fang, X. Microstructural features of biomedical cobalt–chromium–molybdenum (CoCrMo) alloy from powder bed fusion to aging heat treatment. J. Mater. Sci. Technol. 2020, 45, 146–156. [Google Scholar] [CrossRef]
- Li, Z.; Cui, Y.; Wang, J.; Liu, C.; Wang, J.; Xu, T.; Lu, T.; Zhang, H.; Lu, J.; Ma, S.; et al. Characterization of microstructure and mechanical properties of stellite 6 part fabricated by wire arc additive manufacturing. Metals 2019, 9, 474. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.D.; Chattree, A.; Rajendran, S. Stainless Steel Alloys for Dental Application: Corrosion Behaviour in the Presence of Toothpaste Vicco. Der Pharma Chem. 2017, 9, 25–31. [Google Scholar]
- Aroussi, D.; Aour, B.; Bouaziz, A.S. A Comparative Study of 316L Stainless Steel and a Titanium Alloy in an Aggressive Biological Medium. Eng. Technol. Appl. Sci. Res. 2019, 9, 5093–5098. [Google Scholar] [CrossRef]
- Reclaru, L.; Meyer, J. Study of corrosion between a titanium implant and dental alloys. J. Dent. 2003, 31, 1–2. [Google Scholar] [CrossRef]
- Revathi, A.; Magesh, S.; Balla, V.K.; Das, M.; Manivasagam, G. Current advances in enhancement of wear and corrosion resistance of titanium alloys—A review. Mater. Technol. 2016, 31, 696–704. [Google Scholar] [CrossRef]
- Lozano, P.; Peña, M.; Herrero-Climent, M.; Rios-Santos, J.V.; Rios-Carrasco, B.; Brizuela, A.; Gil, J. Corrosion Behavior of Titanium Dental Implants with Implantoplasty. Materials 2022, 15, 1593. [Google Scholar] [CrossRef]
- Figueiredo-Pina, C.G.; Moreira, V.; Colaço, R.; Serro, A.P. Tribocorrosion in dental implants: An in-vitro study. In Proceedings of the VIII International Scientific Conference, Dedicated to 50th Anniversary Year of Tribology, Aleksandras Stulginskis University, Kaunas, Lithuania, 26–27 November 2015; pp. 122–125. [Google Scholar] [CrossRef] [Green Version]
- Alemanno, F.; Peretti, V.; Tortora, A.; Spriano, S. Tribological behaviour of Ti or Ti alloy vs. zirconia in presence of artificial saliva. Coatings 2020, 10, 851. [Google Scholar] [CrossRef]
- Branco, A.C.; Moreira, V.; Reis, J.A.; Colaço, R.; Figueiredo-Pina, C.G.; Serro, A.P. Influence of contact configuration and lubricating conditions on the microtriboactivity of the zirconia-Ti6Al4V pair used in dental applications. J. Mech. Behav. Biomed. Mater. 2019, 91, 164–173. [Google Scholar] [CrossRef]
- Osak, P.; Maszybrocka, J.; Kubisztal, J.; Łosiewicz, B. Effect of amorphous calcium phosphate coatings on tribological properties of titanium grade 4 in protein-free artificial saliva. Biotribology 2022, 32. [Google Scholar] [CrossRef]
- Bulaqi, D.H.A.; Mashhadi, M.M.; Geramipanah, F.; Safari, H.; Paknejad, M. Effect of the coefficient of friction and tightening speed on the preload induced at the dental implant complex with the finite element method. J. Prosthet. Dent. 2015, 113, 405–411. [Google Scholar] [CrossRef]
- Al Jabbari, Y.S. Physico-mechanical properties and prosthodontic applications of Co-Cr dental alloys: A review of the literature. J. Adv. Prosthodont. 2014, 6, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Apratim, B.A.; Eachempati, P.; Salian, K.K.; Singh, V.; Chhabra, S.; Shah, S. Zirconia in dental implantology: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Xiao, Z.; Yangpeng, S.; Deng, F.; Zhiguang, Z. Production of inter-connective porous dental implants by computer-aided design and metal three-dimensional printing. J. Biomater. Appl. 2020, 34, 1227–1238. [Google Scholar] [CrossRef]
- Silva, M.; Felismina, R.; Mateus, A.; Parreira, P.; Malça, C. Application of a Hybrid Additive Manufacturing Methodology to Produce a Metal/Polymer Customized Dental Implant. Procedia Manuf. 2017, 12, 150–155. [Google Scholar] [CrossRef]
- Koutsoukis, T.; Zinelis, S.; Eliades, G.; Al-Wazzan, K.; Al Rifaiy, M.; Al Jabbari, Y.S. Selective Laser Melting Technique of Co-Cr Dental Alloys: A Review of Structure and Properties and Comparative Analysis with Other Available Techniques. J. Prosthodont. 2015, 24, 303–312. [Google Scholar] [CrossRef]
- Hu, M.L.; Lin, H.; Zhang, Y.D.; Han, J.M. Comparison of technical, biological, and esthetic parameters of ceramic and metal-ceramic implant-supported fixed dental prostheses: A systematic review and meta-analysis. J. Prosthet. Dent. 2020, 124, 26–35.e2. [Google Scholar] [CrossRef]
- Rudd, R.W.; Rudd, K.D. A review of 243 errors possible during the fabrication of a removable partial denture: Part I. J. Prosthet. Dent. 2001, 86, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Frias, V.; Lee, K.W.; Wright, R.F. Effect of implant size and shape on implant success rates: A literature review. J. Prosthet. Dent. 2005, 94, 377–381. [Google Scholar] [CrossRef]
- Misch, C.E.; Bidez, M.W.; Sharawy, M. A Bioengineereed Implant for a Predetermined Bone Cellular Response to Loading Forces. A Literature Review and Case Report. J. Periodontol. 2001, 72, 1276–1286. [Google Scholar] [CrossRef] [Green Version]
- Mendenhall, W.M.; Foote, R.L.; Sandow, P.L.; Fernandes, R.P. Oral cavity. In Clinical Radiation Oncology; Gunderson, L.L., Tepper, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 570–596.e3. ISBN 978032324098. [Google Scholar]
- Payvand, K.; Sadiq, N.M. Anatomy, Head and Neck, Oral Cavity (Mouth) Blood Supply and Lymphatics; StatPearls: Treasure Island, FL, USA, 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/31424855/ (accessed on 18 November 2022).
- Baranova, J.; Büchner, D.; Götz, W.; Schulze, M.; Tobiasch, E. Tooth formation: Are the hardest tissues of human body hard to regenerate? Int. J. Mol. Sci. 2020, 21, 4031. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Strucutre, composition and mineralization. Natl. Institue Heal. 2011, 3, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, E.; Holt, A.; Swain, M.; Kilpatrick, N. The hardness and modulus of elasticity of primary molar teeth: An ultra-micro-indentation study. J. Dent. 2000, 28, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Habelitz, S.; Marshall, S.J.; Marshall, G.W.M., Jr.; Balooch, M. Mechanical properties of human dental enamel on the nanometre scale. Arch. Oral Biol. 2001, 46, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.F.; Scholz, T.; Klocke, A.; Schneider, G.A. Determination of the elastic/plastic transition of human enamel by nanoindentation. Dent. Mater. 2009, 25, 1403–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeng, Y.R.; Lin, T.T.; Hsu, H.M.; Chang, H.J.; Shieh, D. Bin Human enamel rod presents anisotropic nanotribological properties. J. Mech. Behav. Biomed. Mater. 2011, 4, 515–522. [Google Scholar] [CrossRef]
- Cuy, J.L.; Mann, A.B.; Livi, K.J.; Teaford, M.F.; Weihs, T.P. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch. Oral Biol. 2002, 47, 281–291. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Wang, X.M.; Ge, J.; Yan, J.X.; Ji, N.; Tian, L.L.; Cui, F.Z. The mechanical anisotropy on a longitudinal section of human enamel studied by nanoindentation. J. Mater. Sci. Mater. Med. 2010, 21, 1811–1816. [Google Scholar] [CrossRef]
- Braly, A.; Darnell, L.A.; Mann, A.B.; Teaford, M.F.; Weihs, T.P. The effect of prism orientation on the indentation testing of human molar enamel. Arch. Oral Biol. 2007, 52, 856–860. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Basu, B. Mechanical and tribological characterization of human tooth. Mater. Charact. 2008, 59, 747–756. [Google Scholar] [CrossRef]
- Flávio, V.B. The Perception of Food Texture—The Philosophy Of The Breakdown Path. J. Texture Stud. 1988, 1, 12–17. [Google Scholar]
- Witt, T.; Stokes, J.R. Physics of food structure breakdown and bolus formation during oral processing of hard and soft solids. Curr. Opin. Food Sci. 2015, 3, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Bongaerts, J.H.H.; Rossetti, D.; Stokes, J.R. The lubricating properties of human whole saliva. Tribol. Lett. 2007, 27, 277–287. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Wang, S.; Yang, X.; Sun, Y. Two-Body and Three-Body Wear Behavior of a Dental Fluorapatite Glass-Ceramic. Coatings 2019, 9, 580. [Google Scholar] [CrossRef] [Green Version]
- Mathew, M.T.; Abbey, S.; Hallab, N.J.; Hall, D.J.; Sukotjo, C.; Wimmer, M.A. Influence of pH on the tribocorrosion behavior of CpTi in the oral environment: Synergistic interactions of wear and corrosion. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2012, 100 B, 1662–1671. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Z.R. Wear behavior of human teeth in dry and artificial saliva conditions. Wear 2001, 249, 980–984. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Barbosa, S.L.; Ariza, E.; Celis, J.P.; Rocha, L.A. Simultaneous degradation by corrosion and wear of titanium in artificial saliva containing fluorides. Wear 2012, 292–293, 82–88. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Si, W.; Feng, H.; Tao, Y.; Ma, Z. Friction and wear behaviors of dental ceramics against natural tooth enamel. J. Eur. Ceram. Soc. 2012, 32, 2599–2606. [Google Scholar] [CrossRef]
- Pulikkottil, V.J.; Chidambaram, S.; Bejoy, P.U.; Femin, P.K.; Paul, P.; Rishad, M. Corrosion resistance of stainless steel, nickel-titanium, titanium molybdenum alloy, and ion-implanted titanium molybdenum alloy archwires in acidic fluoride-containing artificial saliva: An in vitro study. J. Pharm. Bioallied Sci. 2016, 8, S96–S99. [Google Scholar] [CrossRef]
- Andrei, M.; Galateanu, B.; Hudita, A.; Costache, M.; Osiceanu, P.; Calderon Moreno, J.M.; Drob, S.I.; Demetrescu, I. Electrochemical comparison and biological performance of a new CoCrNbMoZr alloy with commercial CoCrMo alloy. Mater. Sci. Eng. C 2016, 59, 346–355. [Google Scholar] [CrossRef]
- Romonti, D.E.; Gomez Sanchez, A.V.; Milošev, I.; Demetrescu, I.; Ceré, S. Effect of anodization on the surface characteristics and electrochemical behaviour of zirconium in artificial saliva. Mater. Sci. Eng. C 2016, 62, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Licausi, M.P.; Igual Muñoz, A.; Amigó Borrás, V. Influence of the fabrication process and fluoride content on the tribocorrosion behaviour of Ti6Al4V biomedical alloy in artificial saliva. J. Mech. Behav. Biomed. Mater. 2013, 20, 137–148. [Google Scholar] [CrossRef]
- Moreira, V.; Serro, A.P.; Colaço, R.; Pina, C.; Reis, J.A.; Art, E.X.P. Tribological Behavior of the Zirconia/Ti6Al4V System Used in Dental Implants. 2014, pp. 1–10. Available online: https://www.semanticscholar.org/paper/Tribological-behavior-of-the-Zirconia-%2F-Ti-6-Al-4-V-Moreira-Serro/a28243cc871504ac4abe06f4024bf54e5e482029 (accessed on 27 September 2021).
- Alshali, R.Z.; Salim, N.A.; Satterthwaite, J.D.; Silikas, N. Long-term sorption and solubility of bulk-fill and conventional resin-composites in water and artificial saliva. J. Dent. 2015, 43, 1511–1518. [Google Scholar] [CrossRef]
- d’Incau, E.; Saulue, P. Understanding dental wear. J. Dentofac. Anom. Orthod. 2012, 15, 104. [Google Scholar] [CrossRef]
- Schwarz, W.H. The Rheology of Saliva. J. Dent. Res. 1987, 66, 660–666. [Google Scholar] [CrossRef]
- Stokes, J.R.; Davies, G.A. Viscoelasticity of human whole saliva collected after acid and mechanical stimulation. Biorheology 2007, 44, 141–160. [Google Scholar]
- Murray, P. Biocompatibility of Biomaterials for Dental Tissue Repair. Biocompat. Dent. Biomater. 2017, 41–62. [Google Scholar] [CrossRef]
- Zafar, M.S.; Ullah, R.; Qamar, Z.; Fareed, M.A.; Amin, F.; Khurshid, Z.; Sefat, F. Properties of dental biomaterials. Adv. Dent. Biomater. 2019, 7–35. [Google Scholar] [CrossRef]
- Pinto, P.; Carvalho, A.; Silva, F.S.; Gomes, J.R.; Carvalho, O.; Madeira, S. Comparative toothbrush abrasion resistance and surface analysis of different dental restorative materials. Tribol. Int. 2022, 175, 107799. [Google Scholar] [CrossRef]
- Ruse, N.D. Fracture mechanics characterization of dental biomaterials. Dent. Biomater. Imaging Test. Model. 2008, 261–293. [Google Scholar] [CrossRef]
- Sajewicz, E. On evaluation of wear resistance of tooth enamel and dental materials. Wear 2006, 260, 1256–1261. [Google Scholar] [CrossRef]
- Hussein, M.A.; Mohammed, A.S.; Al-Aqeeli, N. Wear characteristics of metallic biomaterials: A review. Materials 2015, 8, 2749–2768. [Google Scholar] [CrossRef] [Green Version]
- Mair, L.H.; Stolarski, T.A.; Vowles, R.W.; Lloyd, C.H. Wear: Mechanisms, manifestations and measurement. Report of a workshop. J. Dent. 1996, 24, 141–148. [Google Scholar] [CrossRef]
- Xiang, D.; Tan, X.; Liao, Z.; He, J.; Zhang, Z.; Liu, W.; Wang, C.; Shu, B.T. Comparison of wear properties of Ti6Al4V fabricated by wrought and electron beam melting processes in simulated body fluids. Rapid Prototyp. J. 2020, 26, 959–969. [Google Scholar] [CrossRef]
- Eisenburger, M.; Addy, M. Erosion and attrition of human enamel in vitro Part I: Interaction effects. J. Dent. 2002, 30, 341–347. [Google Scholar] [CrossRef]
- Mayworm, C.D.; Camargo, S.S.; Bastian, F.L. Influence of artificial saliva on abrasive wear and microhardness of dental composites filled with nanoparticles. J. Dent. 2008, 36, 703–710. [Google Scholar] [CrossRef]
- Arsecularatne, J.A.; Hoffman, M. On the wear mechanism of human dental enamel. J. Mech. Behav. Biomed. Mater. 2010, 3, 347–356. [Google Scholar] [CrossRef]
- Borrero-Lopez, O.; Constantino, P.J.; Lawn, B.R. Role of particulate concentration in tooth wear. J. Mech. Behav. Biomed. Mater. 2018, 80, 77–80. [Google Scholar] [CrossRef]
- Gahr, K.H. Zum Modelling of two-body abrasive wear. Wear 1988, 124, 87–103. [Google Scholar] [CrossRef]
- Duyck, J.; Van Oosterwyck, H.; Vander Sloten, J.; De Cooman, M.; Puers, R.; Naert, I. Magnitude and distribution of occlusal forces on oral implants supporting fixed prostheses: An in vivo study. Clin. Oral Implant. Res. 2000, 11, 465–475. [Google Scholar] [CrossRef]
- Wassell, R.W.; Mccabe, J.E.; Walls, A.W.G. A Two-body Frictional Wear Test. J. Dent. Res. 1994, 73, 1546–1553. [Google Scholar] [CrossRef] [Green Version]
- Söderholm, K.J.; Richards, N.D. Wear resistance of composites: A solved problem? Gen. Dent. 1998, 46, 256–263. [Google Scholar]
- Hacker, C.H.; Wagner, W.C.; Razzoog, M.E. An in vitro investigation of the wear of enamel on porcelain and gold in saliva. J. Prosthet. Dent. 1996, 75, 14–17. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Smith, D.T.; Jahanmir, S.; Romberg, E.; Kelly, J.R.; Thompson, V.P.; Rekow, E.D. Indentation damage and mechanical properties of human enamel and dentin. J. Dent. Res. 1998, 77, 472–480. [Google Scholar] [CrossRef]
- Wang, X.; Lussi, A. Assessment and management of dental erosion. Dent. Clin. N. Am. 2010, 54, 565–578. [Google Scholar] [CrossRef]
- Ganss, C. Definition of erosion and links to tooth wear. Monogr. Oral Sci. 2006, 20, 9–16. [Google Scholar] [CrossRef]
- Burtscher, D.; Dalla Torre, D. Dental implant procedures in immunosuppressed organ transplant patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2021, 4–11. [Google Scholar] [CrossRef]
- Misch, C.E. Contemporary Implant Dentistry, 2nd ed.; Mosby: St. Louis, MO, USA, 1999; ISBN 9780815170594. [Google Scholar]
- Winkler, S.; Morris, H.F.; Ochi, S. Implant survival to 36 months as related to length and diameter. Ann. Periodontol. 2000, 5, 22–31. [Google Scholar] [CrossRef]
- Ivanoff, C.J.; Gröndahl, K.; Sennerby, L.; Bergström, C.; Lekholm, U. Influence of variations in implant diameters: A 3- to 5-year retrospective clinical report. Int. Oral Maxillofac Implant. 1999, 14, 173–180. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. Int. J. Oral Maxillofac Surg. 1999, 57, s0278–s2391. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Boring, J.G.; Cooper, R.C., Jr.; Gao, C.; Givaruangsawat, S.; Gilbert, J.A.; Misch, C.M.; Steflik, D. Preliminary evaluation of a new dental implant design in canine models. Implant Dent. 2000, 9, 252–260. [Google Scholar] [CrossRef]
- Linkow, L.I.; Winkler, S.; Shulman, M.; Carlo, L.D.; Pasqualini, M.E.; Rossi, F.; Nardone, M. A new look at the blade implant. J. Oral Implantol. 2016, 42, 373–380. [Google Scholar] [CrossRef]
- Carr, A.; Brown, D. McCracken’s Removable Partial Prosthodontics, 13th ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780323339919. [Google Scholar]
- American Dental Association; Turner, H.F. Ramus frame implant technique. J. Am. Dent. Assoc. 1990, 121, 418–420. [Google Scholar] [CrossRef]
- Dmd, K.U. Effects of Chemical and Prophylactic Agents Used in Dentistry on Titanium Implant Surfaces. Ph.D. Thesis, University of Szeged, Dugonics tér, Hungary, 2013. [Google Scholar]
- Misch, C.E. Single-Tooth Implant Restoration: Maxillary Anterior and Posterior Regions. Dent. Implant. Prosthet. 2015, 499–552. [Google Scholar] [CrossRef]
- Prasad, D.K.; Prasad, D.A.; Bardia, A.; Hegde, C. Questionable abutments: General considerations, changing trends in treatment planning and available options. J. Interdiscip. Dent. 2013, 3, 12. [Google Scholar] [CrossRef]
- Rathi, S.; Verma, A. Material Selection for Single-Tooth Crown Restorations; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128137598. [Google Scholar]
- Makhija, S.K.; Mph, D.D.S.; Lawson, N.C.; Gilbert, G.H.; Litaker, M.S.; Mcclelland, J.A.; Louis, D.R.; Valeria, V.; Ms-ci, D.D.S.M.S.; Pihlstrom, D.J.; et al. Dentist Material Selection for Single-Unit Crowns: Findings from The National Dental Practice-Based Research Network. J. Dent. 2017, 55, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.S.; Lisby, S.; Baadsgaard, O.; Byrialsen, K.; Menné, T. Release of nickel ions from stainless steel alloys used in dental braces and their patch test reactivity in nickel-sensitive individuals. Contact Dermat. 2003, 48, 300–304. [Google Scholar] [CrossRef]
- Almufleh, B.; Emami, E.; Alageel, O.; de Melo, F.; Seng, F.; Caron, E.; Nader, S.A.; Al-Hashedi, A.; Albuquerque, R.; Feine, J.; et al. Patient satisfaction with laser-sintered removable partial dentures: A crossover pilot clinical trial. J. Prosthet. Dent. 2018, 119, 560–567.e1. [Google Scholar] [CrossRef]
- Weiss, C.M.; Weiss, A. Implant dentistry nomenclature, classification, and examples. In Principles and Practice of Implant Dentistry; Mosby Incorporated: St. Louis, MO, USA, 2001; pp. 7–16. [Google Scholar]
- Smith, Y. Procedure for Dental Crowns. Available online: https://www.news-medical.net/health/Procedure-for-Dental-Crowns.aspx (accessed on 11 November 2021).
- Smith, Y. Types of Dental Braces. Available online: https://www.news-medical.net/health/Types-of-Dental-Braces.aspx (accessed on 24 July 2021).
- Smith, D.C. Dental implants: Materials and design considerations. Int. J. Prosthodont. 1993, 6, 106–117. [Google Scholar]
- Poojar, B.; Ommurugan, B.; Adiga, S.; Thomas, H.; Sori, R.K.; Poojar, B.; Hodlur, N.; Tilak, A.; Korde, R.; Gandigawad, P.; et al. Methodology Used in the Study. Asian J. Pharm. Clin. Res. 2017, 7, 1–5. [Google Scholar] [CrossRef]
- Eliasson, A.; Arnelund, C.F.; Johansson, A. A clinical evaluation of cobalt-chromium metal-ceramic fixed partial dentures and crowns: A three- to seven-year retrospective study. J. Prosthet. Dent. 2007, 98, 6–16. [Google Scholar] [CrossRef]
- Svanborg, P.; Hjalmarsson, L. A systematic review on the accuracy of manufacturing techniques for cobalt chromium fixed dental prostheses. Biomater. Investig. Dent. 2020, 7, 31–40. [Google Scholar] [CrossRef]
- Castro, S.M.; Ponces, M.J.; Lopes, J.D.; Vasconcelos, M.; Pollmann, M.C.F. Orthodontic wires and its corrosion—The specific case of stainless steel and beta-titanium. J. Dent. Sci. 2015, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bharate, V.; Kumar, Y.; Koli, D.; Pruthi, G.; Jain, V. Effect of different abutment materials (zirconia or titanium) on the crestal bone height in 1 year: Bone loss with implant abutment materials. J. Oral Biol. Craniofacial Res. 2020, 10, 372–374. [Google Scholar] [CrossRef]
- Liu, R.; Tang, Y.; Zeng, L.; Zhao, Y.; Ma, Z.; Sun, Z.; Xiang, L.; Ren, L.; Yang, K. In vitro and in vivo studies of anti-bacterial copper-bearing titanium alloy for dental application. Dent. Mater. 2018, 34, 1112–1126. [Google Scholar] [CrossRef]
- Homaei, E.; Farhangdoost, K.; Tsoi, J.K.H.; Matinlinna, J.P.; Pow, E.H.N. Static and fatigue mechanical behavior of three dental CAD/CAM ceramics. J. Mech. Behav. Biomed. Mater. 2016, 59, 304–313. [Google Scholar] [CrossRef]
- Janyavula, S.; Lawson, N.; Cakir, D.; Beck, P.; Ramp, L.C.; Burgess, J.O. The wear of polished and glazed zirconia against enamel. J. Prosthet. Dent. 2013, 109, 22–29. [Google Scholar] [CrossRef]
- Vaicelyte, A.; Janssen, C.; Le Borgne, M.; Grosgogeat, B. Cobalt–Chromium Dental Alloys: Metal Exposures, Toxicological Risks, CMR Classification, and EU Regulatory Framework. Crystals 2020, 10, 1151. [Google Scholar] [CrossRef]
- Ramírez-Ledesma, A.L.; Roncagliolo, P.; Álvarez-Pérez, M.A.; Lopez, H.F.; Juárez-Islas, J.A. Corrosion Assessment of an Implantable Dental Co-Cr Alloy in Artificial Saliva and Biocompatibility Behavior. J. Mater. Eng. Perform. 2020, 29, 1657–1670. [Google Scholar] [CrossRef]
- Viennot, S.; Dalard, F.; Lissac, M.; Grosgogeat, B. Corrosion resistance of cobalt-chromium and palladium-silver alloys used in fixed prosthetic restorations. Eur. J. Oral Sci. 2005, 113, 90–95. [Google Scholar] [CrossRef]
- Evans, E.J.; Thomas, I.T. The in vitro toxicity of cobalt-chrome-molybdenum alloy and its constituent metals. Biomaterials 1986, 7, 25–29. [Google Scholar] [CrossRef]
- Craig, R.G.; Hanks, C.T. Reaction of fibroblasts to various dental casting alloys. J. Oral Pathol. Med. 1988, 17, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Mareci, D.; Nemtoi, G.; Aelenei, N.; Bocanu, C. The electrochemical behaviour of various non-precious Ni and Co based alloys in artificial saliva. Eur. Cells Mater. 2005, 10, 1–7. [Google Scholar]
- Wennerberg, A.; Albrektsson, T.; Jimbo, R. Implant Surfaces and Their Biological and Clinical Impact, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Fu, W.; Liu, S.; Jiao, J.; Xie, Z.; Huang, X.; Lu, Y.; Liu, H.; Hu, S.; Zuo, E.; Kou, N.; et al. Wear Resistance and Biocompatibility of Co-Cr Dental Alloys Fabricated with CAST and SLM Techniques. Materials 2022, 15, 3263. [Google Scholar] [CrossRef]
- Puskar, T.; Jevremovic, D.; Williams, R.J.; Eggbeer, D.; Vukelic, D.; Budak, I. A comparative analysis of the corrosive effect of artificial saliva of variable pH on DMLS and cast Co-Cr-Mo dental alloy. Materials 2014, 6, 6486–6501. [Google Scholar] [CrossRef] [Green Version]
- Karimi, S.; Alfantazi, A.M. Ion release and surface oxide composition of AISI 316L, Co-28Cr-6Mo, and Ti-6Al-4V alloys immersed in human serum albumin solutions. Mater. Sci. Eng. C 2014, 40, 435–444. [Google Scholar] [CrossRef]
- Thakur, L.; Arora, N. An investigation on the development and wear performance of chromium-MWCNTs transformed HVOF sprayed nano-WC-CoCr coatings. Surf. Coat. Technol. 2020, 388, 125610. [Google Scholar] [CrossRef]
- Holmes, D.; Sharifi, S.; Stack, M.M. Tribo-corrosion of steel in artificial saliva. Tribol. Int. 2014, 75, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Kerosuo, H.; Moe, G.; Kleven, E. In vitro release of nickel and chromium from different types of simulated orthodontic appliances. Angle Orthod. 1995, 65, 111–116. [Google Scholar] [CrossRef]
- Balamurugan, A.; Rajeswari, S.; Balossier, G.; Rebelo, A.H.S.; Ferreira, J.M.F. Corrosion aspects of metallic implants—An overview. Mater. Corros. 2008, 59, 855–869. [Google Scholar] [CrossRef]
- Zhang, H.; Man, C.; Dong, C.; Wang, L.; Li, W.; Kong, D.; Wang, L.; Wang, X. The corrosion behavior of Ti6Al4V fabricated by selective laser melting in the artificial saliva with different fluoride concentrations and pH values. Corros. Sci. 2021, 179, 109097. [Google Scholar] [CrossRef]
- Zhang, L.C.; Attar, H. Selective Laser Melting of Titanium Alloys and Titanium Matrix Composites for Biomedical Applications: A Review. Adv. Eng. Mater. 2016, 18, 463–475. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Wang, Z.B.; Hu, H.X.; Liu, C.B.; Zheng, Y.G. The effect of fluoride ions on the corrosion behavior of pure titanium in 0.05 M sulfuric acid. Electrochim. Acta 2014, 135, 526–535. [Google Scholar] [CrossRef]
- Sotniczuk, A.; Kuczyńska-Zemła, D.; Kwaśniak, P.; Thomas, M.; Garbacz, H. Corrosion behavior of Ti-29Nb-13Ta-4.6Zr and commercially pure Ti under simulated inflammatory conditions—Comparative effect of grain refinement and non-toxic β phase stabilizers. Electrochim. Acta 2019, 312, 369–379. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.; Bai, L.; Tang, B.; Chu, P.K. Electrochemical surface engineering of titanium-based alloys for biomedical application. Electrochim. Acta 2018, 271, 699–718. [Google Scholar] [CrossRef]
- Izquierdo, J.; González-Marrero, M.B.; Bozorg, M.; Fernández-Pérez, B.M.; Vasconcelos, H.C.; Santana, J.J.; Souto, R.M. Multiscale electrochemical analysis of the corrosion of titanium and nitinol for implant applications. Electrochim. Acta 2016, 203, 366–378. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef]
- Brown, R.P.; Fowler, B.A.; Fustinoni, S.; Costa, M.; Nordberg, M. Toxicity of metals released from implanted medical devices. Handb. Toxicol. Met. Fifth Ed. 2021, 1, 127–136. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Guo, Z.X. Biomechanical and biochemical compatibility in innovative biomaterials. Biocompat. Perform. Med. Devices 2019, 23–46. [Google Scholar] [CrossRef]
- Kim, H.R.; Jang, S.H.; Kim, Y.K.; Son, J.S.; Min, B.K.; Kim, K.H.; Kwon, T.Y. Microstructures and mechanical properties of Co-Cr dental alloys fabricated by three CAD/CAM-based processing techniques. Materials 2016, 9, 596. [Google Scholar] [CrossRef] [PubMed]
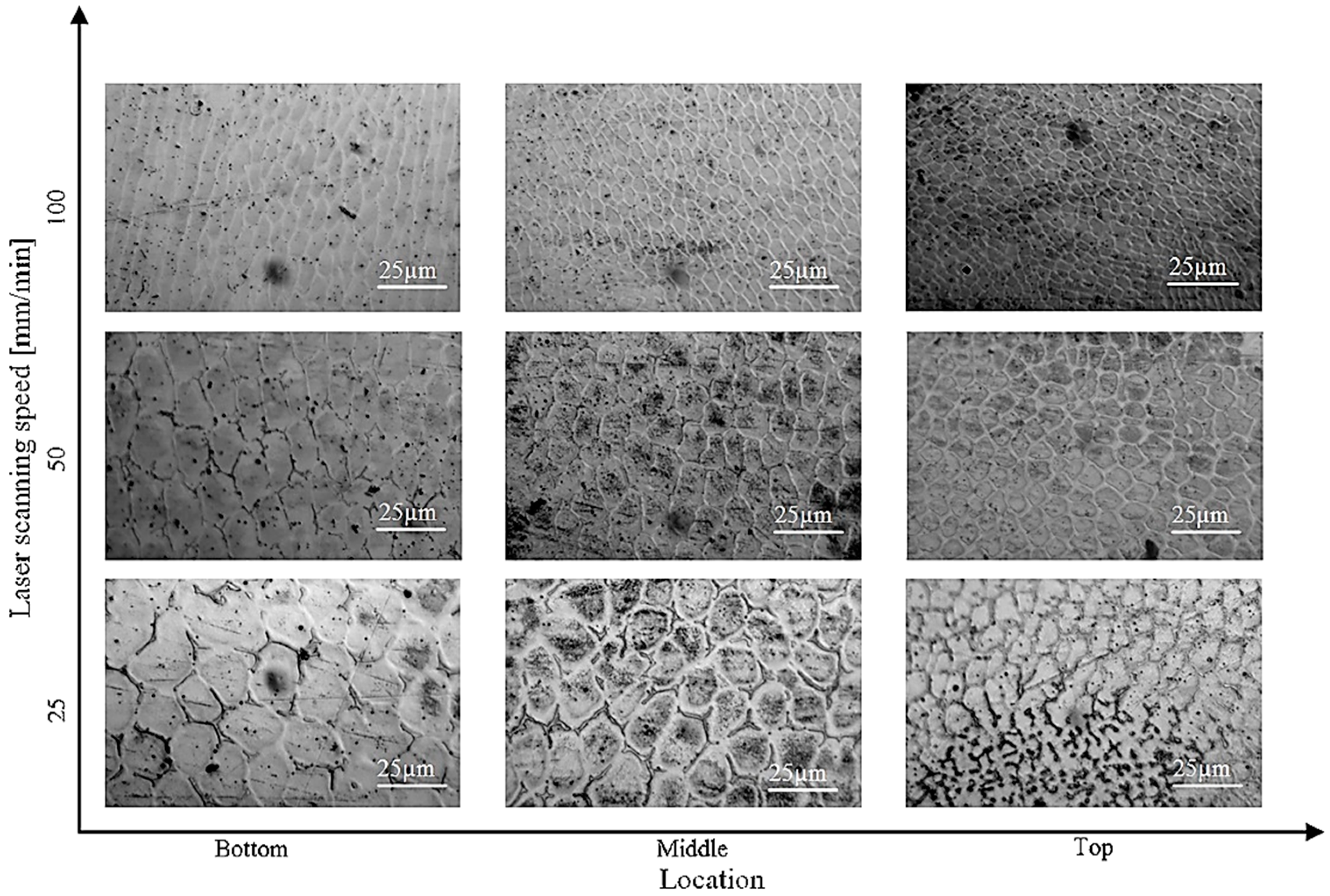
- Moradi, M.; Hasani, A.; Malekshahi Beiranvand, Z.; Ashoori, A. Additive manufacturing of stellite 6 superalloy by direct laser metal deposition—Part 2: Effects of scanning pattern and laser power reduction in differrent layers. Opt. Laser Technol. 2020, 131, 106455. [Google Scholar] [CrossRef]
- Mostafaei, A.; Rodriguez De Vecchis, P.; Buckenmeyer, M.J.; Wasule, S.R.; Brown, B.N.; Chmielus, M. Microstructural evolution and resulting properties of differently sintered and heat-treated binder-jet 3D-printed Stellite 6. Mater. Sci. Eng. C 2019, 102, 276–288. [Google Scholar] [CrossRef]
- Crook, P.; Asphahani, A.I.; Matthews, S.J. Corrosion-And-Wear-Resistant Cobalt-Base Alloy. US Patent 5,002,731, 26 March 1991. [Google Scholar]
- Giacchi, J.V.; Morando, C.N.; Fornaro, O.; Palacio, H.A. Microstructural characterization of as-cast biocompatible Co-Cr-Mo alloys. Mater. Charact. 2011, 62, 53–61. [Google Scholar] [CrossRef]
- Walley, K.C.; Bajraliu, M.; Gonzalez, T.; Nazarian, A. The Chronicle of a Stainless Steel Orthopaedic Implant. Orthop. J. Harv. Med. Sch. 2016, 17, 68–74. [Google Scholar]
- Gotman, I. Characteristics of metals used in implants. J. Endourol. 1997, 11, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Haudrechy, P.; Mantout, B.; Frappaz, A.; Rousseau, D.; Chabeau, G.; Faure, M.; Claudy, A. Nickel release from stainless steels. Contact Dermat. 1997, 37, 113–117. [Google Scholar] [CrossRef]
- Menné, T.; Brandup, F.; Thestrup-Pedersen, K.; Veien, N.K.; Andersen, J.R.; Yding, F.; Valeur, G. Patch test reactivity to nickel alloys. Contact Dermat. 1987, 105–120. [Google Scholar] [CrossRef]
- Park, H.Y.; Shearer, T.R. In vitro release of nickel and chromium from simulated orthodontic appliances. Am. J. Orthod. 1983, 84, 156–159. [Google Scholar] [CrossRef]
- Gjerdet, N.R.; Herø, H. Metal release from heat-treated orthodontic archwires. Acta Odontol. Scand. 1987, 45, 409–414. [Google Scholar] [CrossRef]
- Bieler, T.R.; Trevino, R.M.; Zeng, L. Alloys: Titanium. Ref. Modul. Mater. Sci. Mater. Eng. 2005, 1, 65–76. [Google Scholar] [CrossRef]
- Traini, T.; Mangano, C.; Sammons, R.L.; Mangano, F.; Macchi, A.; Piattelli, A. Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally graded material for manufacture of porous titanium dental implants. Dent. Mater. 2008, 24, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Gahlert, M.; Röhling, S.; Wieland, M.; Sprecher, C.M.; Kniha, H.; Milz, S. Osseointegration of zirconia and titanium dental implants: A histological and histomorphometrical study in the maxilla of pigs. Clin. Oral Implant. Res. 2009, 20, 1247–1253. [Google Scholar] [CrossRef]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implant. Res. 2008, 19, 823–835. [Google Scholar] [CrossRef]
- Givan, D.A. Precious Metals in Dentistry. Dent. Clin. North Am. 2007, 51, 591–601. [Google Scholar] [CrossRef]
- Reclaru, L.; Ardelean, L.C. Alternative Processing Techniques for CoCr Dental Alloys. Encycl. Biomed. Eng. 2019, 1–3, 1–15. [Google Scholar] [CrossRef]
- Ferracane, J.; Powers, J. (Eds.) Restorative materials: Resin composites and polymers. In Craig’s Restorative Dental Materials, 14th ed.; Elsevier: Sakaguchi, Ronald, 2019; pp. 135–170. ISBN 9780323478212. [Google Scholar]
- Aziz, M.N.A.; Rusnaldy; Munyensanga, P.; Widyanto, S.A. Paryanto Application of lost wax casting for manufacturing of orthopedic screw: A review. Procedia CIRP 2018, 78, 149–154. [Google Scholar] [CrossRef]
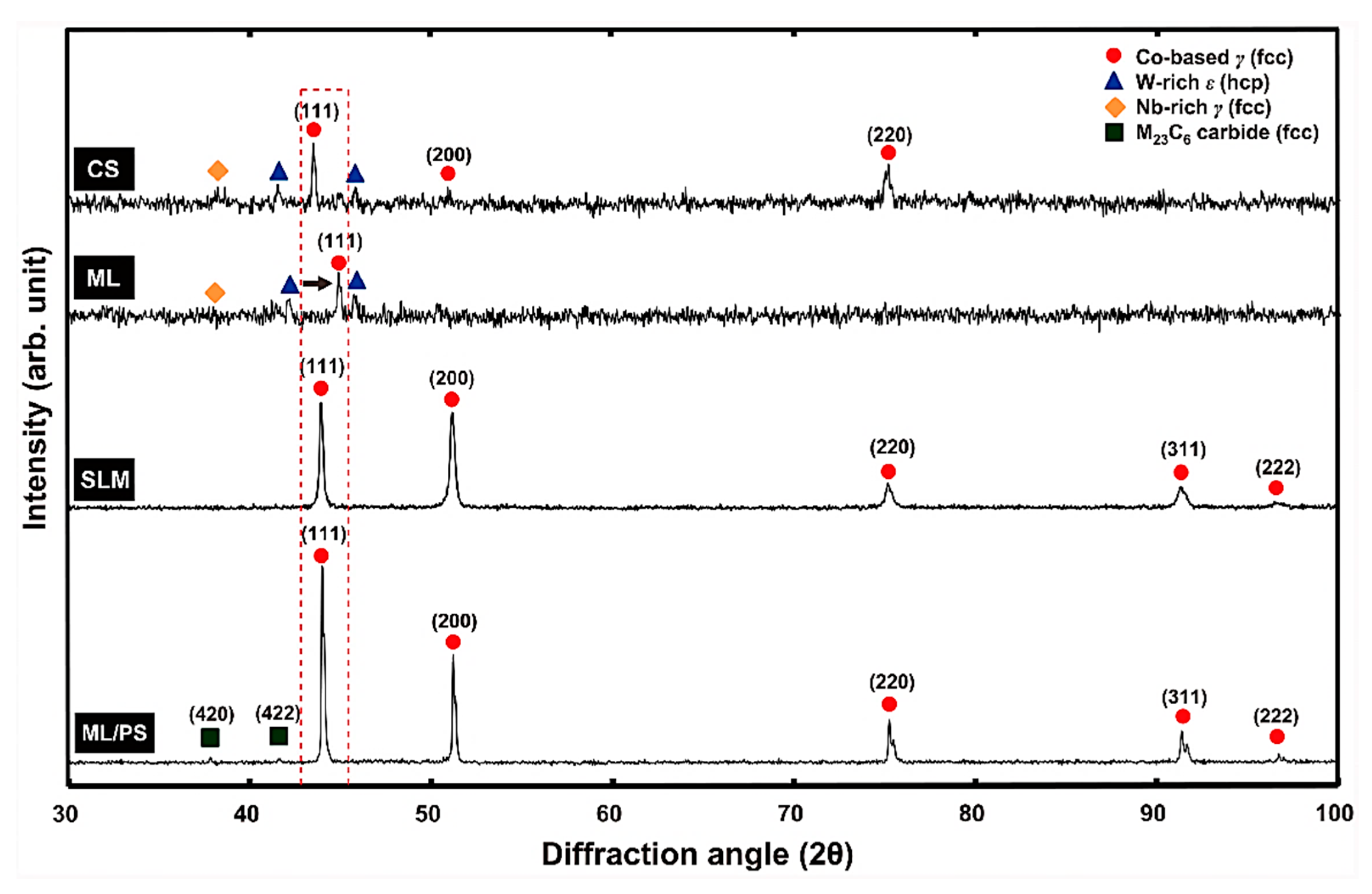
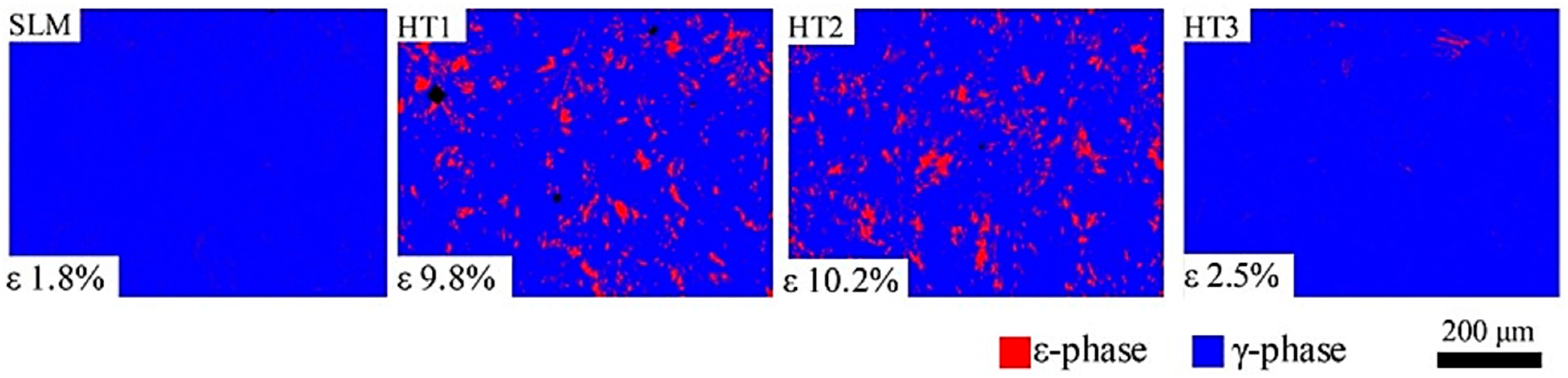
- Wu, M.; Dong, X.; Qu, Y.; Yan, J.; Li, N. Analysis of microstructure and fatigue of cast versus selective laser-melted dental Co-Cr alloy. J. Prosthet. Dent. 2022, 128, 218.e1–218.e7. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, N.; Yan, J.; Zeng, Q. Comparative analysis of the microstructures and mechanical properties of Co-Cr dental alloys fabricated by different methods. J. Prosthet. Dent. 2018, 120, 617–623. [Google Scholar] [CrossRef]
- Li, J.L.Z.; Alkahari, M.R.; Rosli, N.A.B.; Hasan, R.; Sudin, M.N.; Ramli, F.R. Review of wire arc additive manufacturing for 3d metal printing. Int. J. Autom. Technol. 2019, 13, 346–353. [Google Scholar] [CrossRef]
- Sames, W.J.; List, F.A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The Metallurgy and Processing Science of Metal. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- Vandenbroucke, B.; Kruth, J.P. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyp. J. 2007, 13, 196–203. [Google Scholar] [CrossRef]
- Gong, H.; Rafi, K.; Gu, H.; Starr, T.; Stucker, B. Analysis of defect generation in Ti-6Al-4V parts made using powder bed fusion additive manufacturing processes. Addit. Manuf. 2014, 1, 87–98. [Google Scholar] [CrossRef]
- Caiazzo, F.; Alfieri, V. Directed Energy Deposition of stainless steel wire with laser beam: Evaluation of geometry and affection depth. Procedia CIRP 2021, 99, 348–351. [Google Scholar] [CrossRef]
- Sing, S.L.; Tey, C.F.; Tan, J.H.K.; Huang, S.; Yeong, W.Y. 3D printing of metals in rapid prototyping of biomaterials: Techniques in additive manufacturing. Rapid Prototyp. Biomater. Tech. Addit. Manuf. 2019, 17–40. [Google Scholar] [CrossRef]
- Barro, Ó.; Arias-González, F.; Lusquiños, F.; Comesaña, R.; Del Val, J.; Riveiro, A.; Badaoui, A.; Gómez-Baño, F.; Pou, J. Improved commercially pure titanium obtained by laser directed energy deposition for dental prosthetic applications. Metals 2021, 11, 70. [Google Scholar] [CrossRef]
- Mathoho, I.; Akinlabi, E.T.; Arthur, N.; Tlotleng, M. Impact of DED process parameters on the metallurgical characteristics of 17-4 PH SS deposited using DED. CIRP J. Manuf. Sci. Technol. 2020, 31, 450–458. [Google Scholar] [CrossRef]
- Huang, Y.; Ansari, M.; Asgari, H.; Farshidianfar, M.H.; Sarker, D.; Khamesee, M.B.; Toyserkani, E. Rapid prediction of real-time thermal characteristics, solidification parameters and microstructure in laser directed energy deposition (powder-fed additive manufacturing). J. Mater. Process. Technol. 2019, 274, 116286. [Google Scholar] [CrossRef]
- Du, L.; Gu, D.; Dai, D.; Shi, Q.; Ma, C.; Xia, M. Relation of thermal behavior and microstructure evolution during multi-track laser melting deposition of Ni-based material. Opt. Laser Technol. 2018, 108, 207–217. [Google Scholar] [CrossRef]
- Kurz, W.; Giovanola, B.; Trivedi, R. Theory of Microstructural Development During Rapid Solidification. Acta Metall. 1986, 34, 823–830. [Google Scholar] [CrossRef]
- Gan, Z.; Yu, G.; He, X.; Li, S. Numerical simulation of thermal behavior and multicomponent mass transfer in direct laser deposition of Co-base alloy on steel. Int. J. Heat Mass Transf. 2017, 104, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Kavoosi, V.; Abbasi, S.M.; Mirsaed, S.M.G.; Mostafaei, M. Influence of cooling rate on the solidification behavior and microstructure of IN738LC superalloy. J. Alloy. Compd. 2016, 680, 291–300. [Google Scholar] [CrossRef]
- Gao, B.; Sui, Y.; Wang, H.; Chunmin, Z.; Wei, Z.; Wang, R.; Sun, Y. Effects of Cooling Rate on the Solidification and Microstructure of Nickel-Based Superalloy GTD222. Materials 2019, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- Gudur, S.; Nagallapati, V.; Pawar, S.; Muvvala, G.; Simhambhatla, S. A study on the effect of substrate heating and cooling on bead geometry in wire arc additive manufacturing and its correlation with cooling rate. Mater. Today Proc. 2019, 41, 431–436. [Google Scholar] [CrossRef]
- Tan, W.; Wen, S.; Bailey, N.; Shin, Y.C. Multiscale modeling of transport phenomena and dendritic growth in laser cladding processes. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2011, 42, 1306–1318. [Google Scholar] [CrossRef]
- Hama Suleiman, S.; Vult Von Steyern, P. Fracture strength of porcelain fused to metal crowns made of cast, milled or laser-sintered cobalt-chromium. Acta Odontol. Scand. 2013, 71, 1280–1289. [Google Scholar] [CrossRef]
- Myszka, D.; Skrodzki, M. Comparison of Dental Prostheses Cast and Sintered by SLM from Co-Cr-Mo-W Alloy. Arch. Foundry Eng. 2016, 16, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-J.; Koak, J.-Y.; Heo, S.-J.; Kim, S.-K.; Ahn, J.-S.; Park, D.-S. Comparison of the mechanical properties and microstructures of fractured surface for Co-Cr alloy fabricated by conventional cast, 3-D printing laser-sintered and CAD/CAM milled techniques. J. Korean Acad. Prosthodont. 2014, 52, 67. [Google Scholar] [CrossRef] [Green Version]
- Øilo, M.; Nesse, H.; Lundberg, O.J.; Gjerdet, N.R. Mechanical properties of cobalt-chromium 3-unit fixed dental prostheses fabricated by casting, milling, and additive manufacturing. J. Prosthet. Dent. 2018, 120, 156.e1–156.e7. [Google Scholar] [CrossRef]
- Oh, M.S.; Lee, J.Y.; Park, J.K. Continuous cooling β-to-α transformation behaviors of extra-pure and commercially pure Ti. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2004, 35 A, 3071–3077. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Yan, F.; Zhang, Z.; Wu, Y. Microstructure evolution and mechanical properties of AZ80 Mg alloy during annular channel angular extrusion process and heat treatment. Materials 2019, 12, 4223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbin, T.; Velôso, D.V.; Del Rio Silva, L.; Borges, G.A.; Presotto, A.G.C.; Barão, V.A.R.; Mesquita, M.F. 3D metal printing in dentistry: An in vitro biomechanical comparative study of two additive manufacturing technologies for full-arch implant-supported prostheses. J. Mech. Behav. Biomed. Mater. 2020, 108, 103821. [Google Scholar] [CrossRef] [PubMed]
- Burguete, R.L.; Johns, R.B.; King, T.; Patterson, E.A. Tightening characteristics for screwed joints in osseointegrated dental implants. J. Prosthet. Dent. 1994, 71, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Duran, K.; Mindivan, H.; Atapek, Ş.H.; Simov, M.; Dikova, T. Tribological characterization of cast and selective laser melted Co-Cr-Mo alloys under dry and wet conditions. In Proceedings of the 19th International Metallurgy and Materials Congress IMMC, Istanbul, Turkey, 25–17 October 2018; pp. 1212–1215. [Google Scholar]
- Mehkri, S.; Abishek, N.R.; Sumanth, K.S.; Rekha, N. Study of the Tribocorrosion occurring at the implant and implant alloy Interface: Dental implant materials. Mater. Today Proc. 2021, 44, 157–165. [Google Scholar] [CrossRef]
- Pina, V.G.; Amigó, V.; Muñoz, A.I. Microstructural, electrochemical and tribo-electrochemical characterisation of titanium-copper biomedical alloys. Corros. Sci. 2016, 109, 115–125. [Google Scholar] [CrossRef]
- Axente, E.R.; Benea, L.; Bogatu, N.; Celis, J.P. Susceptibility to tribocorrosion degradation of 304 L stainless steel from dental structures in biological solution. Tribol. Int. 2022, 174, 107769. [Google Scholar] [CrossRef]
- Hussein, M.; Adesina, A.Y.; Kumar, M.; Azeem, M.; Sorour, A.; Al-Aqeeli, N. Improvement of in vitro corrosion, wear, and mechanical properties of newly developed Ti alloy by thermal treatment for dental applications. Trans. Nonferrous Met. Soc. China 2021, 31, 952–966. [Google Scholar] [CrossRef]




| Artificial Saliva Constituents (Weight (g) % in 1 L Water) | References | ||||||
|---|---|---|---|---|---|---|---|
| NaCl | KCl | CaCl2 | NaH2PO4 | Na2S | Urea | Others | |
| 0.4 | 0.4 | 0.795 | 0.78 | 0.005 | 1 | [3] | |
| 0.4 | 0.4 | 0.906 | 0.690 | 0.005 | 1 | [68] | |
| 0.4 | 0.4 | 0.795 | 0.78 | 0.005 | 1 | [69] | |
| 0.4 | 0.4 | 0.795 | 0.69 | 0.005 | 1 | [70] | |
| 0.4 | 0.4 | 0.795 | 0.78 | 0.005 | 1 | [71] | |
| 0.4 | 0.4 | 0.795 | 0.69 | 0.005 | 1 | KSCN: 0.3 | [72] |
| 0.7 | 0.4 | 0.795 | 0.13 | Na2HPO4: 0.19, NaHCO3: 0.15, KSCN: 0.33, KH2PO4: 0.26 | [73] | ||
| 0.7 | 1.2 | 1.3 | Na2HPO4: 0.26, NaHCO3: 1.5, KSCN: 0.33 | [74] | |||
| 0.7 | 1.2 | Na2HPO4: 0.26, NaHCO3: 1.5, KSCN: 0.33, KH2PO4: 0.20 | [28,46] | ||||
| 0.4 | 0.4 | 0.6 | 0.58 | 1 | [75] | ||
| 0.3 | 1.12 | 0.17 | MgCl2: 0.06, NaHCO3: 0.63, K2HPO4: 1.5, | [76] | |||
| 0.62 | 0.17 | MgCl2: 0.059, K2HPO4: 0.326, C8H15NaO8: 10, C8H8O3: 2 | [77] | ||||
| Tested Materials | Masticatory Force | References |
|---|---|---|
| Flat enamel (Human tooth) | 20 N | [3] |
| Zirconia ceramic, Gold-Pt alloy, Human tooth | 4 N | [71] |
| CoCr alloy | 22.24 N | [28] |
| Ti-6Al-4V | 3 N | [88] |
| Commercially pure titanium | 20 N | [68] |
| Cusp and polished flat (Human tooth) | 1.9, 3.9, 5.88 N | [89] |
| Human tooth | 20 N | [69] |
| Resin-based composite | 0.98 N | [90] |
| Cusp and flat (Human tooth) | 2, 4, 8 N | [91] |
| Flat enamel (Human tooth) | 30 N | [92] |
| Cast Ti specimen | 5 N | [75] |
| Metal and Alloys | Advantages | Disadvantages |
|---|---|---|
| Cobalt–chromium alloy | Non-magnetic, stain and heat resistant [42] High strength [42,129,130,131] Excellent biocompatibility [132,133] Corrosion resistant [131,134] Wear resistant [135,136] | Cobalt element can result in the greatest release of harmful ions [137]. May cause inflammatory reactions and hyperplasia [138,139]. |
| Stainless steel alloy | High availability Cost effective Formation of passive chromium oxide film resulting corrosion resistance [140] | Nickel and chromium from the alloy can cause hypersensitivity in some people [141]. Can be susceptible to pitting corrosion due to inclusion of dissimilar material in manufacturing process [142]. |
| Titanium and its alloys | Appropriate young modulus High biocompatibility Superior corrosion resistance [143,144,145] | Compromise in corrosion resistance in environment containing fluoride ions [146,147,148,149]. Dissolution of aluminum and vanadium ions can have toxic effects on the host tissue [150,151,152]. |
| Trade Name | Chemical Composition (wt %) | References | |||||
|---|---|---|---|---|---|---|---|
| Co | Cr | Mo | W | Si | Others | ||
| Remanium 2001 | 63 | 23 | 7.3 | 4.3 | 1.6 | Mn < 1, N < 1 | [28] |
| Wirnoit LA | 63.4 | 29 | 5 | - | 1.2 | ||
| Colado CC | 59 | 25.5 | 5.5 | 5 | - | ||
| Heraenium P | 59 | 25 | 4 | 10 | 1 | ||
| MTI China | Bal | 25.7 | 5.9 | 5.6 | <1.5 | C < 0.03, O < 0.05 | [29] |
| Remanium GM 380+ | 64.6 | 29 | 4.5 | - | <1 | C < 1, Mn < 1, N < 1 | [24] |
| Sandvik Osprey F75 | Bal | 27–30 | 5–7 | - | <1 | C < 0.35, Mn < 1, Fe < 0.75, Ni < 0.5 | |
| Wironium plus | 62.5 | 29.5 | 5.0 | <1 | C < 1, Mn < 1, N < 1, Fe < 1 | [42] | |
| Wironit LA | 63.5 | 29.0 | 5.0 | 1.2 | C < 1, Mn < 1, N < 1 | ||
| Brealloy F400 | 64.7 | 29.0 | 5.0 | 0.5 | C: 0.4, Mn: 0.4 | ||
| Bego, German | 63.9 | 24.7 | 5.0 | 5.4 | 1.0 | [30] | |
| Soft Metal™ | 53 | 29 | 6 | 10 | <1 | Fe < 0.1 | [153] |
| Stellite 6 | Bal | 28 | - | 4.5 | 0.9 | C: 1, Mn: 1, Fe: 3. Others < 3 | [31] |
| Stellite 6 | Bal | 31 | - | 3 | - | C: 1.3, Fe: 1.21, P: 0.42, Mn: 0.22 | [154] |
| Stellite 6 | Bal | 30.2 | 0.7 | 4.5 | 1.0 | C: 1.1, Mn: 1.2, Ni: 1.9, Fe: 0.9, p < 0.005, S < 0.005 | [155] |
| Metals | Chemical Composition (wt %) | References | |||||
|---|---|---|---|---|---|---|---|
| Fe | Cr | Ni | C | Mo | Others | ||
| SS 316L | Balance | 16.5–18.5 | 11.0–14 | <0.03 | 2–25 | Si < 1, Mn < 2, p < 0.045, S < 0.03 | [115] |
| SS 305 | Balance | 17–19 | 11.0–13 | <0.07 | Si < 1, Mn < 2, p < 0.045, S < 0.03 | ||
| SS 18/8 | 73.75 | 18 | 8 | 0.25 | [32] | ||
| SS 316L | Balance | 18 | 12 | <0.03 | 2.5 | ||
| SS 316L (ASTM F-1982) | Balance | 17–20 | 12–14 | <0.03 | 2–4 | Mn < 2, p < 0.045, S < 0.03, Si < 0.75, N2 < 0.1, Cu < 0.5 | [159] |
| SS 316L (ASTM F138-1986) | Balance | 17–19 | 13–15.5 | <0.03 | 2–3 | Mn < 2, p < 0.025, S < 0.010, Si < 0.75 | |
| SS 316L | Balance | 16.64 | 10.355 | 0.024 | 2.037 | Mn: 1.519, P: 0.029, S: 0.025, Si: 0.407, Ti: 0.006, N: 0.047, Cu: 0.296, Co: 0.188 | [33] |
| Material | Ti | Fe | O2 | N | H2 | C | |
|---|---|---|---|---|---|---|---|
| Commercially Pure Titanium (Cp Ti) | Grade 1 | 99% | 0.2% | 0.18% | 0.03% | 0.15% | 0.1% |
| Grade 2 | 0.2% | 0.25 | 0.03% | 0.15% | 0.1% | ||
| Grade 3 | 0.2% | 0.35% | 0.05% | 0.15% | 0.1% | ||
| Grade 4 | 0.3% | 0.4% | 0.05% | 0.15% | 0.1% | ||
| Ti-6Al-4V | 90% | 0.25% | 0.2% | - | - | - | |
| Approximate Cooling Rate (°C/s) | Approximate Dendritic Arm Spacing (µm) |
|---|---|
| 450–550 | 10.5 |
| 1050–1150 | 9.4 |
| 1400–1600 | 8 |
| 2250–2350 | 7 |
| Material Used | Manufacturing Process | Tribological Experimental Parameters | Wear Mechanisms | References |
|---|---|---|---|---|
| Co-Cr alloy | Casting, and SLM | Ball-on-disc tribo experiment under dry condition. Used load: 5 N Rotational speed: 200 rpm | Primary wear mechanism was abrasive and fatigue. Plastic deformation was lower for SLM sample and showed overall higher wear resistance. | [136] |
| Co-Cr alloy (Remanium 2001, Wironit LA, Colado CC, Heraenium P) | Casting | Abrasive wear test on Miller apparatus under SiC and artificial saliva solution condition. Used load: 22.24 N Frequency: 48 rpm | Prominent wear mechanism was micro-scratching with a minor degree of micro-ridging. | [28] |
| Co-Cr-Mo alloy | Casting and SLM process | Reciprocating sliding wear test in dry and artificial saliva lubricated condition. Used load: 5 N Sliding speed: 1.7 cm/s | Higher wear rate in cast sample due to tendency of hard carbides leaving the matrix. Formation of micro-cracks was observed in SLM-processed sample under wet condition | [198] |
| Ti-6Al-4V | Wrought and EBM process | Ball-on-disc reciprocating sliding experiment under dry and lubricated (simulated body fluid) condition. Used load: 3 N Sliding frequency: 1 Hz | For both alloys, under dry condition, adhesive and abrasive wear occurred. Abrasive wear dominated the dry condition. | [88] |
| Stainless steel | Wrought | Micro-scale abrasion testing with artificial saliva mixed with abrasive particle. Used load range: 0.5 to 4 N Sliding velocity 150 rpm | With increasing load, the micro-abrasion rate increases. Ridge-dominated 2-body wear mechanism occurred at higher load. | [140] |
| Ti-6Al-4V, yttria-stabilized zirconia and zirconia toughened alumina | Yttria-stabilized zirconia and zirconia toughened alumina: powder sintering and hydraulic pressing | Pin-on-disc reciprocating tribo experiment under artificial saliva lubricated condition. Used load: 20 N load Sliding speed: 200 rpm | Zirconia-toughened alumina was better suited to resist material loss. | [199] |
| Cp-Ti and Ti-Cu alloy (TixCu, x = 3, 7.1 and 12 wt %) | Conventional powder metallurgy compaction: Hidruded-dehidruded (HDH) technique | Ball-on-disk tribometer with integrated electrochemical cell with artificial saliva. Used loads: 1, 5 and 10 N Rotational speed 60 rpm | Increasing Cu content in the alloy results in eutectoid formation along the grain boundary and increases hardness and material loss due to wear also reduced. Cp-Ti experienced plastic deformation, while third bodies and larger debris particle dominated Ti alloy with higher Cu content. | [200] |
| AISI 304 L stainless steel | Wrought | Pin-on-disc tribo experiment in hank biological solution. Used load: 3 N and 5 N Sliding speed: 120 rpm | Bigger wear track and surface crack formed for higher load. | [201] |
| Ti-6Al-4V | Cast and powder compaction sintering | Ball-on-flat type tribo experiemnt in Fusayama Meyer artificial saliva solution. Used load: 5 N Sliding: speed 60 rpm | Along with predominant adhesive wear, isolated wear debris was also observed. | [75] |
| Ti20Nb13Zr Water-cooled (WC) Air-cooled (AC) Furnace-cooled (FC) Aged | Spark plasma sintering | Ball-on-flat reciprocating tribo experiment in artificial saliva lubricated condition. Used load: 10 N Reciprocating frequency: 5 Hz | Primary wear mechanism was abrasive. Trapped debris contributed as ploughing component. Oxide film formation was on air-cooled and aged samples. | [202] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, S.; Roy, S. Metallic Dental Implants Wear Mechanisms, Materials, and Manufacturing Processes: A Literature Review. Materials 2023, 16, 161. https://doi.org/10.3390/ma16010161
Saha S, Roy S. Metallic Dental Implants Wear Mechanisms, Materials, and Manufacturing Processes: A Literature Review. Materials. 2023; 16(1):161. https://doi.org/10.3390/ma16010161
Chicago/Turabian StyleSaha, Sudip, and Sougata Roy. 2023. "Metallic Dental Implants Wear Mechanisms, Materials, and Manufacturing Processes: A Literature Review" Materials 16, no. 1: 161. https://doi.org/10.3390/ma16010161
APA StyleSaha, S., & Roy, S. (2023). Metallic Dental Implants Wear Mechanisms, Materials, and Manufacturing Processes: A Literature Review. Materials, 16(1), 161. https://doi.org/10.3390/ma16010161








