Controllable Preparation of Ag-SiO2 Composite Nanoparticles and Their Applications in Fluorescence Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ag Nanoparticles
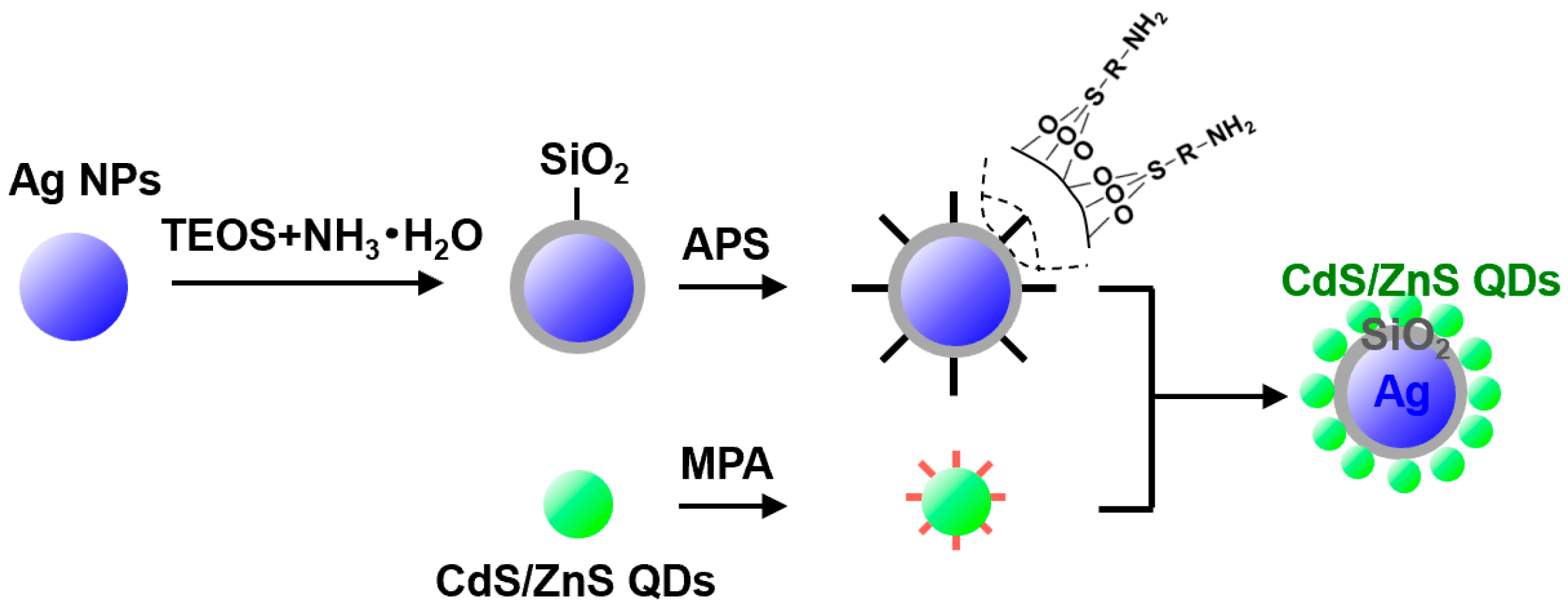
2.3. Preparation of Ag@SiO2 Nanoparticles
2.4. Preparation of Ag@SiO2@CdS/ZnS Composite Nanoparticles
2.5. Characterization Method
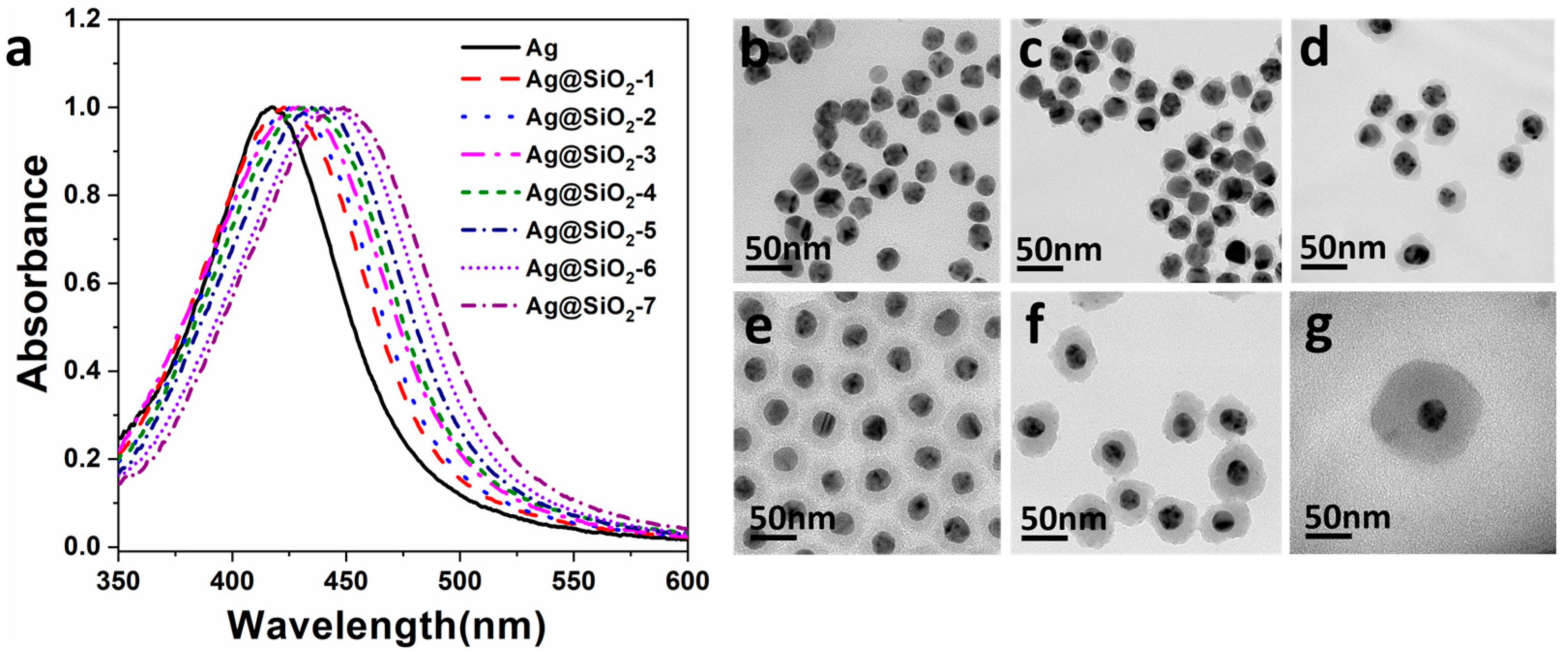
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, Y.; Huang, C.; Li, J.Z.; Grewe, B.F.; Zhang, Y.; Eismann, S.; Schnitzer, M.J. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 2015, 350, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, W.; Schmidt, J.F.; Zhao, W.; Lu, X.; Raab, T.; Diederichs, C.; Gao, W.; Seletskiy, D.V.; Xiong, Q. Correlated fluorescence blinking in two-dimensional semiconductor heterostructures. Nature 2017, 541, 62–67. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Zhang, W.; Yu, S.; Wang, X.; Gao, N. New advances in fluorescence excitation-emission matrix spectroscopy for the characterization of dissolved organic matter in drinking water treatment: A review. Chem. Eng. J. 2020, 381, 122676. [Google Scholar] [CrossRef]
- Banerjee, S.; Gangopadhyay, G. Radiative decay of nonstationary system. J. Chem. Phys. 2004, 120, 6152–6162. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, S.; Li, B.; Yang, K.; Lan, M.; Zeng, L. Advances and perspectives in carbon dot-based fluorescent probes: Mechanism, and application. Coord. Chem. Rev. 2021, 431, 21368. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Jiang, R.; Li, B.; Fang, C.; Wang, J. Metal/Semiconductor Hybrid Nanostructures for Plasmon-Enhanced Applications. Adv. Mater. 2014, 26, 5274–5309. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, H.; Park, E.; Kim, T.; Chung, D.R.; Choi, Y.M.; Kang, M. Paper-Based Multiplex Surface-Enhanced Raman Scattering Detection Using Polymerase Chain Reaction Probe Codification. Anal. Chem. 2021, 93, 3677–3685. [Google Scholar] [CrossRef]
- Fong, K.E.; Yung, L.Y.L. Localized surface plasmon resonance: A unique property of plasmonic nanoparticles for nucleic acid detection. Nanoscale 2013, 5, 12043–12071. [Google Scholar] [CrossRef]
- Aslan, K.; Gryczynski, I.; Malicka, J.; Matveeva, E.; Lakowicz, J.R.; Geddes, C.D. Metal-enhanced fluorescence: An emerging tool in biotechnology. Curr. Opin. Biotechnol. 2005, 16, 55–62. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Malicka, J.; D’Auria, S.; Gryczynski, I. Release of the self-quenching of fluorescence near silver metallic surfaces. Anal. Biochem. 2003, 320, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Gouesbet, G. Asymptotic quantum inelastic generalized Lorenz-Mie theory. Opt. Commun. 2007, 278, 215–220. [Google Scholar] [CrossRef]
- Mortensen, N.A.; Raza, S.; Wubs, M.; Sondergaard, T.; Bozhevolnyi, S.I. A generalized non-local optical response theory for plasmonic nanostructures. Nat. Commun. 2014, 5, 3809. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, S. Generalization of the Finite-Difference-Based Time-Domain Methods Using the Method of Moments. IEEE Trans. Antennas Propag. 2006, 54, 2515–2524. [Google Scholar] [CrossRef]
- Wyss, R.M.; Parzefall, M.; Schlichting, K.P.; Gruber, C.M.; Busschaert, S.; Lightner, C.R.; Lortscher, E.; Novotny, L.; Heeg, S. Freestanding and Permeable Nanoporous Gold Membranes for Surface-Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2022, 14, 16558–16567. [Google Scholar] [CrossRef]
- Li, C.R.; Lei, Y.L.; Li, H.; Ni, M.; Yang, D.R.; Xie, X.Y.; Wang, Y.F.; Ma, H.B.; Xu, W.G.; Xia, X.H. Suppressing Non-Radiative Relaxation through Single-Atom Metal Modification for Enhanced Fluorescence Efficiency in Molybdenum Disulfide Quantum Dots. Angew. Chem. Int. Ed. 2022, 61, e2022073. [Google Scholar]
- Xu, J.; Morton, W.; Jones, D.; Tabish, T.A.; Ryan, M.P.; Xie, F. Significant quantum yield enhancement for near infrared fluorescence dyes by silica templated silver nanorods. Appl. Phys. Rev. 2022, 9, 031406. [Google Scholar] [CrossRef]
- Gahlaut, S.K.; Pathak, A.; Gupta, B.D. Recent Advances in Silver Nanostructured Substrates for Plasmonic Sensors. Biosensors 2022, 12, 713. [Google Scholar] [CrossRef]
- Aladesuyi, O.A.; Lebepe, T.C.; Maluleke, R.; Oluwafemi, O.S. Biological applications of ternary quantum dots: A review. Nanotechnol. Rev. 2022, 11, 2304–2319. [Google Scholar] [CrossRef]
- Zong, J.; Yang, X.; Trinchi, A.; Hardin, S.; Cole, I.; Zhu, Y.; Li, C.; Muster, T.; Wei, G. Carbon dots as fluorescent probes for “off-on” detection of Cu2+ and L-cysteine in aqueous solution. Biosens. Bioelectron. 2014, 51, 330–335. [Google Scholar] [CrossRef]
- Hou, S.; Chen, Y.; Lu, D.; Xiong, Q.; Lim, Y.; Duan, H. A Self-Assembled Plasmonic Substrate for Enhanced Fluorescence Resonance Energy Transfer. Adv. Mater. 2020, 32, 19064. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, Y.; Zhang, B.; Feng, P.; Dang, C.; Li, M.; Zhao, L.; Gao, L. Metal-Enhanced Circularly Polarized Luminescence of Self-Assembled Au@SiO2 Triangular Nanoprisms and Fluorophores in Chiral Cellulose Nanocrystal Films. Adv. Opt. Mater. 2021, 9, 2100907. [Google Scholar] [CrossRef]
- Mishra, H.; Mali, B.L.; Karolin, J.; Dragan, A.I.; Geddes, C.D. Experimental and theoretical study of the distance dependence of metal-enhanced fluorescence, phosphorescence and delayed fluorescence in a single system. Phys. Chem. Chem. Phys. 2013, 15, 19538–19544. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, H.; Chen, Y.; Liu, F.; Huang, C.Z.; Li, N. A distance-dependent metal-enhanced fluorescence sensing platform based on molecular beacon design. Biosens. Bioelectron. 2014, 52, 367–373. [Google Scholar] [CrossRef]
- Hahm, E.; Jo, A.; Lee, S.H.; Kang, H.; Pham, X.H.; Jun, B.H. Silica Shell Thickness-Dependent Fluorescence Properties of SiO2@Ag@SiO2@QDs Nanocomposites. Int. J. Mol. Sci. 2022, 23, 10041. [Google Scholar] [CrossRef]
- Liao, C.; Tang, L.; Gao, X.; Xu, R.; Zhang, H.; Yu, Y.; Lu, C.; Cui, Y.; Zhang, J. Bright white-light emission from Ag/SiO2/CdS-ZnS core/shell/shell plasmon couplers. Nanoscale 2015, 7, 20607–20613. [Google Scholar] [CrossRef]
- Kang, J.; Li, Y.; Chen, Y.; Wang, A.; Yue, B.; Qu, Y.; Zhao, Y.; Chu, H. Core-shell Ag@SiO2 nanoparticles of different silica shell thicknesses: Preparation and their effects on photoluminescence of lanthanide complexes. Mater. Res. Bull. 2015, 71, 116–121. [Google Scholar] [CrossRef]
- Rao, J.G.; Delande, D.; Taylor, K.T. Quantum manifestations of closed orbits in the photoexcitation scaled spectrum of the hydrogen atom in crossed fields. J. Phys. B At. Mol. Opt. Phys. 2001, 34, L391–L399. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, L.; Ge, R.; Zhang, S.; Miao, Y.; Zou, W.; Yi, C.; Sun, Y.; Cao, Y.; Yang, R.; et al. Perovskite light-emitting diodes based on solution-processed self-organized multiple quantum wells. Nat. Photonics 2016, 10, 699–704. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering: Biophysical and biomedical applications. Anal. Biochem. 2001, 298, 1–24. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; Cai, C.; Xu, T.; Zhang, B.; Jin, S.; Wan, Z.; Wei, X. Plasmon-Enhanced Surface-State Emission of CdSe Quantum Dots and Its Application to Microscale Luminescence Pattern. J. Phys. Chem. C 2012, 116, 11283–11291. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; Zhang, B.; Du, L.; Xu, T.; Chen, Y.; Zhang, Y. Designable Luminescence with Quantum Dot–Silver Plasmon Coupler. Small 2014, 10, 3099–3109. [Google Scholar] [CrossRef]
- Ozel, T.; Soganci, I.M.; Nizamoglu, S.; Huyal, I.O.; Mutlugun, E.; Sapra, S.; Gaponik, N.; Eychmuller, A.; Demir, H.V. Selective enhancement of surface-state emission and simultaneous quenching of interband transition in white-luminophor CdS nanocrystals using localized plasmon coupling. New J. Phys. 2008, 10, 083035. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Liao, C.; Guo, Y.; Zhang, Y. Controllable Preparation of Ag-SiO2 Composite Nanoparticles and Their Applications in Fluorescence Enhancement. Materials 2023, 16, 201. https://doi.org/10.3390/ma16010201
Tang L, Liao C, Guo Y, Zhang Y. Controllable Preparation of Ag-SiO2 Composite Nanoparticles and Their Applications in Fluorescence Enhancement. Materials. 2023; 16(1):201. https://doi.org/10.3390/ma16010201
Chicago/Turabian StyleTang, Luping, Chen Liao, Yingqing Guo, and Yangyang Zhang. 2023. "Controllable Preparation of Ag-SiO2 Composite Nanoparticles and Their Applications in Fluorescence Enhancement" Materials 16, no. 1: 201. https://doi.org/10.3390/ma16010201
APA StyleTang, L., Liao, C., Guo, Y., & Zhang, Y. (2023). Controllable Preparation of Ag-SiO2 Composite Nanoparticles and Their Applications in Fluorescence Enhancement. Materials, 16(1), 201. https://doi.org/10.3390/ma16010201






