Study of 211Bi and 211Pb Recoils Release from 223Ra Labelled TiO2 Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Study Design
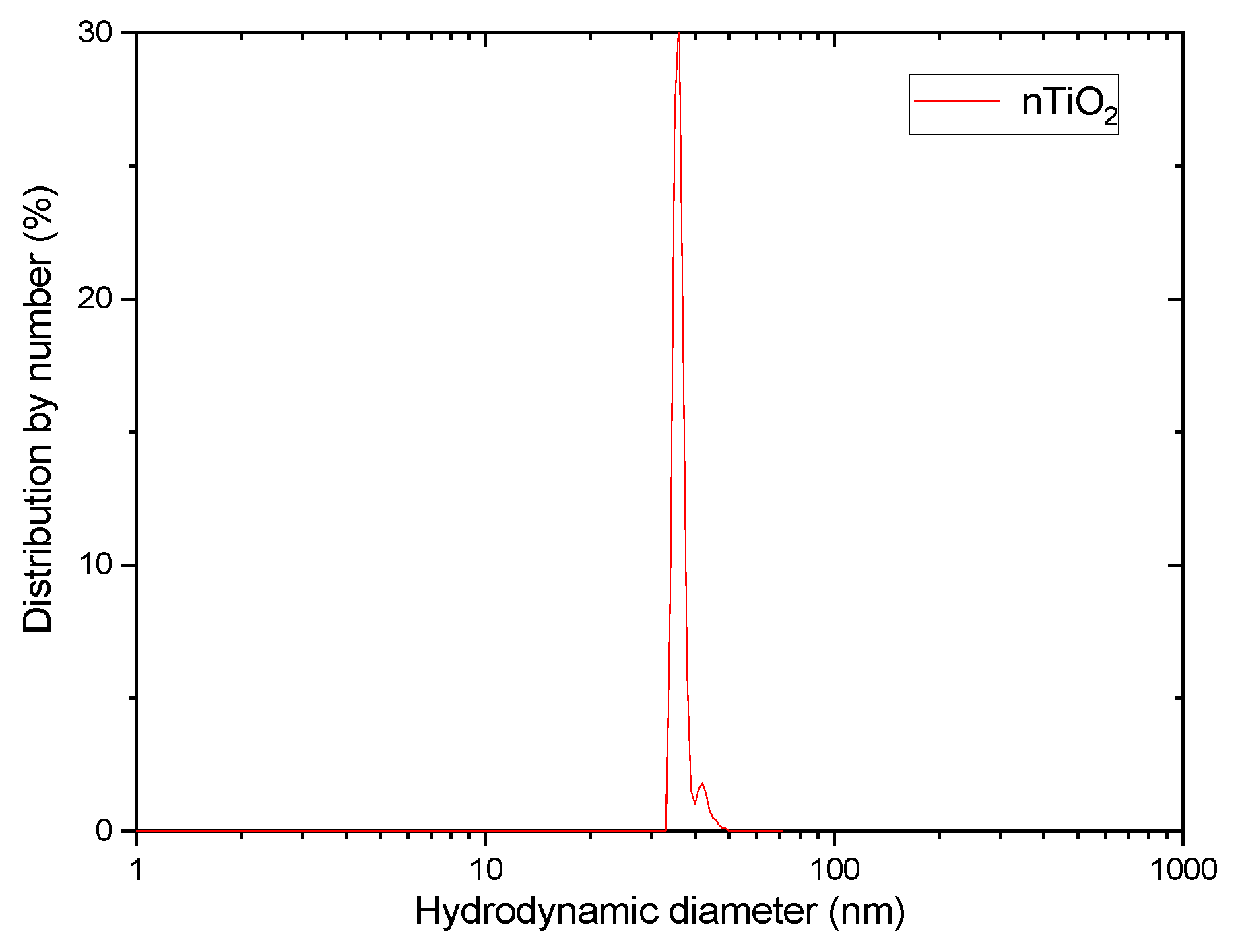
2.2. TiO2 NPs Preparation and Radiolabelling
2.3. Activity Measurements
2.4. Phase Separation Methods
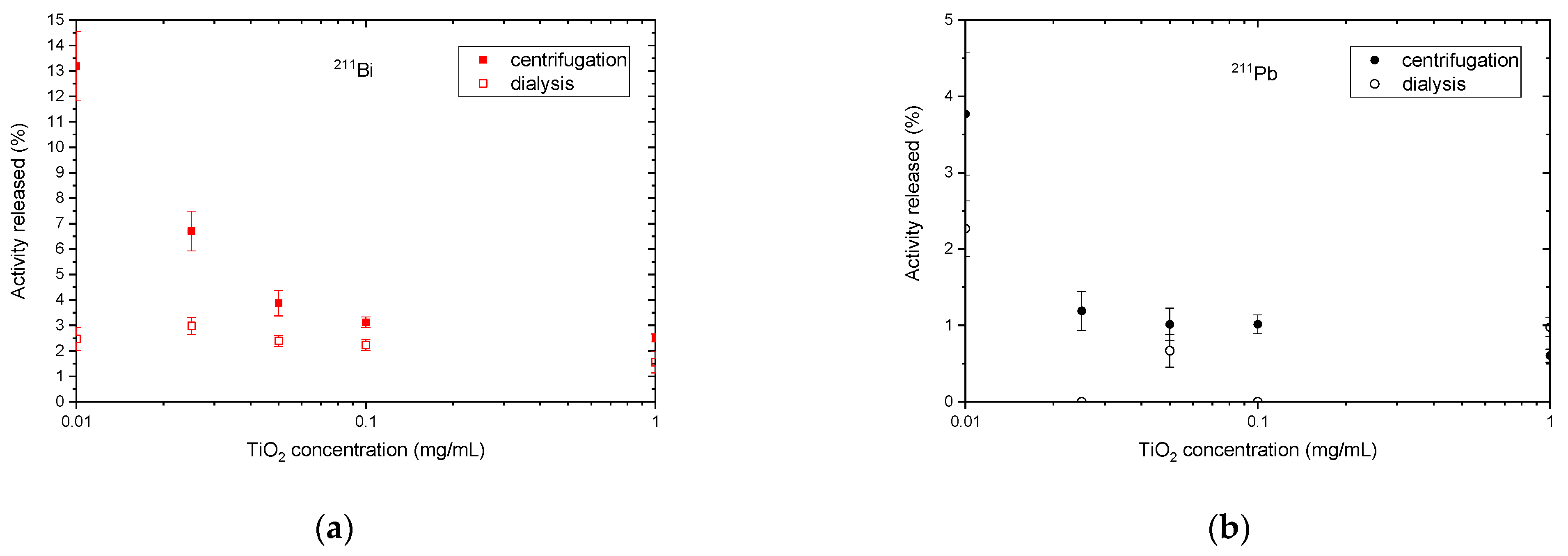
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guerra Liberal, F.D.C.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted alpha therapy: Current clinical applications. Cancer Biother. Radiopharm. 2020, 35, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Brechbiel, M.W. An overview of targeted alpha therapy. Tumour Biol. 2012, 33, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Bayer Xofigo, SPC. Available online: https://www.ema.europa.eu/en/documents/product-information/xofigo-epar-product-information_en.pdf (accessed on 4 October 2022).
- Hallqvist, A.; Bergmark, K.; Back, T.; Andersson, H.; Dahm-Kahler, P.; Johansson, M.; Lindegren, S.; Jensen, H.; Jacobsson, L.; Hultborn, R.; et al. Intraperitoneal alpha-Emitting Radioimmunotherapy with 211At in Relapsed Ovarian Cancer: Long-Term Follow-up with Individual Absorbed Dose Estimations. J. Nucl. Med. 2019, 60, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Liu, Y.; Ooe, K.; Teramoto, T.; Toyoshima, A.; Shimosegawa, E.; Nakano, T.; Kanai, Y.; et al. Targeted Alpha Therapy Using Astatine (211At)-Labeled Phenylalanine: A Preclinical Study in Glioma Bearing Mice. Oncotarget 2020, 11, 1388–1398. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Cosma, L.; Brunotti, G.; Pani, A.; Spanu, A.; Nuvoli, S.; De Cristofaro, F.; Civitelli, L.; De Vincentis, G. Targeted Alpha Therapy with Thorium-227. Cancer Biother. Radiopharm. 2020, 35, 437–445. [Google Scholar] [CrossRef]
- Satapathy, S.; Sharma, A.; Sood, A.; Maheshwari, P.; Gill, H.J.S. Delayed Nephrotoxicity After 225Ac-PSMA-617 Radioligand Therapy. Clin. Nucl. Med. 2022, 47, e466–e467. [Google Scholar] [CrossRef]
- Lawal, I.O.; Morgenstern, A.; Vorster, M.; Knoesen, O.; Mahapane, J.; Hlongwa, K.N.; Maserumule, L.C.; Ndlovu, H.; Reed, J.D.; Popoola, G.O.; et al. Hematologic toxicity profile and efficacy of [225Ac]Ac-PSMA-617 α-radioligand therapy of patients with extensive skeletal metastases of castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3581–3592. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. Ac-225-DOTATOC—An Empiric Dose Finding for Alpha Particle Emitter Based Radionuclide Therapy of Neuroendocrine Tumors. J. Nucl. Med. 2015, 56, 1232. [Google Scholar]
- Atallah, E.; Berger, M.; Jurcic, J.; Roboz, G.; Tse, W.; Mawad, R.; Rizzieri, D.; Begna, K.; Orozco, J.; Craig, M.; et al. A Phase 2 Study of Actinium-225 (225Ac)-Lintuzumab in Older Patients with Untreated Acute Myeloid Leukemia (AML). J. Med. Imaging Radiat. Sci. 2019, 50, 37. [Google Scholar] [CrossRef]
- De Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef]
- Lin, W. Introduction: Nanoparticles in medicine. Chem. Rev. 2015, 115, 10407–10409. [Google Scholar] [CrossRef] [PubMed]
- Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators. Molecules 2018, 23, 581. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska, M.; Fracasso, G.; Pruszyński, M.; Bilewicz, A.; Kruszewski, M.; Majkowska-Pilip, A.; Lankoff, A. Design and Evaluation of (223)Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy—Part I. Materials 2020, 13, 3875. [Google Scholar] [CrossRef] [PubMed]
- Kleynhans, J.; Sathekge, M.; Ebenhan, T. Obstacles and Recommendations for Clinical Translation of Nanoparticle System-Based Targeted Alpha-Particle Therapy. Materials 2021, 14, 4784. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; De Kruijff, R.M.; Rol, A.; Thijssen, L.; Mendes, E.; Morgenstern, A.; Bruchertseifer, F.; Stuart, M.C.A.; Wolterbeek, H.T.; Denkova, A.G. Retention studies of recoiling daughter nuclides of 225Ac in polymer vesicles. Appl. Radiat. Isot. 2014, 85, 45–53. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Seideman, J.; Sofou, S. Enhanced loading efficiency and retention of 225Ac in rigid liposomes for potential targeted therapy of micrometastases. Bioconjug. Chem. 2008, 19, 1274–1282. [Google Scholar] [CrossRef]
- Souza, B.N.R.F.; Ribeiro, E.R.F.R.; da Silva de Barros, A.O.; Pijeira, M.S.O.; Kenup-Hernandes, H.O.; Ricci-Junior, E.; Diniz Filho, J.F.S.; dos Santos, C.C.; Alencar, L.M.R.; Attia, M.F.; et al. Nanomicelles of Radium Dichloride [223Ra]RaCl2 Co-Loaded with Radioactive Gold [198Au]Au Nanoparticles for Targeted Alpha–Beta Radionuclide Therapy of Osteosarcoma. Polymers 2022, 14, 1405. [Google Scholar] [CrossRef]
- Pijeira, M.S.O.; Viltres, H.; Kozempel, J.; Sakmár, M.; Vlk, M.; İlem-Özdemir, D.; Ekinci, M.; Srinivasan, S.; Rajabzadeh, A.R.; Ricci-Junior, E.; et al. Radiolabeled nanomaterials for biomedical applications: Radiopharmacy in the era of nanotechnology. EJNMMI Radiopharm. Chem. 2022, 7, 8. [Google Scholar] [CrossRef]
- Gaweda, W.; Pruzynski, M.; Cedrowska, E.; Rodak, M.; Majokwska-Pilip, A.; Gawel, D.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Traztuzumab modified barium ferrite magnetic nanoparticles labeled with radium-223: A new potential radiobioconjuagte for alpha radioimmunotherapy. Nanomaterials 2020, 10, 2067. [Google Scholar] [CrossRef]
- Pellico, J.; Gawne, P.J.; De Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 5, 3355–3423. [Google Scholar] [CrossRef] [PubMed]
- Toro-González, M.; Dame, A.N.; Mirzadeh, S.; Rojas, J.V. Encapsulation and retention of 225Ac, 223Ra, 227Th, and decay daughters in zircon-type gadolinium vanadate nanoparticles. Radiochim. Acta 2020, 108, 967–977. [Google Scholar] [CrossRef]
- Salvanou, E.A.; Stellas, D.; Tsoukalas, C.; Mavroidi, B.; Paravatou-Petsotas, M.; Kalogeropoulos, N.; Xanthopoulos, S.; Denat, F.; Laurent, G.; Bazzi, R.; et al. A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics 2020, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Suchánková, P.; Kukleva, E.; Štamberg, K.; Nykl, P.; Vlk, M.; Kozempel, J. Study of 223Ra uptake mechanism on hydroxyapatite and titanium dioxide nanoparticles as a function of pH. RSC Adv. 2020, 10, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Suchánková, P.; Kukleva, E.; Nykl, E.; Nykl, P.; Sakmár, M.; Vlk, M.; Kozempel, J. Hydroxyapatite and Titanium Dioxide Nanoparticles: Radiolabelling and In Vitro Stability of Prospective Theranostic Nanocarriers for 223Ra and 99mTc. Nanomaterials 2020, 10, 1632. [Google Scholar] [CrossRef]
- Arazi, L.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. Treatment of solid tumors by interstitial release of recoiling short-lived alpha emitters. Phys. Med. Biol. 2007, 52, 5025–5042. [Google Scholar] [CrossRef]
- Nishri, Y.; Vatarescu, M.; Luz, I.; Epstein, L.; Dumančić, M.; Del Mare, S.; Shai, A.; Schmidt, M.; Deutsch, L.; Den, R.B.; et al. Diffusing alpha-emitters radiation therapy in combination with temozolomide or bevacizumab in human glioblastoma multiforme xenografts. Front. Oncol. 2022, 12, 888100. [Google Scholar] [CrossRef]
- Krolicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Krolicki, B.; Jakucinski, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Prolonged survival in secondary glioblastoma following local injection of targeted alpha therapy with 213Bi-substance P analogue. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1636–1644. [Google Scholar] [CrossRef]
- Gorin, J.-B.; Guilloux, Y.; Morgenstern, A.; Chérel, M.; Davodeau, F.; Gaschet, J. Using α radiation to boost cancer immunity? OncoImmunology 2014, 3, e954925. [Google Scholar] [CrossRef]
- Confino, H.; Schmidt, M.; Efrati, M.; Hochman, I.; Umansky, V.; Kelson, I.; Keisari, Y. Inhibition of mouse breast adenocarcinoma growth by ablation with intratumoral alpha-irradiation combined with inhibitors of immunosuppression and CpG. Cancer Immunol. Immunother. 2016, 65, 1149–1158. [Google Scholar] [CrossRef]
- Gorin, J.-B.; Ménager, J.; Gouard, S.; Maurel, C.; Guilloux, Y.; Faivre-Chauvet, A.; Morgenstern, A.; Bruchertseifer, F.; Chérel, M.; Davodeau, F.; et al. Antitumor Immunity Induced after α Irradiation. Neoplasia 2014, 16, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Kukleva, E.; Suchánková, P.; Štamberg, K.; Vlk, M.; Šlouf, M.; Kozempel, J. Surface protolytic property characterization of hydroxyapatite and titanium dioxide nanoparticles. RSC Adv. 2019, 9, 21989–21995. [Google Scholar] [CrossRef] [PubMed]
- Suchánková, P.; Kukleva, E.; Štamberg, K.; Nykl, P.; Sakmár, M.; Vlk, M.; Kozempel, J. Determination, Modeling and Evaluation of Kinetics of 223Ra Sorption on Hydroxyapatite and Titanium Dioxide Nanoparticles. Materials 2020, 13, 1915. [Google Scholar] [CrossRef] [PubMed]
- Live Chart of Nuclides, IAEA Vienna Austria. Available online: https://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html (accessed on 22 December 2022).
- Holzwarth, U.; Ojea Jimenez, I.; Calzolai, L. A random walk approach to estimate the confinement of α-particle emitters in nanoparticles for targeted radionuclide therapy. EJNMMI Radiopharm. Chem. 2018, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Sweeney, E.; Wilson, J.; Cusey, P.W.; Babich, J.W. A suitable time point for quantifying the radiochemical purity of 225Ac-labeled radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2021, 6, 38. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozempel, J.; Sakmár, M.; Janská, T.; Vlk, M. Study of 211Bi and 211Pb Recoils Release from 223Ra Labelled TiO2 Nanoparticles. Materials 2023, 16, 343. https://doi.org/10.3390/ma16010343
Kozempel J, Sakmár M, Janská T, Vlk M. Study of 211Bi and 211Pb Recoils Release from 223Ra Labelled TiO2 Nanoparticles. Materials. 2023; 16(1):343. https://doi.org/10.3390/ma16010343
Chicago/Turabian StyleKozempel, Ján, Michal Sakmár, Tereza Janská, and Martin Vlk. 2023. "Study of 211Bi and 211Pb Recoils Release from 223Ra Labelled TiO2 Nanoparticles" Materials 16, no. 1: 343. https://doi.org/10.3390/ma16010343





