Effect of Solution-to-Binder Ratio and Alkalinity on Setting and Early-Age Properties of Alkali-Activated Slag-Fly Ash Binders
Abstract
:1. Introduction
| Method | Criterion | Mix Scale Initial Set | Final Set | |
|---|---|---|---|---|
| UPV | Velocity threshold (m/s) | Paste | 1500 [36,51], 1440–1550 [52] * | 1750–1850 [51], 1650–1725 [52] * |
| Mortar | 800–980 [29] | 1200–1400 [29], 1500 [28] | ||
| Concrete | 1000–1500 [53], 2300–2700 [49], 1100–2000 [29] | 3000 [53], 2790–3180 [49], 2000–3000 [29] | ||
| UPV | First inflection point | [28,36,54,55,56] * | - | |
| UPV | Intersection of tangent lines | [31,35,42,57] * | ||
| UPV & Calorimetry | Minimum reaction degree required for setting to occur (%) | Concrete Paste | Schindler [50] prediction = 7.5% Shiva [52] * approximation = 2.1–3.2% | Schindler [50] = 13% Herb [49] bilinear approx. = 8.5% Shiva [52] * = 3.5–4.5% |
2. Raw Materials and Experimental Methods
2.1. Raw Materials
2.2. Mix Design and Specimen Preparation
2.3. Experimetnal Methods
2.3.1. Slump Flow
2.3.2. Vicat Test
2.3.3. Isothermal Calorimetry
2.3.4. Ultrasonic Measurements
2.3.5. Compressive Strength
3. Individual Test Results
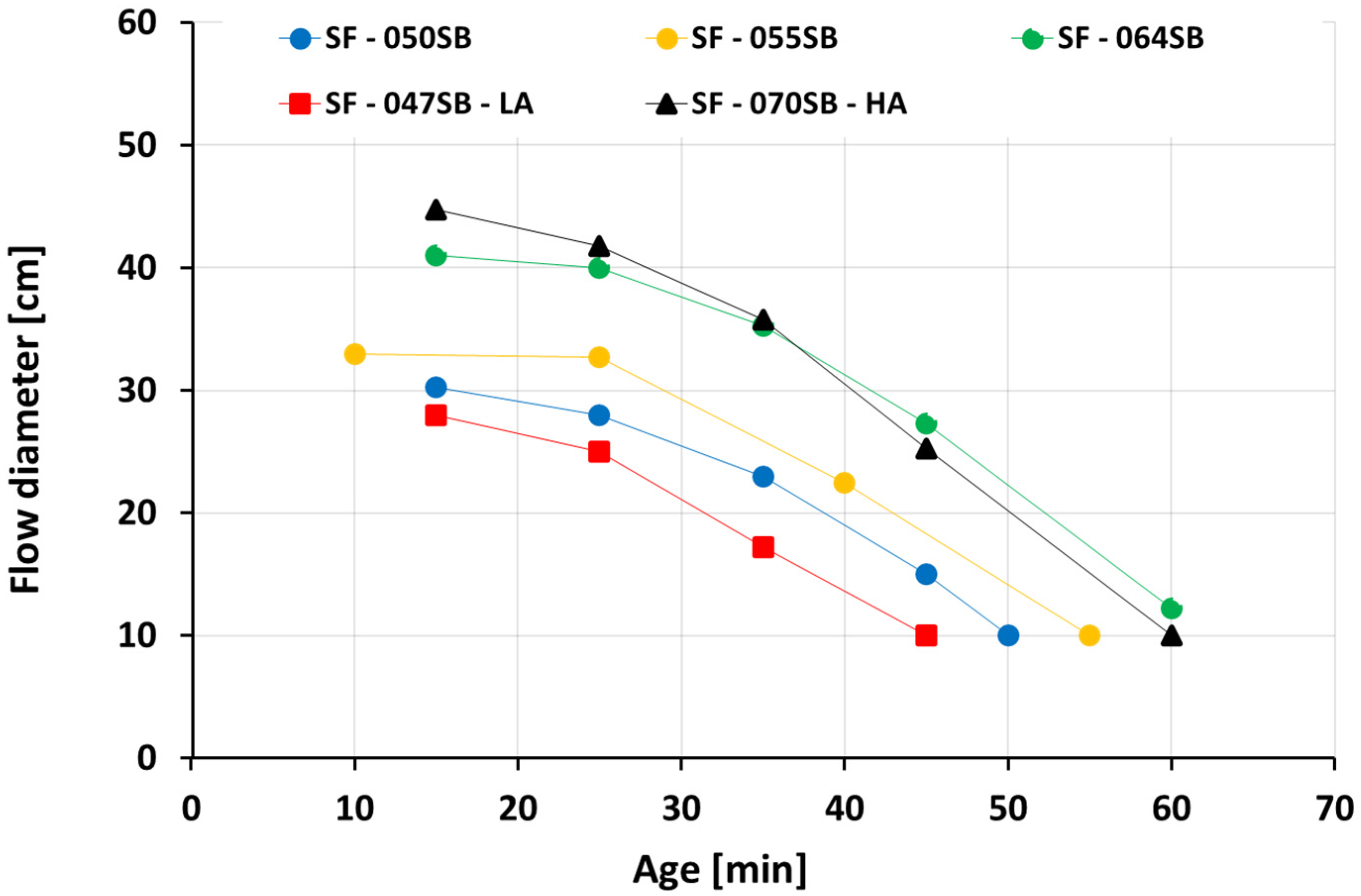
3.1. Slump Flow
3.2. Vicat Test
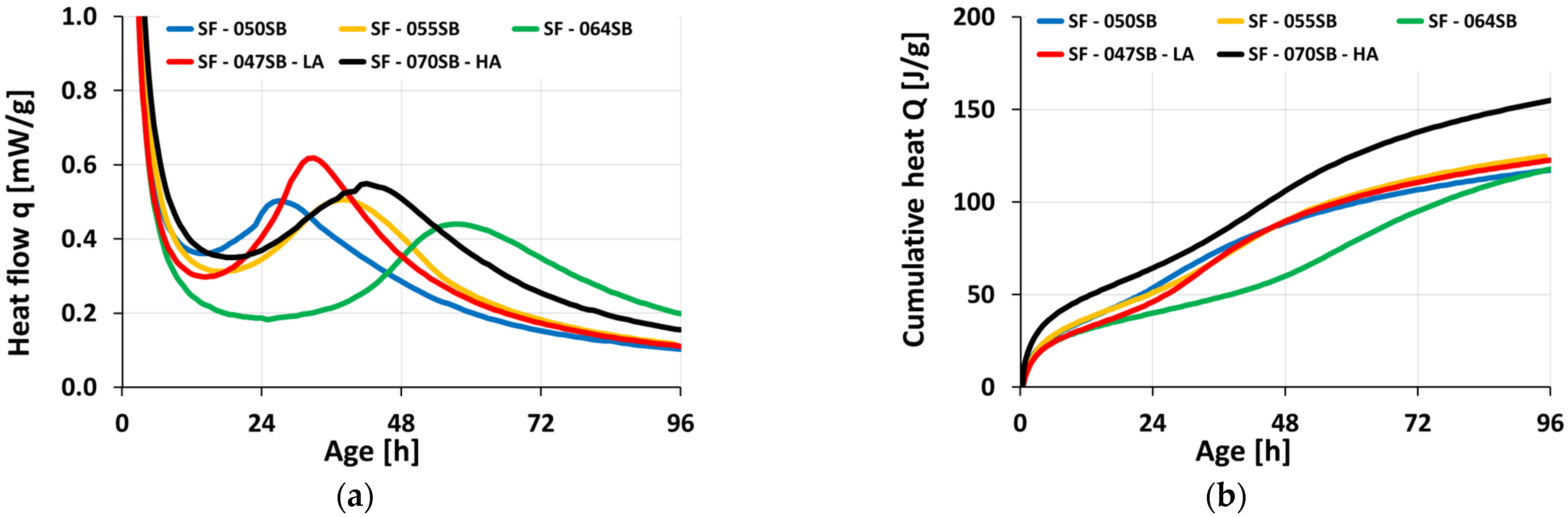
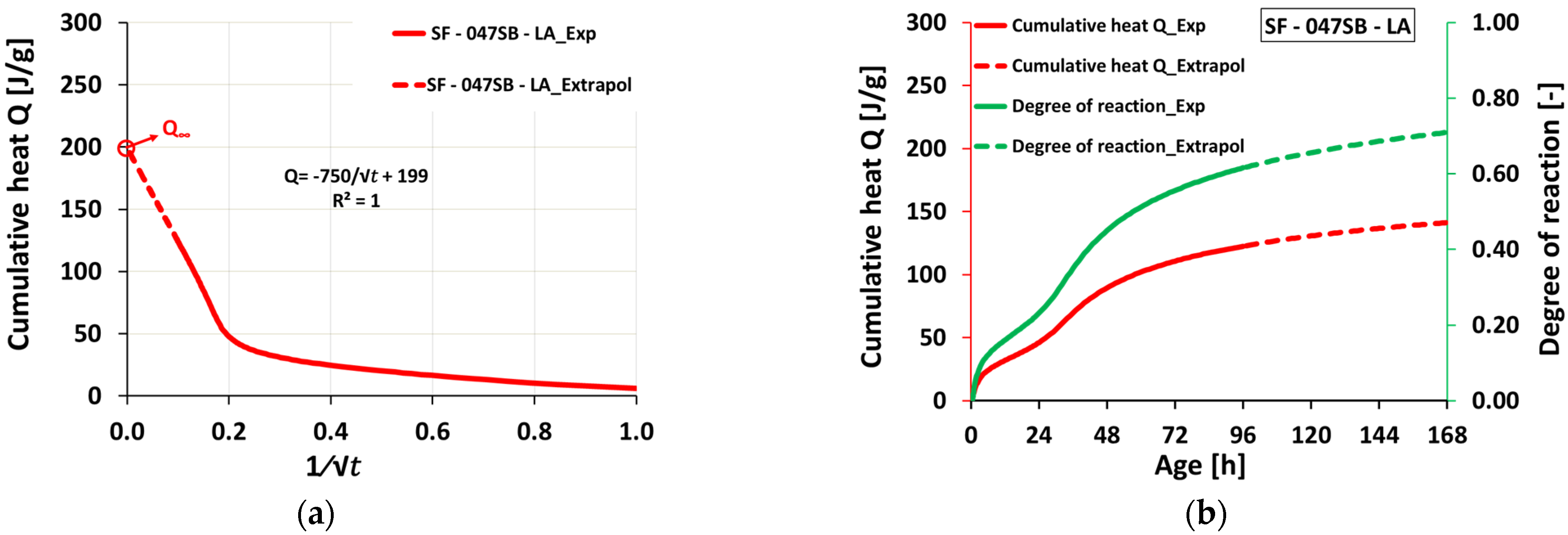
3.3. Isotheral Calorimetry
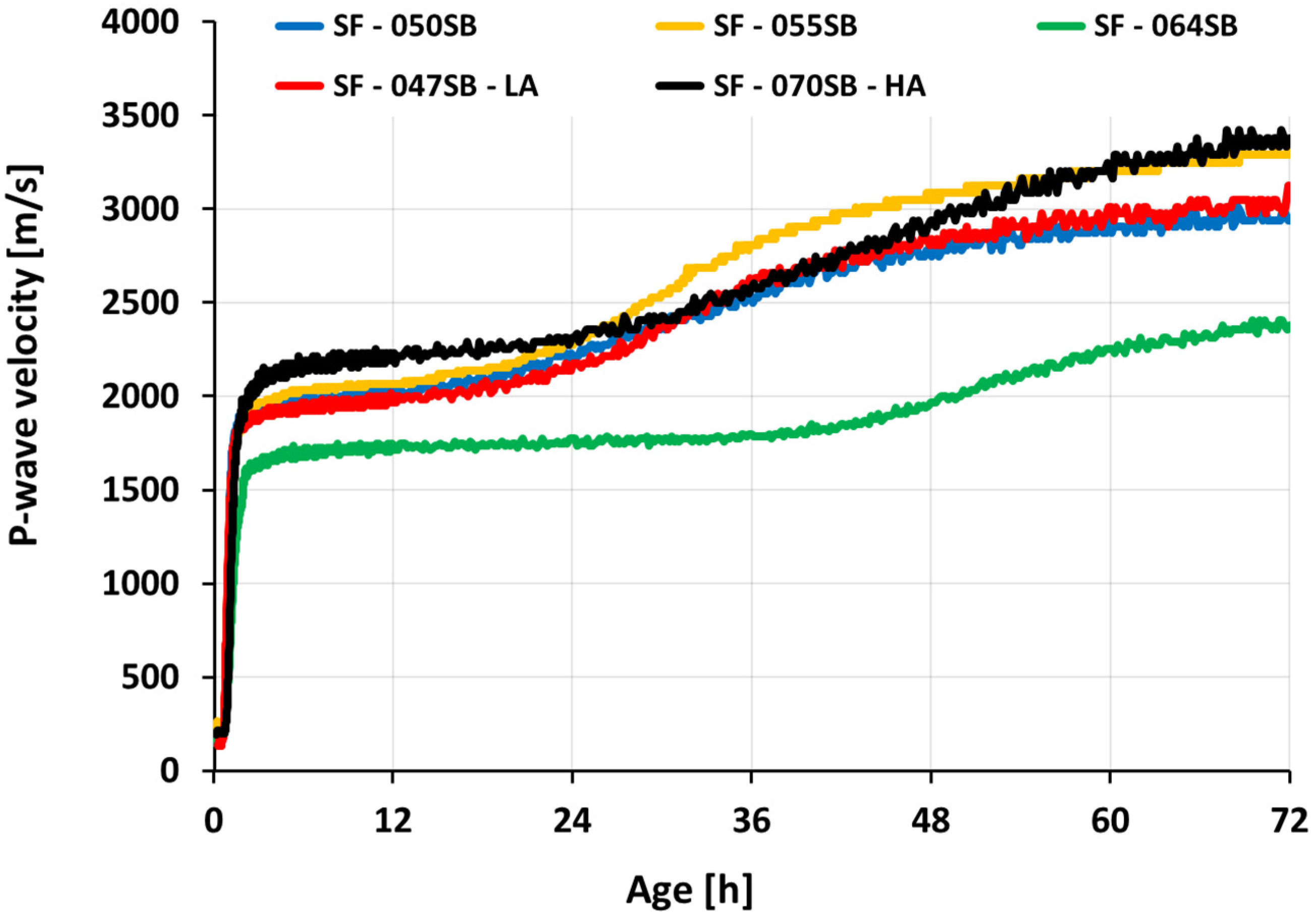
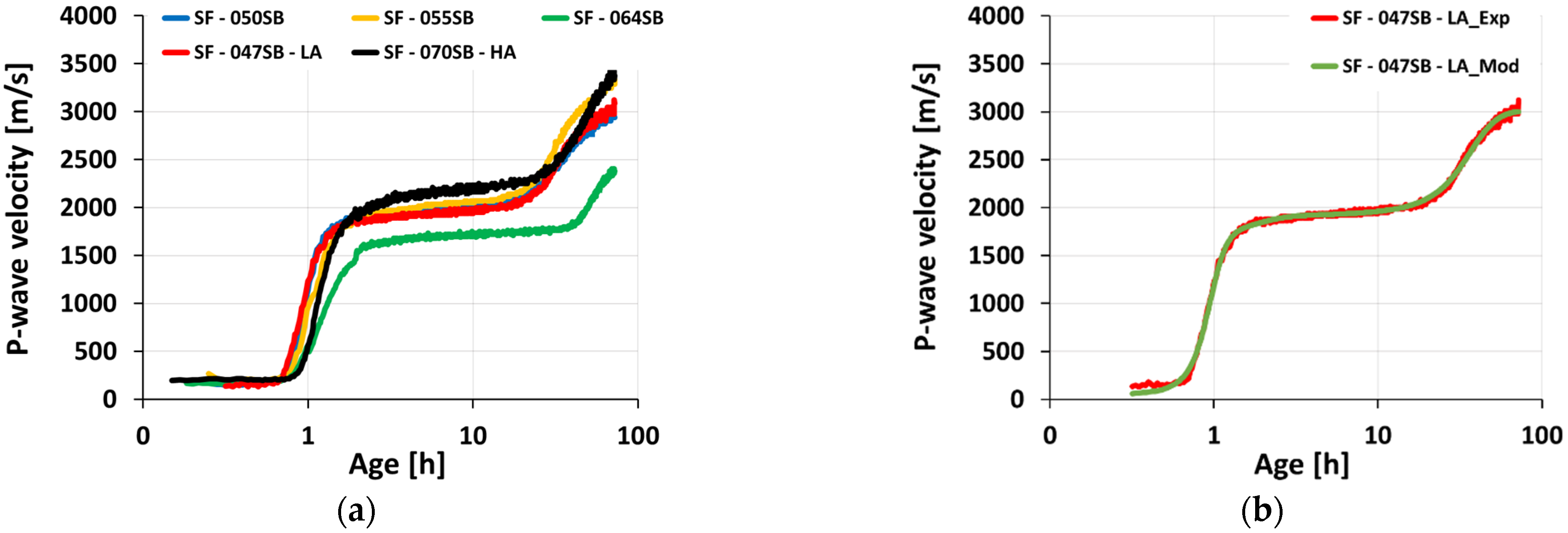
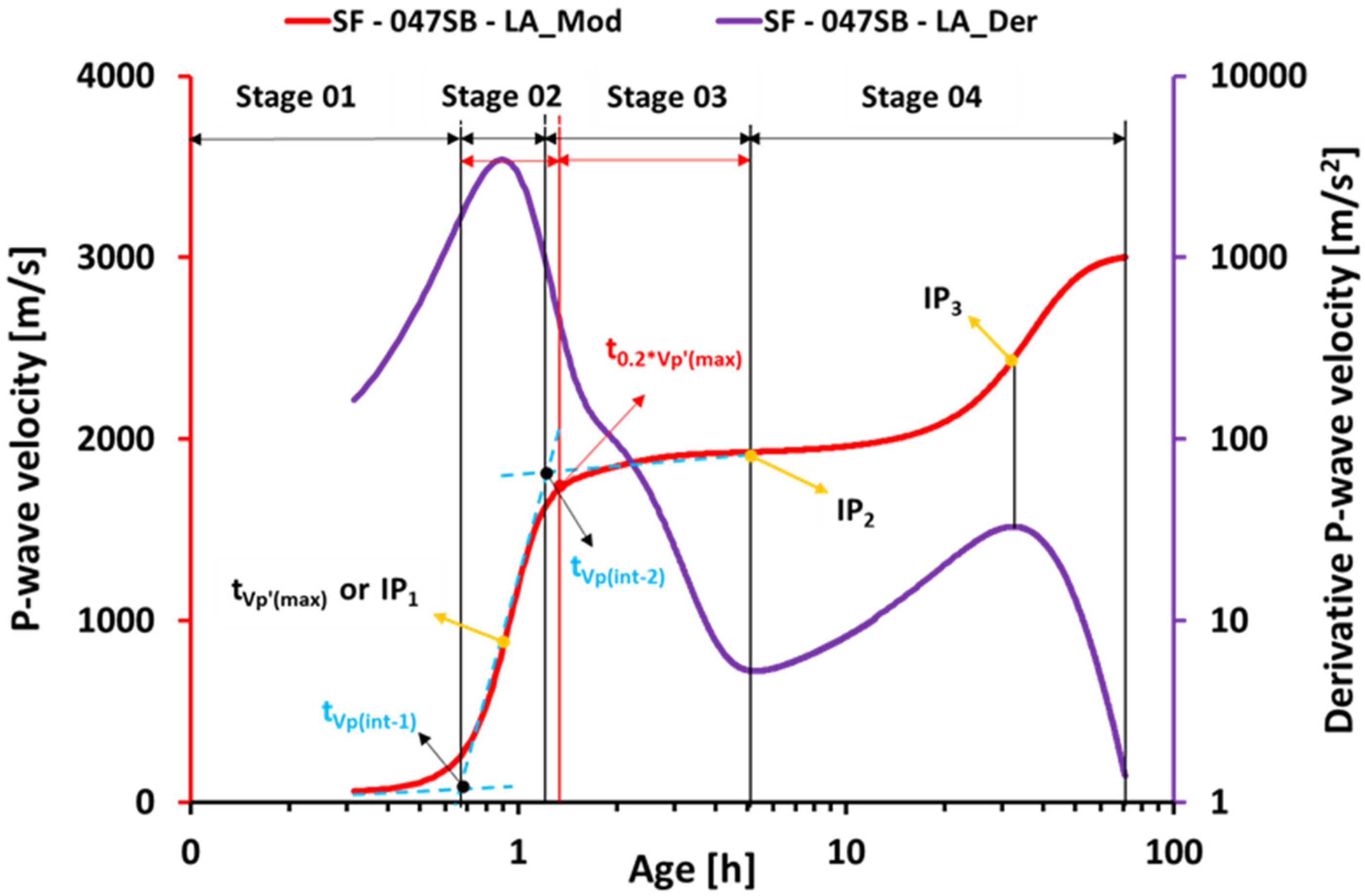
3.4. Ultrasonic Measurements
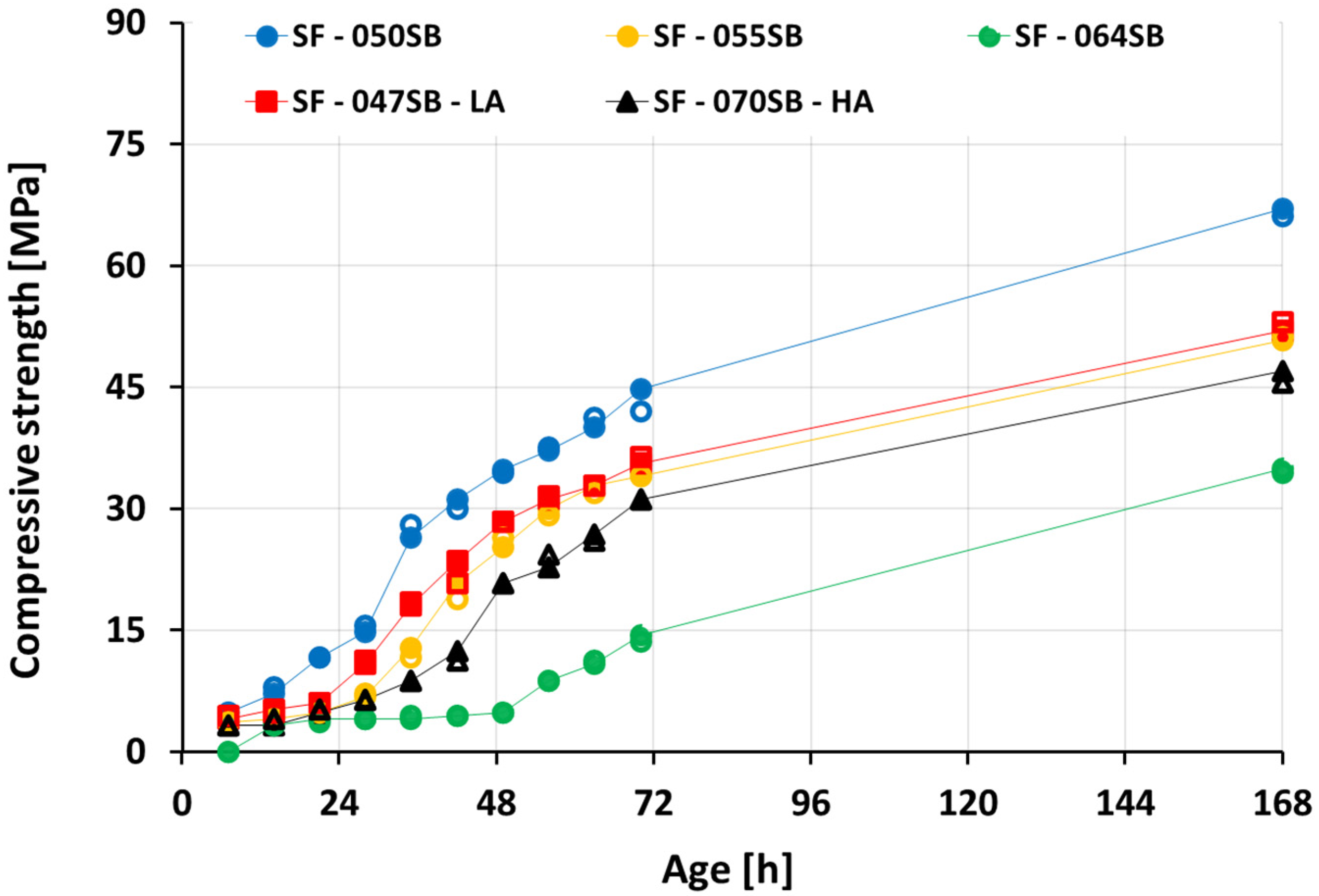
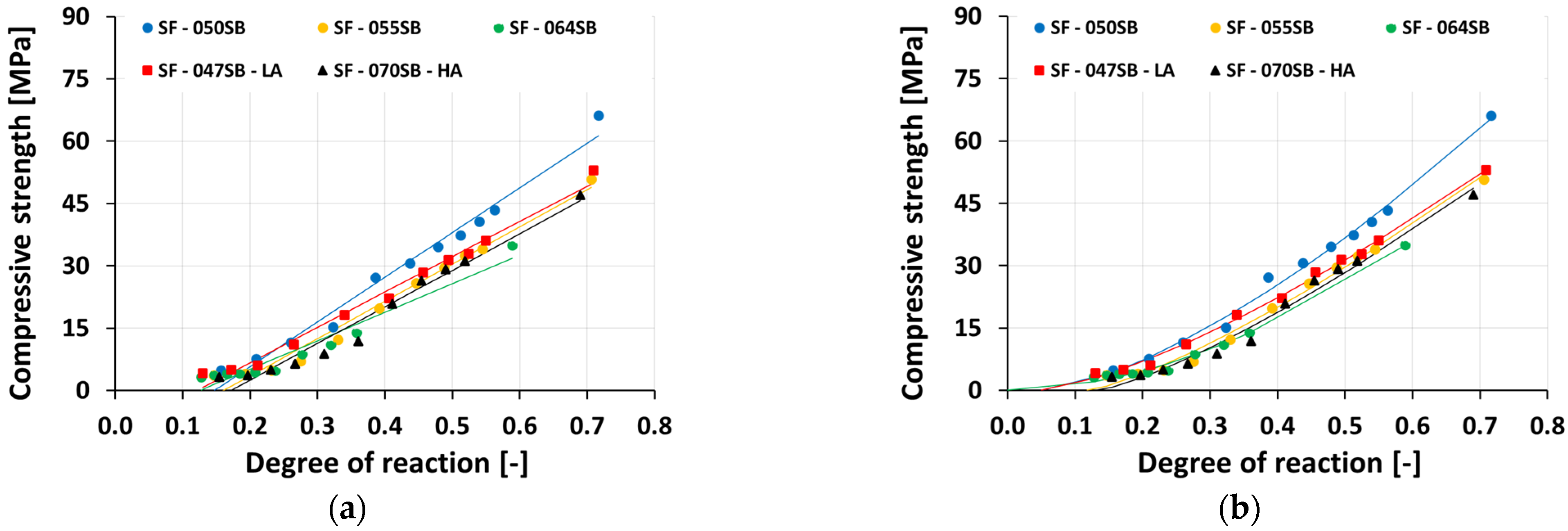
3.5. Compressive Strength Evolution
- A linear function of the form
- Alternatively, a power function of the form
4. Discussion
5. Conclusions and Outlook
- The ultrasonic pulse velocity (UPV) measurements for all slag-fly ash binders revealed four characteristic stages of velocity evolutions, clearly sensitive to the alkali and silica dosages as well as to the s/b ratio.
- The UPV-derived initial setting times showed good agreement with the slump flow measurements for all the studied mixes. Interestingly, the classical Vicat tests were accurate for mixes with low s/b ratios of 0.5 or smaller; for increasing s/b ratios, Vicat initial setting times occurred significantly later than UPV-derived initial setting times, and then the slump loss.
- P-wave velocities at the identified (initial and final) setting times are very different from one mix to another. Thus, P-wave velocity thresholds cannot be used for setting quantification of the studied AASF mixes.
- Heat flow evolutions obtained from isothermal calorimetry could not be used to determine setting times, as the characteristic minimum and maximum heat flow occurred at later ages (dormant period, acceleration stage, and appearance of main hydration peak).
- For mixes with the same alkali and silica content, the compressive strength decreased with an increasing solution-to-binder (s/b) ratio. This trend was expected, as a lower s/b ratio facilitates the closer packing of precursor particles, fewer pores, and, thus, a stronger microstructure. The compressive strength increased progressively with increasing calorimetry-derived reaction degrees. The correlation could be fitted reasonably well using a power-law function, but extrapolation attempts to predict the setting were not accurate.
6. Limitation of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United States Geological Survey. Mineral Commodity Summaries 2020; United States Geological Survey: Reston, VA, USA, 2020; ISBN 9781411343627.
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Readily Implementable Techniques Can Cut Annual CO2 Emissions from the Production of Concrete by over 20%. Environ. Res. Lett. 2016, 11, 074029. [Google Scholar] [CrossRef] [Green Version]
- Zosin, A.P.; Priimak, T.I.; Avsaragov, K.B. Geopolymer Materials Based on Magnesia-Iron Slags for Normalization and Storage of Radioactive Wastes. At. Energy 1998, 85, 510–514. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Investigations about the Effect of Aggregates on Strength and Microstructure of Geopolymeric Mine Waste Mud Binders. Cem. Concr. Res. 2007, 37, 933–941. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Jiménez, A.; Palomo, J.G.; Puertas, F. Alkali-Activated Slag Mortars: Mechanical Strength Behaviour. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Mehrotra, S.P. Influence of Granulated Blast Furnace Slag on the Reaction, Structure and Properties of Fly Ash Based Geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Li, Z.; Alfredo Flores Beltran, I.; Chen, Y.; Šavija, B.; Ye, G. Early-Age Properties of Alkali-Activated Slag and Glass Wool Paste. Constr. Build. Mater. 2021, 291, 123326. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Alkali Activation of Australian Slag Cements. Cem. Concr. Res. 1999, 29, 113–120. [Google Scholar] [CrossRef]
- Chen, W.; Xie, Y.; Li, B.; Li, B.; Wang, J.; Thom, N. Role of Aggregate and Fibre in Strength and Drying Shrinkage of Alkali-Activated Slag Mortar. Constr. Build. Mater. 2021, 299, 7–9. [Google Scholar] [CrossRef]
- Palacios, M.; Banfill, P.F.G.; Puertas, F. Rheology and Setting of Alkali-Activated Slag Pastes and Mortars: Effect If Organic Admixture. ACI Mater. J. 2008, 105, 140–148. [Google Scholar] [CrossRef]
- Palomo, A.; Alonso, S.; Fernández-Jiménez, A.; Sobrados, I.; Sanz, J. Alkaline activation of fly ashes: NMR study of the reaction products. J. Am. Ceram. Soc. 2004, 87, 1141–1145. [Google Scholar]
- Ruengsillapanun, K.; Udtaranakron, T.; Pulngern, T.; Tangchirapat, W.; Jaturapitakkul, C. Mechanical Properties, Shrinkage, and Heat Evolution of Alkali Activated Fly Ash Concrete. Constr. Build. Mater. 2021, 299, 123954. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-Activated Ground Fly Ash Geopolymer Cured at Ambient Temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Das, B.B.; Narayanan, N. Sustainable Construction and Building Materials: Select Proceedings of ICSCBM 2018; Springer: Berlin/Heidelberg, Germany, 2019; Volume 25, ISBN 978-981-13-3317-0. [Google Scholar]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vázquez, T. Alkali-Activated Fly Ash/Slag Cements. Strength Behaviour and Hydration Products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and Mechanical Properties of Alkali-Activated Fly Ash/Slag Concrete Manufactured at Room Temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Fang, G.; Bahrami, H.; Zhang, M. Mechanisms of Autogenous Shrinkage of Alkali-Activated Fly Ash-Slag Pastes Cured at Ambient Temperature within 24 h. Constr. Build. Mater. 2018, 171, 377–387. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, N.K.; Lee, H.K. Fresh and Hardened Properties of Alkali-Activated Fly Ash/Slag Pastes with Superplasticizers. Constr. Build. Mater. 2014, 50, 169–176. [Google Scholar] [CrossRef]
- Sarıdemir, M.; Çelikten, S. Investigation of Fire and Chemical Effects on the Properties of Alkali-Activated Lightweight Concretes Produced with Basaltic Pumice Aggregate. Constr. Build. Mater. 2020, 260, 119969. [Google Scholar] [CrossRef]
- Hewlett, P. Lea’s Chemistry of Cement and Concrete, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2003. [Google Scholar]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 3rd ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Pinto, R.C.A.; Schindler, A.K. Unified Modeling of Setting and Strength Development. Cem. Concr. Res. 2010, 40, 58–65. [Google Scholar] [CrossRef]
- Struble, L.; Kim, T.Y.; Zhang, H. Setting of Cement and Concrete. Cem. Concr. Aggregates 2001, 23, 88–93. [Google Scholar]
- Fernandez-Jimenez, A.; Puertas, F. Setting of Alkali-Activated Slag Cement. Influence of Activator Nature. Adv. Cem. Res. 2001, 13, 115–121. [Google Scholar] [CrossRef]
- Darquennes, A.; Staquet, S.; Espion, B. Determination of Time-Zero and Its Effect on Autogenous Deformation Evolution. Eur. J. Environ. Civ. Eng. 2011, 15, 1017–1029. [Google Scholar] [CrossRef]
- Boulay, C.; Staquet, S.; Delsaute, B.; Carette, J.; Crespini, M.; Yazoghli-Marzouk, O.; Merliot, É.; Ramanich, S. How to Monitor the Modulus of Elasticity of Concrete, Automatically since the Earliest Age? Mater. Struct. Constr. 2014, 47, 141–155. [Google Scholar] [CrossRef]
- Serdar, M.; Gabrijel, I.; Schlicke, D.; Staquet, S. Advanced Techniques for Testing of Cement-Based Materials; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-39737-1. [Google Scholar]
- Reinhardt, H.W.; Grosse, C.U. Continuous Monitoring of Setting and Hardening of Mortar and Concrete. Constr. Build. Mater. 2004, 18, 145–154. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, K.M.; Kim, Y.H.; Yim, H.; Bae, D.B. Ultrasonic In-Situ Monitoring of Setting Process of High-Performance Concrete. Cem. Concr. Res. 2004, 34, 631–640. [Google Scholar] [CrossRef]
- De Belie, N.; Grosse, C.U.; Kurz, J.; Reinhardt, H.W. Ultrasound Monitoring of the Influence of Different Accelerating Admixtures and Cement Types for Shotcrete on Setting and Hardening Behaviour. Cem. Concr. Res. 2005, 35, 2087–2094. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.; Chotard, T.; Gimet-Breart, N.; Fargeot, D. Correlation between Hydration Mechanism and Ultrasonic Measurements in an Aluminous Cement: Effect of Setting Time and Temperature on the Early Hydration. J. Eur. Ceram. Soc. 2002, 22, 1947–1958. [Google Scholar] [CrossRef]
- Lawson, J.L. On the Determination of the Elastic Properties of Geopolymeric Materials Using Non-Destructive Ultrasonic Techniques. Mech. Eng. 2008, 121, 1457480. [Google Scholar]
- D’Angelo, R.; Plona, T.J.; Schwartz, L.M.; Coveney, P. Ultrasonic Measurements on Hydrating Cement Slurries. Adv. Cem. Based Mater. 1995, 2, 8–14. [Google Scholar] [CrossRef]
- Carette, J.; Staquet, S. Monitoring the Setting Process of Mortars by Ultrasonic P and S-Wave Transmission Velocity Measurement. Constr. Build. Mater. 2015, 94, 196–208. [Google Scholar] [CrossRef]
- Chotard, T.; Gimet-Breart, N.; Smith, A.; Fargeot, D.; Bonnet, J.P.; Gault, C. Application of Ultrasonic Testing to Describe the Hydration of Calcium Aluminate Cement at the Early Age. Cem. Concr. Res. 2001, 31, 405–412. [Google Scholar] [CrossRef]
- Trtnik, G.; Turk, G.; Kavčič, F.; Bosiljkov, V.B. Possibilities of Using the Ultrasonic Wave Transmission Method to Estimate Initial Setting Time of Cement Paste. Cem. Concr. Res. 2008, 38, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Krüger, M.; Bregar, R.; David, G.A.; Juhart, J. Non-Destructive Evaluation of Eco-Friendly Cementituous Materials by Ultrasound. In Proceedings of the International RILEM Conference: Materials, Systems and Structures in Civil Engineering, Segment on Service Life of Cement-Based Materials and Structures, Lyngby, Denmark, 22–24 August 2016; pp. 503–512. [Google Scholar]
- Buchwald, A.; Tatarin, R.; Stephan, D. Reaction Progress of Alkaline-Activated Metakaolin-Ground Granulated Blast Furnace Slag Blends. J. Mater. Sci. 2009, 44, 5609–5617. [Google Scholar] [CrossRef]
- Kar, A.; Halabe, U.B.; Ray, I.; Unnikrishnan, A. Nondestructive Characterizations of Alkali Activated Fly Ash and/or Slag Concrete. Eur. Sci. J. 2013, 9, 1857–7881. [Google Scholar]
- Ren, W.; Xu, J.; Bai, E. Strength and Ultrasonic Characteristics of Alkali-Activated Fly Ash-Slag Geopolymer Concrete after Exposure to Elevated Temperatures. J. Mater. Civ. Eng. 2016, 28, 04015124. [Google Scholar] [CrossRef]
- Panchmatia, P.; Zhou, N.S.; Juenger, M.; van Oort, E. Monitoring the Strength Development of Alkali-Activated Materials Using an Ultrasonic Cement Analyzer. J. Pet. Sci. Eng. 2019, 180, 538–544. [Google Scholar] [CrossRef]
- Ryu, G.S.; Choi, S.; Koh, K.T.; Ahn, G.H.; Kim, H.Y.; You, Y.J. A Study on Initial Setting and Modulus of Elasticity of AAM Mortar Mixed with CSA Expansive Additive Using Ultrasonic Pulse Velocity. Materials 2020, 13, 4432. [Google Scholar] [CrossRef]
- Tekle, B.H.U.; Hertwig, L.; Holschemacher, K. Setting Time and Strength Monitoring of Alkali-Activated Cement Mixtures by Ultrasonic Testing. Materials 2021, 14, 1889. [Google Scholar] [CrossRef]
- DENER, M. Effect of Alkali Modulus on the Compressive Strength and Ultrasonic Pulse Velocity of Alkali-Activated BFS/FS Cement. Türk Doğa ve Fen Derg. 2022, 11, 63–68. [Google Scholar] [CrossRef]
- Nelson, E.B. Well Cementing, 2nd ed.; Schlumberger: Houston, TX, USA, 1990. [Google Scholar]
- Bentz, D.P.; Coveney, P.V.; Garboczi, E.J.; Kleyn, M.F.; Stutzman, P.E. Cellular Automaton Simulations of Cement Hydration and Microstructure Development. Model. Simul. Mater. Sci. Eng. 1994, 2, 783–808. [Google Scholar] [CrossRef]
- Ye, G. Experimental Study and Numerical Simulation of the Development of the Microstructure and Permeability of Cementitious Materials. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2003. [Google Scholar]
- Li, Z.; Wyrzykowski, M.; Dong, H.; Granja, J.; Azenha, M.; Lura, P.; Ye, G. Internal Curing by Superabsorbent Polymers in Alkali-Activated Slag. Cem. Concr. Res. 2020, 135, 106123. [Google Scholar] [CrossRef]
- Herb, A.T. Indirekte Beobachtung des Erstarrens und Erhärtens von Zementleim, Mörtel und Beton Mittels Schallwellenausbreitung. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2003. [Google Scholar]
- Schindler, A.K. Concrete Hydration, Temperature Development, and Setting at Early-Ages; University of Texas: Austin, TX, USA, 2002. [Google Scholar]
- Kamada, T.; Uchida, S.; Rokugo, K. Nondestructive Evaluation of Setting and Hardening of Cement Paste Based on Ultrasonic Propagation Characteristics. J. Adv. Concr. Technol. 2005, 3, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Uppalapati, S. Early-Age Structural Development and Autogenous Shrinkage of Alkali-Activated Slag/Fly Ash Cements. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2020. [Google Scholar]
- Van der Winden, N.G. Ultrasonic measurement for setting control of concrete. In Testing during Concrete Construction; CRC Press: Boca Raton, FL, USA, 1991; pp. 122–137. [Google Scholar]
- Voigt, T.; Grosse, C.U.; Sun, Z.; Shah, S.P.; Reinhardt, H.W. Comparison of Ultrasonic Wave Transmission and Reflection Measurements with P- and S-Waves on Early Age Mortar and Concrete. Mater. Struct. Constr. 2005, 38, 729–738. [Google Scholar] [CrossRef]
- Robeyst, N.; Gruyaert, E.; Grosse, C.U.; De Belie, N. Monitoring the Setting of Concrete Containing Blast-Furnace Slag by Measuring the Ultrasonic p-Wave Velocity. Cem. Concr. Res. 2008, 38, 1169–1176. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; De Schutter, G. Early Age Reaction, Rheological Properties and Pore Solution Chemistry of NaOH-Activated Slag Mixtures. Cem. Concr. Compos. 2022, 133, 104715. [Google Scholar] [CrossRef]
- Garnier, V.; Corneloup, G.; Sprauel, J.M.; Perfumo, J.C. Setting Time Study of Roller Compacted Concrete by Spectral Analysis of Transmitted Ultrasonic Signals. NDT E Int. 1995, 28, 15–22. [Google Scholar] [CrossRef]
- Dai, X.; Aydın, S.; Yardımcı, M.Y.; Lesage, K.; De Schutter, G. Effects of Activator Properties and GGBFS/FA Ratio on the Structural Build-up and Rheology of AAC. Cem. Concr. Res. 2020, 138, 106253. [Google Scholar] [CrossRef]
- Nedeljković, M.; Li, Z.; Ye, G. Setting, Strength, and Autogenous Shrinkage of Alkali-Activated Fly Ash and Slag Pastes: Effect of Slag Content. Materials 2018, 11, 2121. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Bernal, S.A.; Provis, J.L. Reproducible Mini-Slump Test Procedure for Measuring the Yield Stress of Cementitious Pastes. Mater. Struct. Constr. 2017, 50, 235. [Google Scholar] [CrossRef] [Green Version]
- ASTM C230; Standard Specification for Flow Table for Use in Tests of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2010; pp. 4–9. [CrossRef]
- BS EN 196; Part 3, 1995 Methods of Testing Cement. Part 3: Determination of Setting Time and Soundness. Mark Wright, Bechtel Ltd.: London, UK, 1995; p. 10.
- Carette, J.; Staquet, S. Monitoring the Setting Process of Eco-Binders by Ultrasonic P-Wave and S-Wave Transmission Velocity Measurement: Mortar vs Concrete. Constr. Build. Mater. 2016, 110, 32–41. [Google Scholar] [CrossRef]
- Adam, A. Strength and Durability Properties of Alkali Activated Slag and Fly Ash-Based Geopolymer Concrete. Ph.D. Thesis, RMIT University, Melbourne, Australia, 2009. [Google Scholar]
- Reddy, K.C.; Subramaniam, K.V.L. Investigation on the Roles of Solution-Based Alkali and Silica in Activated Low-Calcium Fly Ash and Slag Blends. Cem. Concr. Compos. 2021, 123, 104175. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía De Gutierrez, R. Evolution of Binder Structure in Sodium Silicate-Activated Slag-Metakaolin Blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Carette, J.; Staquet, S. Monitoring and Modelling the Early Age and Hardening Behaviour of Eco-Concrete through Continuous Non-Destructive Measurements: Part I. Hydration and Apparent Activation Energy. Cem. Concr. Compos. 2016, 73, 10–18. [Google Scholar] [CrossRef]
- Uppalapati, S.; Vandewalle, L.; Cizer, Ö. Monitoring the Setting Process of Alkali-Activated Slag-Fly Ash Cements with Ultrasonic P-Wave Velocity. Constr. Build. Mater. 2021, 271, 121592. [Google Scholar] [CrossRef]
- Gargouri, A.; Daoud, A.; Loulizi, A.; Kallel, A. Laboratory Investigation of Self-Consolidating Waste Tire Rubberized Concrete. ACI Mater. J. 2016, 113, 661–668. [Google Scholar] [CrossRef]
- Povey, M. Ultrasonic Techniques for Fluids Characterization; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Shi, Z.; Shi, C.; Wan, S.; Li, N.; Zhang, Z. Effect of Alkali Dosage and Silicate Modulus on Carbonation of Alkali-Activated Slag Mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z.; Gao, X.; Zhang, C.; Wu, Q. Effect of Fly Ash Microsphere on the Rheology and Microstructure of Alkali-Activated Fly Ash/Slag Pastes. Cem. Concr. Res. 2018, 109, 198–207. [Google Scholar] [CrossRef]
- Sarıdemir, M.; Çelikten, S. Effects of Ms Modulus, Na Concentration and Fly Ash Content on Properties of Vapour-Cured Geopolymer Mortars Exposed to High Temperatures. Constr. Build. Mater. 2023, 363, 129868. [Google Scholar] [CrossRef]
- Cavallini, F. Fitting a Logistic Curve to Data. Coll. Math. J. 1993, 24, 247–253. [Google Scholar] [CrossRef]
- Trtnik, G.; Kavčič, F.; Turk, G. Prediction of Concrete Strength Using Ultrasonic Pulse Velocity and Artificial Neural Networks. Ultrasonics 2009, 49, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhang, Y.; Li, Z. Ultrasonic Monitoring of Setting and Hardening of Slag Blended Cement under Different Curing Temperatures by Using Embedded Piezoelectric Transducers. Constr. Build. Mater. 2018, 159, 553–560. [Google Scholar] [CrossRef]
- Uppalapati, S.; Vandewalle, L.; Cizer, Ö. Autogenous Shrinkage of Slag-Fly Ash Blends Activated with Hybrid Sodium Silicate and Sodium Sulfate at Different Curing Temperatures. Constr. Build. Mater. 2020, 265, 121276. [Google Scholar] [CrossRef]
- De Schutter, G.; Taerwe, L. Degree of Hydration-Based Description of Mechanical Properties of Early Age Concrete. Mater. Struct. Constr. 1996, 29, 335–344. [Google Scholar] [CrossRef]
- Zhu, J.; Cao, J.N.; Bate, B.; Khayat, K.H. Determination of Mortar Setting Times Using Shear Wave Velocity Evolution Curves Measured by the Bender Element Technique. Cem. Concr. Res. 2018, 106, 1–11. [Google Scholar] [CrossRef]
- Carette, J.; Staquet, S. Monitoring and Modelling the Early Age and Hardening Behaviour of Eco-Concrete through Continuous Non-Destructive Measurements: Part II. Mechanical Behaviour. Cem. Concr. Compos. 2016, 73, 1–9. [Google Scholar] [CrossRef]
- Latif Al-Mufti, R.; Fried, A.N. The Early Age Non-Destructive Testing of Concrete Made with Recycled Concrete Aggregate. Constr. Build. Mater. 2012, 37, 379–386. [Google Scholar] [CrossRef]
- Robeyst, N. Monitoring Setting and Microstructure Development in Fresh Concrete with the Ultrasonic Through-Transmission Method Opvolging van Binding En Microstructuurontwikkeling in Vers Beton Met de Ultrasone-Transmissiemethode. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2010. [Google Scholar]
- Lu, Y.; Ma, H.; Li, Z. Ultrasonic Monitoring of the Early-Age Hydration of Mineral Admixtures Incorporated Concrete Using Cement-Based Piezoelectric Composite Sensors. J. Intell. Mater. Syst. Struct. 2015, 26, 280–291. [Google Scholar] [CrossRef]
- Bentz, D.P.; Garboczi, E.J. Percolation of Phases in a Three-Dimensional Cement Paste Microstructural Model. Cem. Concr. Res. 1991, 21, 325–344. [Google Scholar] [CrossRef]
- Torrenti, J.M.; Benboudjema, F. Mechanical Threshold of Cementitious Materials at Early Age. Mater. Struct. Constr. 2005, 38, 299–304. [Google Scholar] [CrossRef]
- Delsaute, B. Influence of Cyclic Movement on the Hardening Process of Grout: Case of Offshore Wind Turbine Installation. In Proceedings of the 10th International Conference on Fracture Mechanics of Concrete and Concrete Structures, Bayonne, France, 24–26 June 2019. [Google Scholar]



| Oxide Composition | Slag [%] | Fly Ash [%] |
|---|---|---|
| CaO | 40.80 | 4.33 |
| SiO2 | 33.30 | 56.70 |
| Al2O3 | 12.30 | 23.50 |
| MgO | 7.84 | 1.43 |
| SO3 | 2.30 | 1.16 |
| TiO2 | 1.29 | 1.23 |
| K2O | 0.67 | 2.65 |
| Na2O | 0.44 | 0.91 |
| Fe2O3 | 0.39 | 5.92 |
| MnO | 0.36 | - |
| BaO | 0.11 | 0.21 |
| SrO | - | 0.15 |
| P2O5 | - | 1.49 |
| Parameters | SF—050SB | SF—055SB | SF—064SB | SF—047SB—LA | SF—070SB—HA |
|---|---|---|---|---|---|
| Slag [g] | 50 | 50 | 50 | 50 | 50 |
| Fly ash [g] | 50 | 50 | 50 | 50 | 50 |
| NaOH (8 M aq) [g] | 2.49 | 2.49 | 2.49 | 2.13 | 3.11 |
| Na2SiO3 (aq) [g] | 26.53 | 26.53 | 26.53 | 22.91 | 33.79 |
| Water [g] | 20.85 | 25.85 | 34.85 | 22.07 | 32.47 |
| s/b [-] | 0.50 | 0.55 | 0.64 | 0.47 | 0.70 |
| w/b [-] | 0.37 | 0.42 | 0.51 | 0.36 | 0.53 |
| Na2O content [%] | 5.26 | 5.26 | 5.26 | 4.54 | 6.69 |
| SiO2 content [%] | 7.56 | 7.56 | 7.56 | 6.53 | 9.63 |
| Ms value [-] | 1.44 | 1.44 | 1.44 | 1.44 | 1.44 |
| Ultimate Heat Release | SF—050SB | SF—055SB | SF—064SB | SF—047SB—LA | SF—070SB—HA |
|---|---|---|---|---|---|
| 187.6 | 204.7 | 258.8 | 199.2 | 262.2 |
| SF—050SB | SF—055SB | SF—064SB | SF—047SB—LA | SF—070SB—HA | |
|---|---|---|---|---|---|
| 19.83 | 27.85 | 18.88 | 25.46 | 26.33 |
| Initial Setting Time [min] | Final Setting Time [min] | ||||||
|---|---|---|---|---|---|---|---|
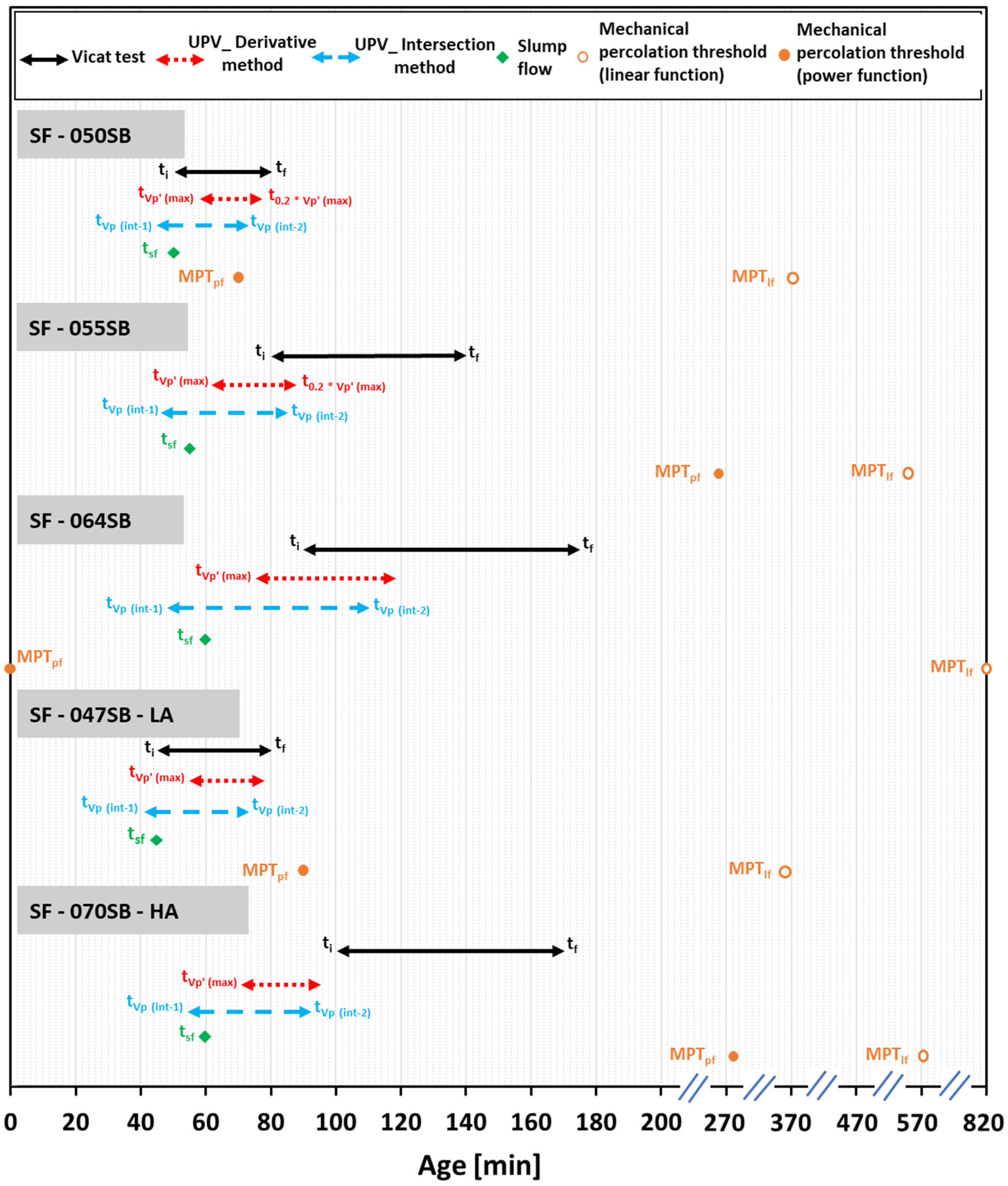
| Mix ID | Slump Flow [tsf] | Vicat Test [ti] | FreshCON [tVp’(max)] | FreshCON [tVp(int-1)] | Vicat Test [tf] | FreshCON [t0.2*Vp’(max)] | FreshCON [tVp(int-2)] |
| SF—050SB | 50 | 50 | 58 | 45 | 80 | 77 | 73 |
| SF—055SB | 55 | 80 | 62 | 46 | 140 | 88 | 85 |
| SF—064SB | 60 | 90 | 75 | 48 | 175 | 118 | 110 |
| SF—047SB—LA | 45 | 45 | 55 | 41 | 80 | 78 | 73 |
| SF—070SB—HA | 60 | 100 | 71 | 54 | 170 | 95 | 92 |
| Mix ID | UPV Values at Initial Set [m/s] | UPV Values at Final Set [m/s] | |||||
|---|---|---|---|---|---|---|---|
| Slump Flow [tsf] | Vicat Test [ti] | FreshCON [tVp’(max)] | FreshCON [tVp(int-1)] | Vicat Test [tf] | FreshCON [t0.2*Vp’(max)] | FreshCON [tVp(int-2)] | |
| SF—050SB | 525 | 525 | 928 | 344 | 1775 | 1730 | 1678 |
| SF—055SB | 620 | 1581 | 892 | 380 | 1910 | 1696 | 1675 |
| SF—064SB | 516 | 1209 | 840 | 322 | 1631 | 1526 | 1466 |
| SF—047SB—LA | 411 | 411 | 857 | 282 | 1725 | 1694 | 1635 |
| SF—070SB—HA | 565 | 1847 | 1020 | 397 | 2037 | 1798 | 1771 |
| Mix ID | Linear Function, MPTlf | Power Function, MPTpf | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Slope k | RMSE | Age | Compressive Strength | Power Law Exponent | RMSE | Age | |||
| [MPa] | [MPa] | [-] | [min] | [MPa] | [-] | [MPa] | [-] | [min] | |
| SF—050SB | 64.89 | 2.53 | 0.147 | 370 | 70.31 | 1.46 | 1.48 | 0.052 | 70 |
| SF—055SB | 52.81 | 2.28 | 0.161 | 530 | 56.94 | 1.30 | 1.51 | 0.118 | 260 |
| SF—064SB | 42.90 | 2.32 | 0.126 | 820 | 54.72 | 1.86 | 0.85 | 0.000 | 00 |
| SF—047SB—LA | 53.46 | 1.81 | 0.122 | 360 | 57.59 | 1.38 | 0.93 | 0.050 | 90 |
| SF—070SB—HA | 50.97 | 2.62 | 0.172 | 570 | 55.50 | 1.30 | 1.20 | 0.132 | 280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naqi, A.; Delsaute, B.; Königsberger, M.; Staquet, S. Effect of Solution-to-Binder Ratio and Alkalinity on Setting and Early-Age Properties of Alkali-Activated Slag-Fly Ash Binders. Materials 2023, 16, 373. https://doi.org/10.3390/ma16010373
Naqi A, Delsaute B, Königsberger M, Staquet S. Effect of Solution-to-Binder Ratio and Alkalinity on Setting and Early-Age Properties of Alkali-Activated Slag-Fly Ash Binders. Materials. 2023; 16(1):373. https://doi.org/10.3390/ma16010373
Chicago/Turabian StyleNaqi, Ali, Brice Delsaute, Markus Königsberger, and Stéphanie Staquet. 2023. "Effect of Solution-to-Binder Ratio and Alkalinity on Setting and Early-Age Properties of Alkali-Activated Slag-Fly Ash Binders" Materials 16, no. 1: 373. https://doi.org/10.3390/ma16010373





