Production of Activated Biochar Derived from Residual Biomass for Adsorption of Volatile Organic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Raw Material Carbonization
2.3. Physiochemical Analysis of Biochar Samples
2.4. Adsorption and Desorption of VOCs
3. Results and Discussion
3.1. Biochars and Chemically Activated Biochars
3.2. Morphological Structure of Non and Activated Biochars
3.3. VOCs Removal
3.4. VOC Adsorption on Biochar Samples
3.5. Main Factors Influencing Adsorption on Activated Biochars of VOCs
3.6. Effects of VOCs on Environment and Human Health, and Abatement Methods
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kung, S.-S.; Zhang, L.; Gong, X.; Kung, C.-C. A sector-wide economic and environmental analysis on bioenergy production and emission consequences. Energy Explor. Exploit. 2019, 37, 1408–1425. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, E.; Khapre, A.; Bordoloi, N.; Kumar, S. Sorption of volatile organic compounds on non-activated biochar. Bioresour. Technol. 2020, 297, 122469. [Google Scholar] [CrossRef]
- Jia, L.; Ma, J.; Shi, Q.; Long, C. Prediction of Adsorption Equilibrium of VOCs onto Hyper-Cross-Linked Polymeric Resin at Environmentally Relevant Temperatures and Concentrations Using Inverse Gas Chromatography. Environ. Sci. Technol. 2017, 51, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wu, L.; Wu, B.; Wang, Z.; Yu, L.; Crocker, M.; Zhu, A.; Shi, C. Catalytic materials for low concentration vocs removal through ‘‘storage-regeneration” cycling. Chem. Cat. Chem. 2019, 11, 3644–3659. [Google Scholar] [CrossRef]
- Cabanes, A.; Valdés, F.J.; Fullana, A. A review on VOCs from recycled plastics. Sustain. Mater. Technol. 2020, 25, e00179. [Google Scholar] [CrossRef]
- Peluso, A.; Gargiulo, N.; Aprea, P.; Pepe, F.; Caputo, D. Nanoporous Materials as H2S Adsorbents for Biogas Purification: A Review. Sep. Purif. Rev. 2018, 48, 78–89. [Google Scholar] [CrossRef]
- Li, L.; Kang, Y.T. Bubble behaviors and CO2 absorption characteristics in nanoabsorbents. J. CO2 Util. 2019, 33, 488–499. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into Multiple and Multilevel Structures of Biochars and Their Potential Environmental Applications: A Critical Review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef] [PubMed]
- Luengas, A.; Barona, A.; Hort, C.; Gallastegui, G.; Platel, V.; Elias, A. A review ofindoor air treatment technologies. Rev. Environ. Sci. Bio/Technol. 2015, 14, 499–522. [Google Scholar] [CrossRef]
- Du, Y.R.; Fan, Z.; Guo, T.X.; Xu, J.P.; Han, Z.H.; Pan, Y.F.; Xiao, H.N.; Sun, Y.M.; Yan, Q. Characteristics of as-prepared biochar derived from catalytic pyrolysis within moderate-temperature ionic liquid for CO2 uptake. Can. J. Chem. Eng. 2019, 98, 690–704. [Google Scholar] [CrossRef]
- Vikrant, K.; Kumar, V.; Kim, K.-H.; Kukkar, D. Metal–organic frameworks (MOFs): Potential and challenges for capture and abatement of ammonia. J. Mater. Chem. A 2017, 5, 22877–22896. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Wu, J.; Liu, Y. Novel carbon-based sorbents for elemental mercury removal from gas streams: A review. Chem. Eng. J. 2020, 391, 123514. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J.; O’Connor, D. Remediation of mercury contaminated soil, water, and air: A review of emerging materials and innovative technologies. Environ. Int. 2020, 134, 105281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ruan, G.; Jalilov, A.S.; Tarkunde, Y.R.; Fei, H.; Tour, J.M. Biochar as a renewable source for high-performance CO2 sorbent. Carbon 2016, 107, 344–351. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Rosales, E.; Sanromán, M.A. Unravelling the Environmental Application of Biochar as Low-Cost Biosorbent: A Review. Appl. Sci. 2020, 10, 7810. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Gaunt, J.L.; Lehmann, J. Energy Balance and Emissions Associated with Biochar Sequestration and Pyrolysis Bioenergy Production. Environ. Sci. Technol. 2008, 42, 4152–4158. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Zhang, L.; Sun, Z. Preparation of high performance H2S removal biochar by direct fluidized bed carbonization using potato peel waste. Process. Saf. Environ. Prot. 2017, 107, 281–288. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, D.; Liu, R.R.; Wan, S.G.; Lu, X.J. Coadsorption of gaseous xylene, ethyl acetate and water onto porous biomass carbon foam pellets derived from liquefied Vallisneria natans waste. J. Chem. Technol. Biotechnol. 2020, 95, 1348–1360. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation—A review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Feng, D.D.; Guo, D.W.; Zhang, Y.; Sun, S.Z.; Zhao, Y.J.; Shang, Q.; Sun, H.L.; Wu, J.Q.; Tan, H.P. Functionalized construction of biochar with hierarchical pore structures and surface O-/N-containing groups for phenol adsorption. Chem. Eng. J. 2021, 410, 127707. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Bamdad, H.; Hawboldt, K.; MacQuarrie, S. A review on common adsorbents for acid gases removal: Focus on biochar. Renew. Sustain. Energy Rev. 2018, 81, 1705–1720. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.S. Insight into biochar properties and its cost analysis. Biomass Bioenergy 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Balasubramanian, P. The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application—A review. Ind. Crops Prod. 2019, 128, 405–423. [Google Scholar]
- Igalavithana, A.D.; Choi, S.W.; Dissanayake, P.D.; Shang, J.; Wang, C.-H.; Yang, X.; Kim, S.; Tsang, D.C.W.; Lee, K.B.; Ok, Y.S. Gasification biochar from biowaste (food waste and wood waste) for effective CO2 adsorption. J. Hazard. Mater. 2020, 391, 121147. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Mosa, A.; Zhang, L.; Gao, B. Biochar modulates mineral nitrogen dynamics in soil and terrestrial ecosystems: A critical review. Chemosphere 2021, 278, 130378. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B.S. Black carbon (biochar) in water/soil environments:molecular structure, sorption, stability, and potential risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef] [PubMed]
- Usevičiūtė, L.; Baltrėnaitė-Gedienė, E. Dependence of pyrolysis temperature and lignocellulosic physical-chemical properties of biochar on its wettability. Biomass Convers. Biorefin. 2020, 11, 2775–2793. [Google Scholar] [CrossRef]
- Song, G.; Qin, F.; Yu, J.; Tang, L.; Pang, Y.; Zhang, C.; Wang, J.; Deng, L. Tailoring biochar for persulfate-based environmental catalysis: Impact of biomass feedstocks. J. Hazard. Mater. 2022, 424, 127663. [Google Scholar] [CrossRef]
- Guo, S.; Dong, X.; Zhu, C.; Han, Y.; Ma, F.; Wu, T. Pyrolysis behaviors and thermodynamics properties of hydrochar from bamboo (Phyllostachys heterocycla cv. pubescens) shoot shell. Bioresour. Technol. 2017, 233, 92–98. [Google Scholar] [CrossRef]
- Flora, J.F.R.; Lu, X.; Li, L.; Flora, J.R.V.; Berge, N.D. The effects of alkalinity and acidity of process water and hydrochar washing on the adsorption of atrazine on hydrothermally produced hydrochar. Chemosphere 2013, 93, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol. Environ. Saf. 2017, 138, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gao, B.; Fang, J. Recent advances in engineered biochar productions and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2158–2207. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Al-Wabel, M.; Elfaki, J.; Usman, A.; Hussain, Q.; Ok, Y.S. Performance of dry water- and porous carbon-based sorbents for carbon dioxide capture. Environ. Res. 2019, 174, 69–79. [Google Scholar] [CrossRef]
- Toulene. Available online: https://en.wikipedia.org/wiki/Toluene (accessed on 2 November 2022).
- Acetone. Available online: https://en.wikipedia.org/wiki/Acetone (accessed on 2 November 2022).
- Lamplugh, A.; Harries, M.; Nguyen, A.; Montoya, L.D. VOC emissions from nail salon products and their effective removal using affordable adsorbents and synthetic jets. Build. Environ. 2020, 168, 106499. [Google Scholar] [CrossRef]
- Yorgun, S.; Yıldız, D. Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J. Taiwan Inst. Chem. Eng. 2015, 53, 122–131. [Google Scholar] [CrossRef]
- Thubsuang, U.; Laebang, S.; Manmuanpom, N.; Wongkasemjit, S.; Chaisuwan, T. Tuning pore characteristics of porous carbon monoliths prepared from rubber wood waste treated with H3PO4 or NaOH and their potential as supercapacitor electrode materials. J. Mater. Sci. 2017, 52, 6837–6855. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Lima, I.M.; Klasson, K.T. Sorption of Deisopropylatrazine on Broiler Litter Biochars. J. Agric. Food Chem. 2010, 58, 12350–12356. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Ojala, S.; Pitkäaho, S.; Laitinen, T.; Koivikko, N.N.; Brahmi, R.; Gaálová, J.; Matejova, L.; Kucherov, A.; Päivärinta, S.; Hirschmann, C.; et al. Catalysis in VOC Abatement. Top. Catal. 2011, 54, 1224–1256. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Fang, J.; Zou, W.; Dong, L.; Cao, C.; Zhang, J.; Li, Y.; Wang, H. Chemically activated hydrochar as an effective adsorbent for volatile organic compounds (VOCs). Chemosphere 2019, 218, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Q.; Song, J.-F.; Yao, X.-L.; Huang, G.-J.; Liu, Z.; Tang, L. Adsorption of volatile organic compounds on three activated carbon samples: Effect of pore structure. J. Central South Univ. 2012, 19, 3530–3539. [Google Scholar] [CrossRef]
- Nasri, N.S.; Mohammed, J.; Zaini, M.A.A.; Hamza, U.D.; Zain, H.M.; Ani, F.N. Equilibrium and Kinetic Studies of Benzene and Toluene Adsorption onto Microwave Irradiated-Coconut Shell Activated Carbon. Adv. Mater. Res. 2014, 1043, 219–223. [Google Scholar] [CrossRef]
- Son, H.K.; Sivakumar, S.; Rood, M.J.; Kim, B.J. Electrothermal adsorption and desorption of volatile organic compounds on activated carbon fiber cloth. J. Hazard. Mater. 2016, 301, 27–34. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Zheng, Y.; Hu, X.; Creamer, A.E.; Annable, M.D.; Li, Y. Biochar for volatile organic compound (VOC) removal: Sorption performance and governing mechanisms. Bioresour. Technol. 2017, 245, 606–614. [Google Scholar] [CrossRef]
- Lei, B.; Liu, B.; Zhang, H.; Yan, L.; Xie, H.; Zhou, G. CuO-modified activated carbon for the improvement of toluene removal in air. J. Environ. Sci. 2020, 88, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Kim, K.-J.; Kang, C.-S.; You, Y.-J.; Chung, M.-C.; Woo, M.-W.; Jeong, W.-J.; Park, N.-C.; Ahn, H.-G. Adsorption–desorption characteristics of VOCs over impregnated activated carbons. Catal. Today 2006, 111, 223–228. [Google Scholar] [CrossRef]
- Subramanian, S.; Pande, G.; De Weireld, G.; Giraudon, J.-M.; Lamonier, J.-F.; Batra, V.S. Sugarcane bagasse fly ash as an attractive agro-industry source for VOC removal on porous carbon. Ind. Crops Prod. 2013, 49, 108–116. [Google Scholar] [CrossRef]
- Niu, Y.; Shang, T.; Zeng, J.; Wang, S.; Gong, Y.; Hui, S. Effect of Pulverized Coal Preheating on NOx Reduction during Combustion. Energy Fuels 2017, 31, 4436–4444. [Google Scholar] [CrossRef]
- Oh, K.-J.; Park, D.-W.; Kim, S.-S.; Park, S.-W. Breakthrough data analysis of adsorption of volatile organic compounds on granular activated carbon. Korean J. Chem. Eng. 2010, 27, 632–638. [Google Scholar] [CrossRef]
- Tsai, J.H.; Chiang, H.M.; Huang, G.Y.; Chiang, H.L. Adsorption characteristics of acetone, chloroform and acetonitrile on sludge-derived adsorbent, commercial granular activated carbon and activated carbon fibers. J. Hazard. Mater. 2008, 154, 1183–1191. [Google Scholar] [CrossRef]
- Zhou, K.; Ma, W.; Zeng, Z.; Ma, X.; Xu, X.; Guo, Y.; Li, H.; Li, L. Experimental and DFT study on the adsorption of VOCs on activated carbon/metal oxides composites. Chem. Eng. J. 2019, 372, 1122–1133. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N. Facile synthesis of porous carbons from silica-rich rice husk char for volatile organic compounds (VOCs) sorption. Bioresour. Technol. 2019, 282, 294–300. [Google Scholar] [CrossRef]
- Tehrani, N.H.M.H.; Alivand, M.S.; Rashidi, A.; Shamskar, K.R.; Samipoorgiri, M.; Esrafili, M.D.; Maklavany, D.M.; Shafiei-Alavijeh, M. Preparation and characterization of a new waste-derived mesoporous carbon structure for ultrahigh adsorption of benzene and toluene at ambient conditions. J. Hazard. Mater. 2020, 384, 121317. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Y.; Wu, F.; Peng, S.; Li, J.; Liu, Z. Tubular activated carbons made from cotton stalk for dynamic adsorption of airborne toluene. J. Taiwan Inst. Chem. Eng. 2017, 80, 399–405. [Google Scholar] [CrossRef]
- Tang, M.; Huang, X.; Peng, Y.; Lu, S. Hierarchical porous carbon as a highly efficient adsorbent for toluene and benzene. Fuel 2020, 270, 117478. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Q.; Deng, Q.; Guo, Z.; Seok, I. Modification of coconut shell activated carbon and purification of volatile organic waste gas acetone. Adv. Compos. Hybrid Mater. 2022, 5, 491–503. [Google Scholar] [CrossRef]
- Baur, G.B.; Yuranov, I.; Renken, A.; Kiwi-Minsker, L. Activated carbon fibers for efficient VOC removal from diluted streams: The role of surface morphology. Adsorption 2015, 21, 479–488. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Benzene and toluene adsorption at low concentration on activated carbon fibres. Adsorption 2011, 17, 473–481. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Marco-Lozar, J.P.; Cazorla-Amorós, D.; Linares-Solano, A. Activated carbons prepared by pyrolysis of mixtures of carbon precursor/alkaline hydroxide. J. Anal. Appl. Pyrolysis 2007, 80, 166–174. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Larsson, S.H.; Mathieu, M.; Thyrel, M.; Pham, T.N. Application of design of experiments (DoE) for optimised production of micro- and mesoporous Norway spruce bark activated carbons. Biomass Convers. Biorefinery 2021, 1–19. [Google Scholar] [CrossRef]
- Guy, M.; Mathieu, M.; Anastopoulos, I.P.; Martínez, M.G.; Rousseau, F.; Dotto, G.L.; de Oliveira, H.P.; Lima, E.C.; Thyrel, M.; Larsson, S.H.; et al. Process Parameters Optimization, Characterization, and Application of KOH-Activated Norway Spruce Bark Graphitic Biochars for Efficient Azo Dye Adsorption. Molecules 2022, 27, 456. [Google Scholar] [CrossRef]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Qian, Q.; Gong, C.; Zhang, Z.; Yuan, G. Removal of VOCs by activated carbon microspheres derived from polymer: A comparative study. Adsorption 2015, 21, 333–341. [Google Scholar] [CrossRef]
- Mao, H.; Zhou, D.; Hashisho, Z.; Wang, S.; Chen, H.; Wang, H.; Lashaki, M.J. Microporous activated carbon from pinewood and wheat straw bymicrowave-assisted koh treatment for the adsorption of toluene and acetone vapors. RSC Adv. 2015, 5, 36051–36058. [Google Scholar] [CrossRef]
- Mao, H.; Huang, R.; Hashisho, Z.; Wang, S.; Chen, H.; Wang, H.; Zhou, D. Adsorption of toluene and acetone vapors on microwave-prepared activated carbon from agricultural residues: Isotherms, kinetics, and thermodynamics studies. Res. Chem. Intermed. 2016, 42, 3359–3371. [Google Scholar] [CrossRef]
- Jaramillo, J.; Álvarez, P.M.; Gómez-Serrano, V. Preparation and ozone-surface modification of activated carbon. Thermal stability of oxygen surface groups. Appl. Surf. Sci. 2010, 256, 5232–5236. [Google Scholar] [CrossRef]
- Deng, Z.; Deng, Q.; Wang, L.; Xiang, P.; Lin, J.; Murugadoss, V.; Song, G. Modifying coconut shell activated carbon for improved purification of benzene from volatile organic waste gas. Adv. Compos. Hybrid Mater. 2021, 4, 751–760. [Google Scholar] [CrossRef]
- Wang, H.; Jahandar Lashaki, M.; Fayaz, M.; Hashisho, Z.; Philips, J.H.; Anderson, J.E.; Nichols, M. Adsorption and desorption of mixtures of organicvapors on beaded activated carbon. Environ. Sci. Technol. 2012, 46, 8341–8350. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmospheric Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Patnaik, P. A Comprehensive Guide to the Hazardous Properties of Chemical Substances; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]


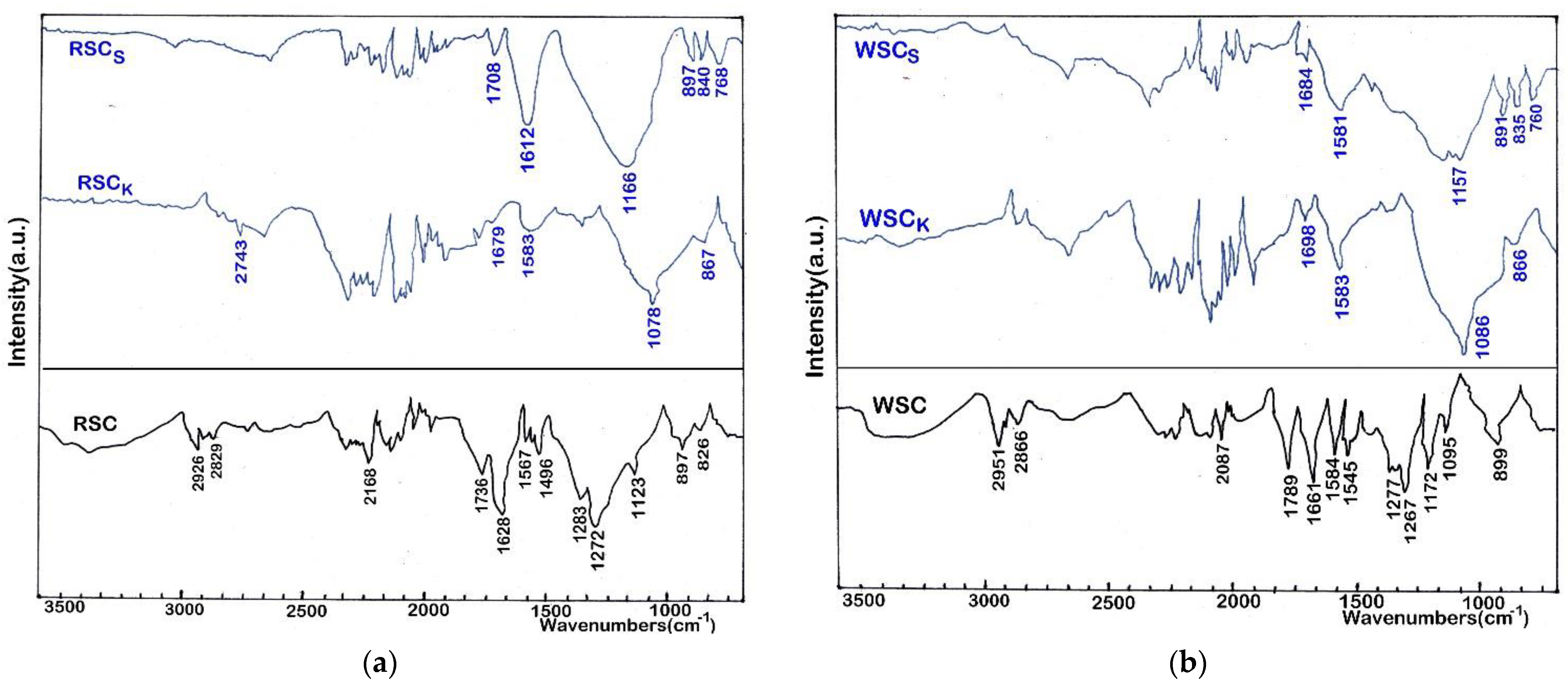
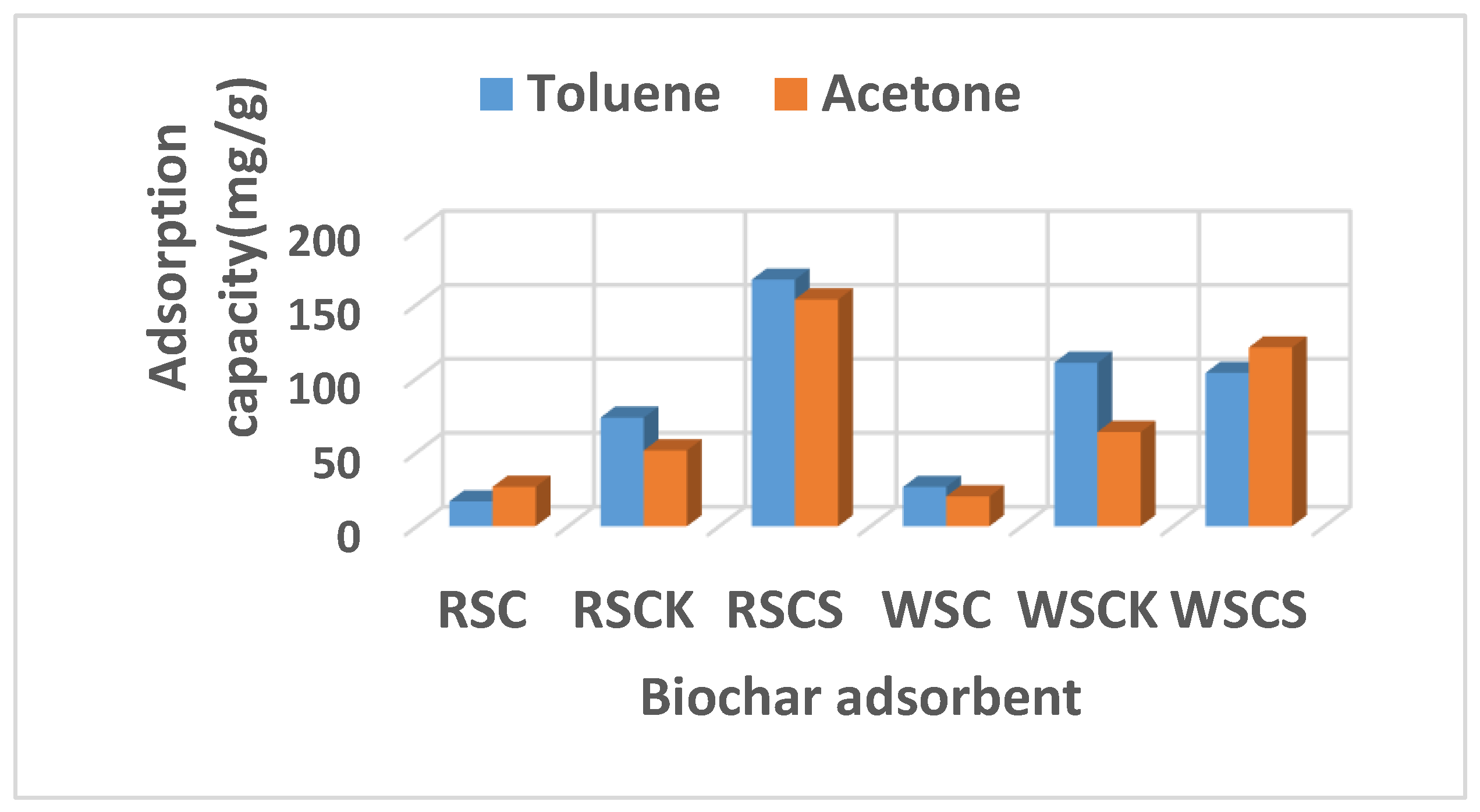
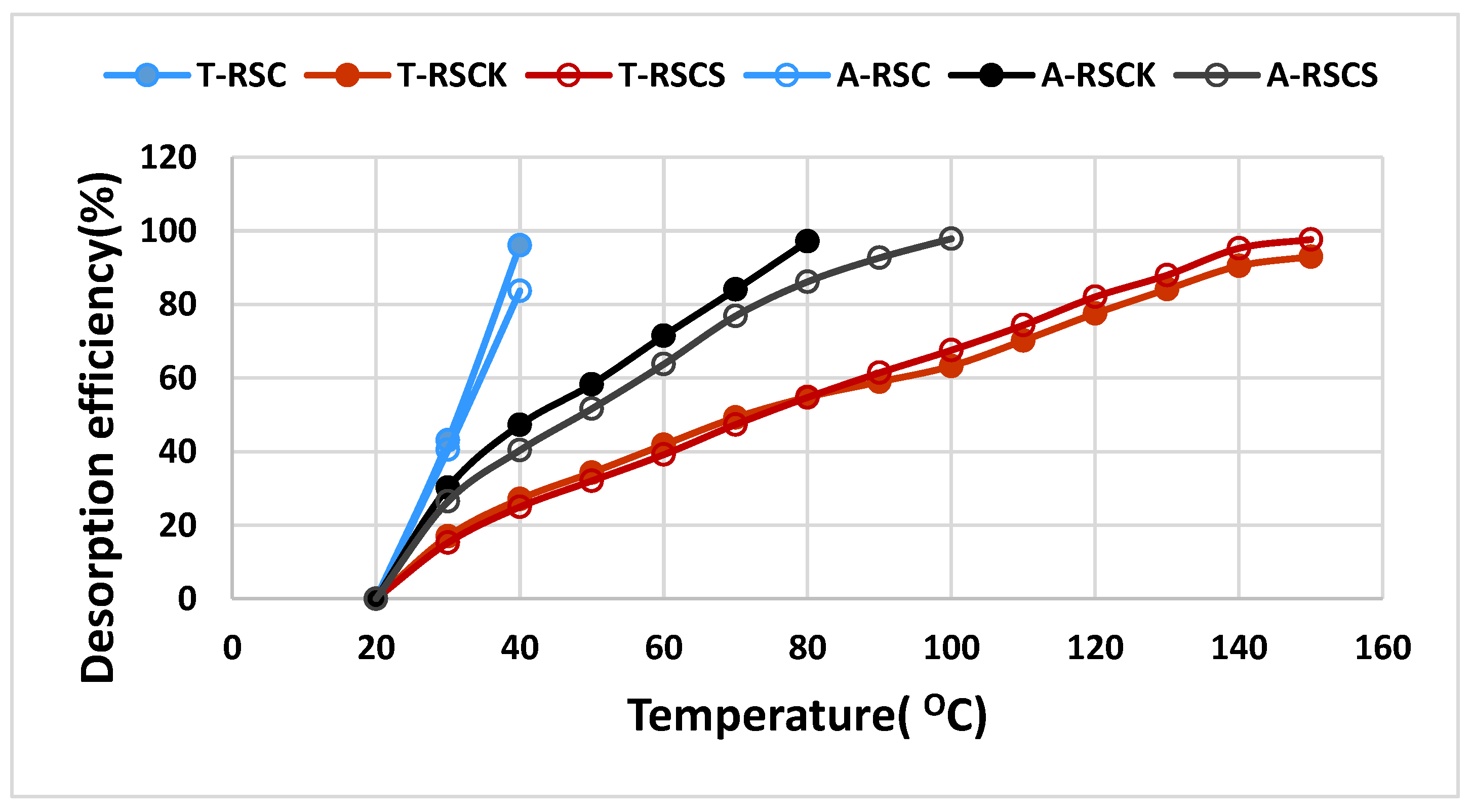

| Sample | SABET | PV | Dp | C | H | N | S | O | Ash | O/C |
|---|---|---|---|---|---|---|---|---|---|---|
| (m2/g) | (cm3/g) | (nm) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (wt.%) | (mol/mol) | |
| RSC | 8.5 | 0.124 | 0.92 | 46.05 | 6.14 | 6.68 | 0.85 | 39.62 | 6.24 | 0.645 |
| WSC | 9.6 | 0.126 | 1.12 | 47.62 | 5.72 | 0.35 | 0.68 | 45.63 | 7.26 | 0.718 |
| RSCK | 583 | 0.64 | 67.56 | 85.52 | 1.85 | 1.85 | 0.63 | 10.14 | 4.45 | 0.089 |
| RSCS | 1114 | 1.47 | 89.16 | 91.71 | 1.73 | 1.28 | 0.62 | 4.65 | 4.05 | 0.038 |
| WSCK | 235 | 0.47 | 57.45 | 83.34 | 1.76 | 0.32 | 0.64 | 13.93 | 6.45 | 0.125 |
| WSCS | 1020 | 1.29 | 97.05 | 89.73 | 1.87 | 0.28 | 0.37 | 7.74 | 6.12 | 0.064 |
| VOCs | FM | WM (g/mol) | ρ (g/mL) | Bp (°C) | Dm (D) | DK (nm) |
|---|---|---|---|---|---|---|
| Toluene | C7H8 | 92.141 | 0.87 | 111 | 0 | 0.58 |
| Acetone | C3H6O | 58.08 | 0.7845 | 56.05 | 2.91 | 0.46 |
| Material | SBET | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|
| Commercial AC | 934 | 41 | [60] |
| AC (rice husk) | 1818 | 264 | [61] |
| AC/ZrO | 837 | 127 | [60] |
| AC (petroleum waste) | 2692 | 659.9 | [62] |
| AC-Z | 795 | 258 | [63] |
| HPC-900 | 578 | 182 | [64] |
| RSCS | 1114 | 166.72 | [This work] |
| WSCS | 1020 | 152.34 | [This work] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, E. Production of Activated Biochar Derived from Residual Biomass for Adsorption of Volatile Organic Compounds. Materials 2023, 16, 389. https://doi.org/10.3390/ma16010389
David E. Production of Activated Biochar Derived from Residual Biomass for Adsorption of Volatile Organic Compounds. Materials. 2023; 16(1):389. https://doi.org/10.3390/ma16010389
Chicago/Turabian StyleDavid, Elena. 2023. "Production of Activated Biochar Derived from Residual Biomass for Adsorption of Volatile Organic Compounds" Materials 16, no. 1: 389. https://doi.org/10.3390/ma16010389
APA StyleDavid, E. (2023). Production of Activated Biochar Derived from Residual Biomass for Adsorption of Volatile Organic Compounds. Materials, 16(1), 389. https://doi.org/10.3390/ma16010389







