Effect of Warm-Water Retting Pretreatment on the Physical Properties of Banana Stem and Its Fibre
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of Banana Stem Warm Water Retting
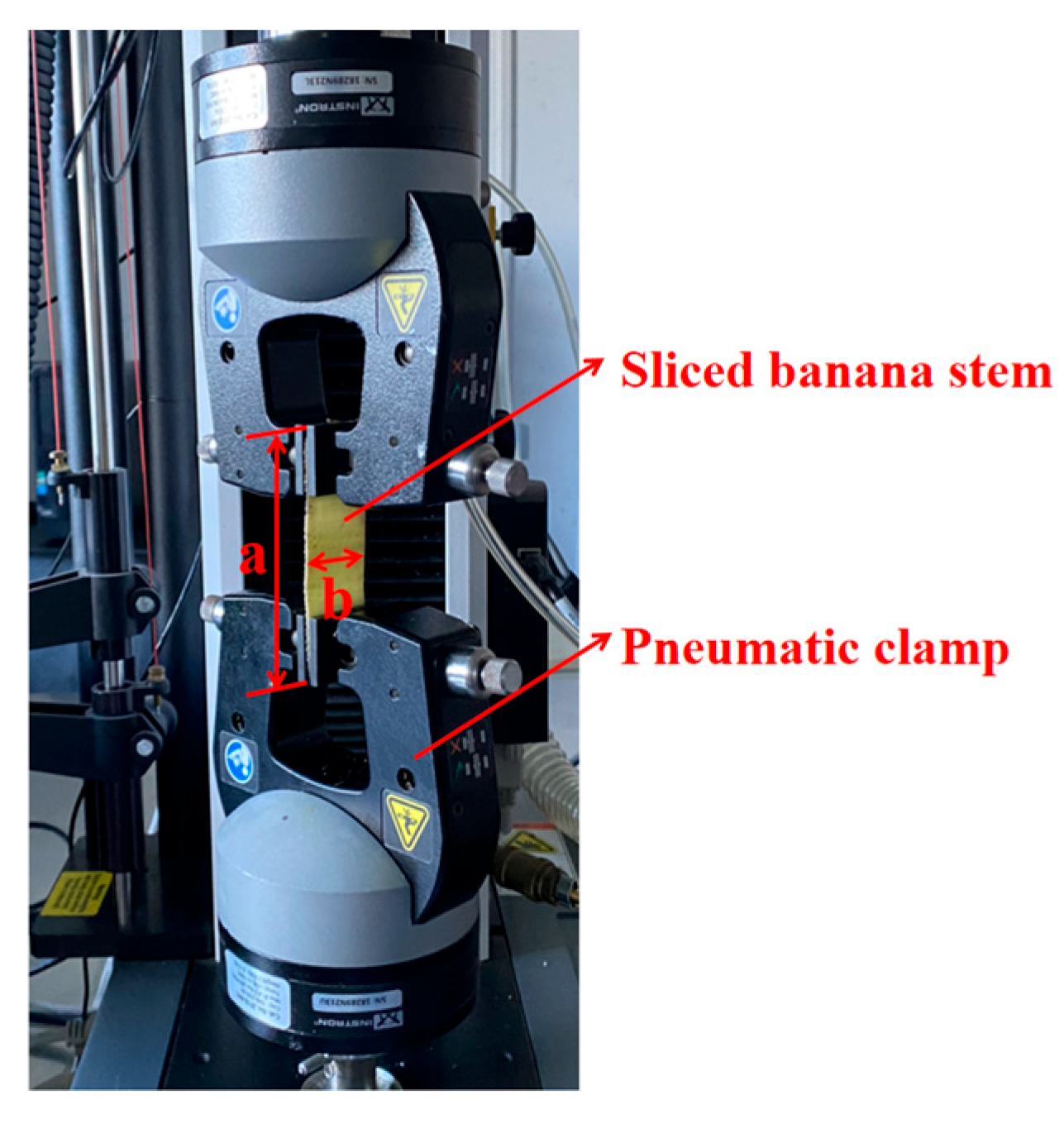
2.3. Bundle Tensile Test
2.4. Determination of Fibre Diameter of Banana Stem
2.5. Determination of Moisture Regain of Banana-Stem Fibre
2.6. Banana Stem Tensile Separation Test
2.7. Statistical Methods and Tools
2.8. Microscopic Observation on the Outer Skin of Stem Slice
2.9. FTIR Spectroscopy Analysis
3. Discussion of Results
3.1. Bundle Tensile Test
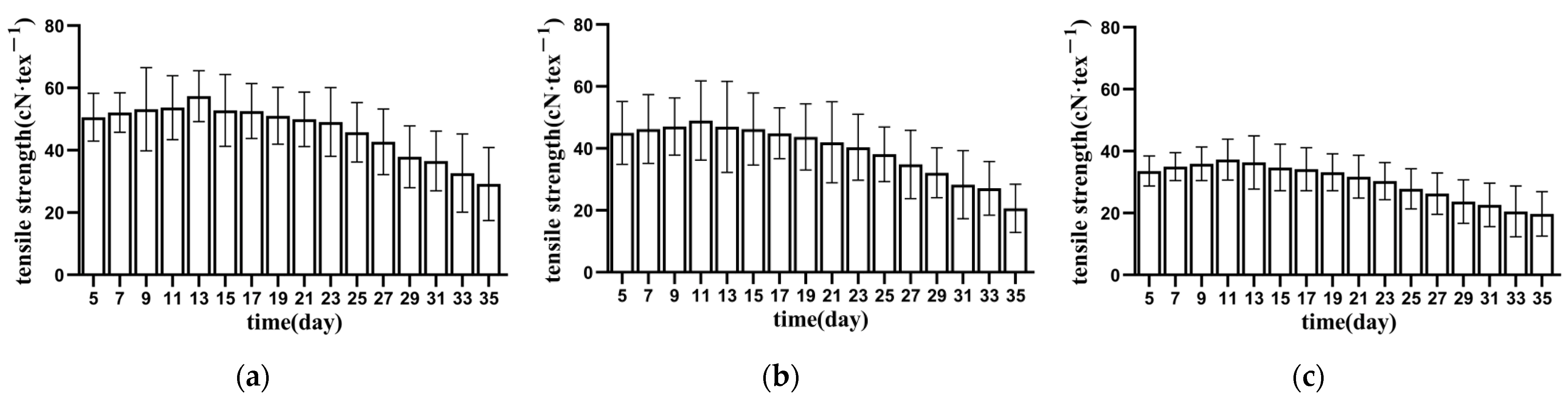
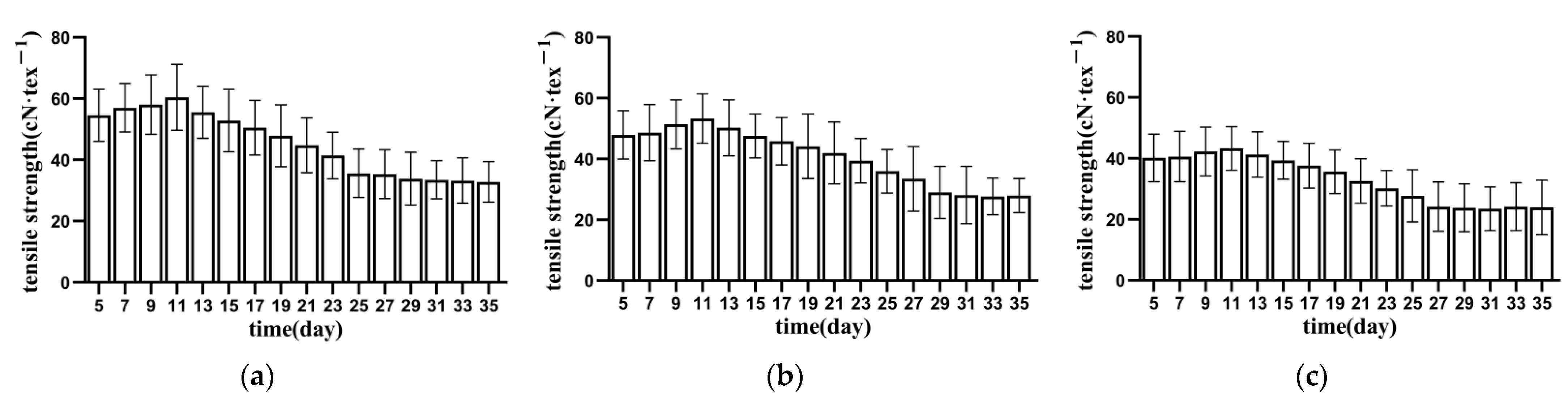
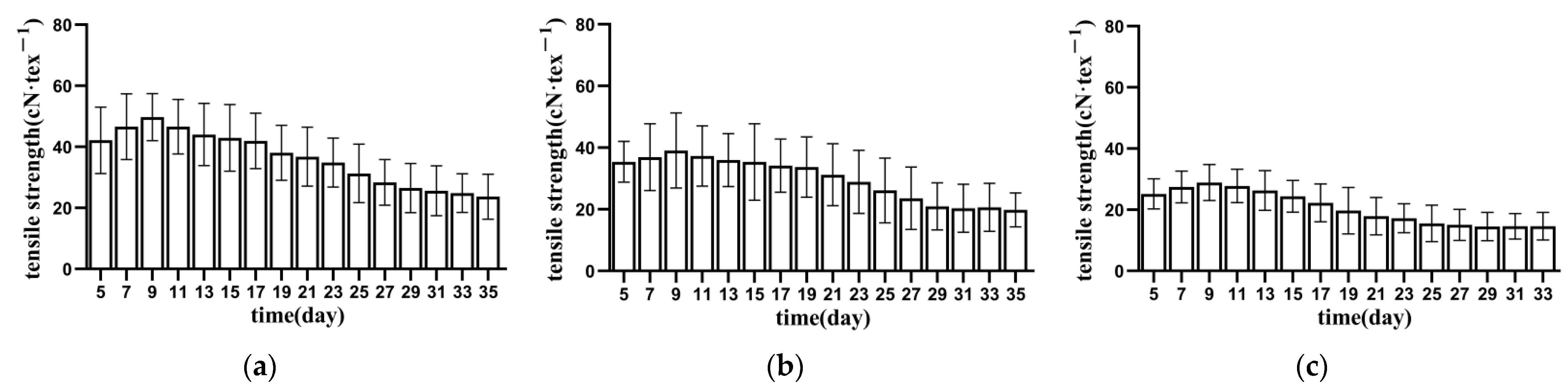
3.1.1. Tensile Strength of Bundle Fibre
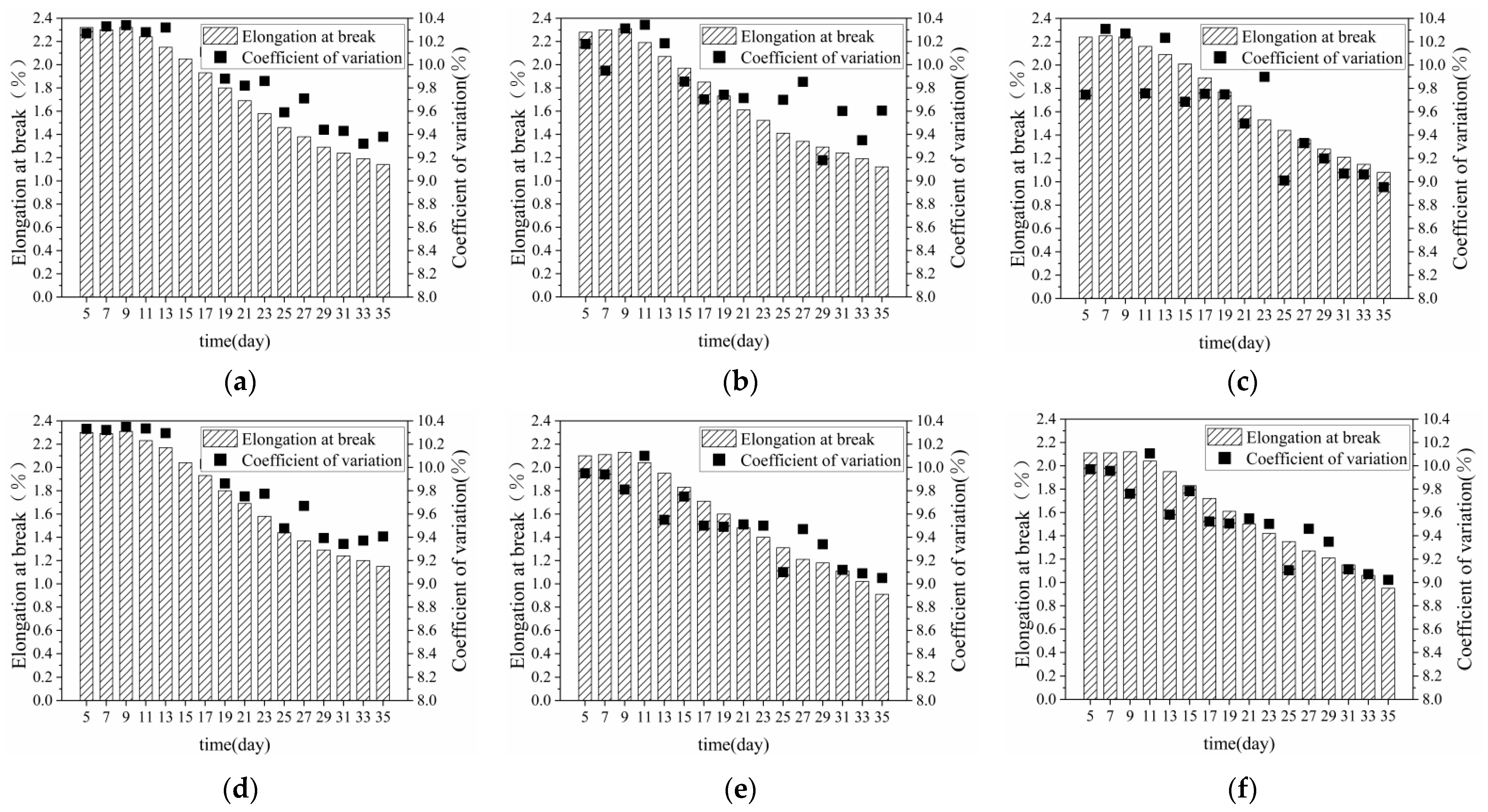
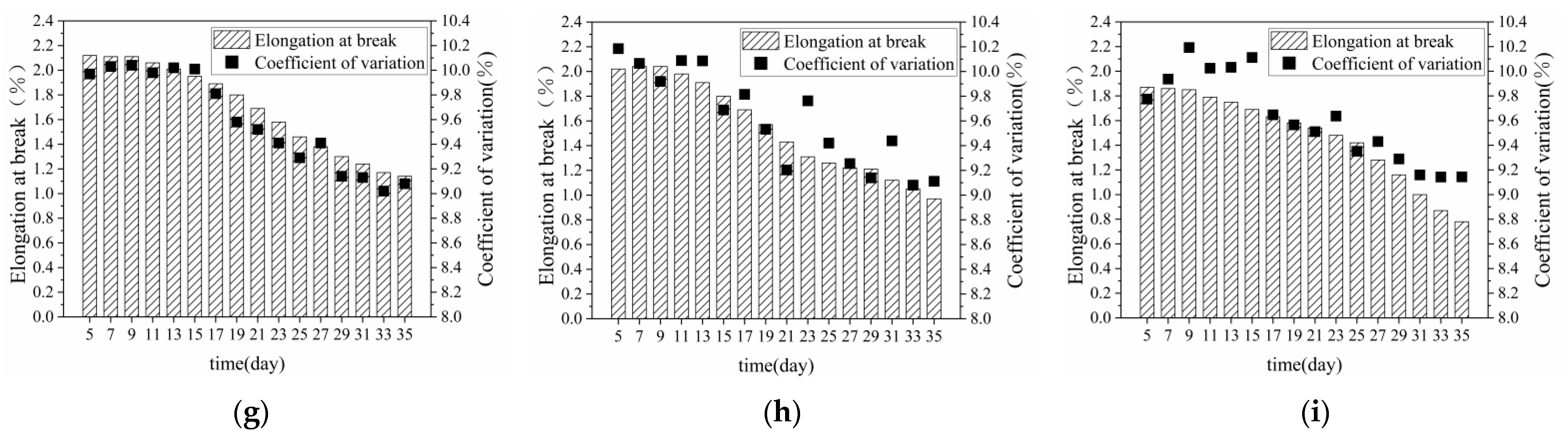
3.1.2. Breaking Elongation of Bundle Fibre
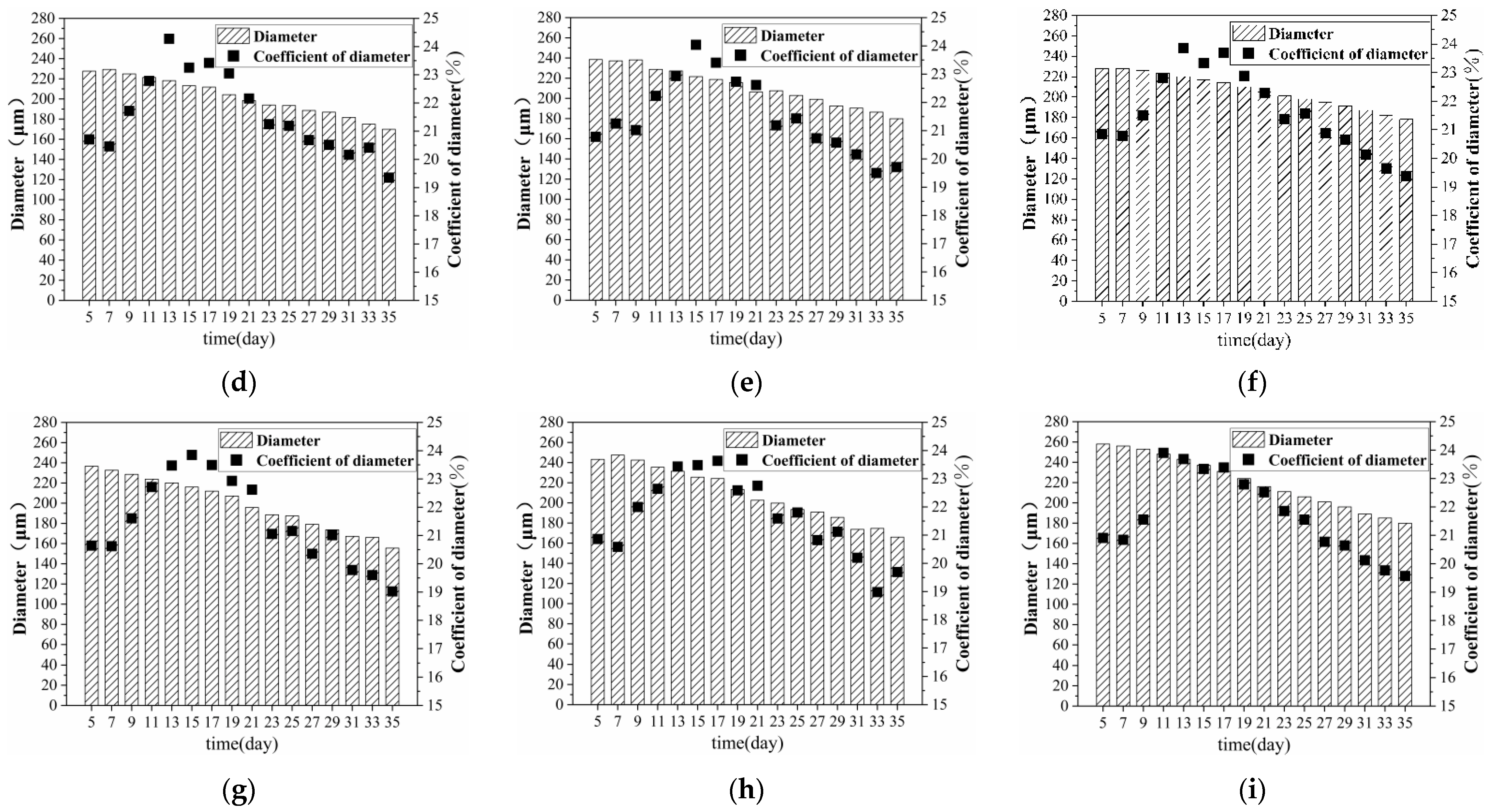
3.2. Effect of Retting Flax on Fibre Diameter
3.3. Effect of Retting Flax on Fibre-Moisture Regain
3.4. Separation of Fibre and Plant Tissue
3.4.1. Straw Tensile Separation Test
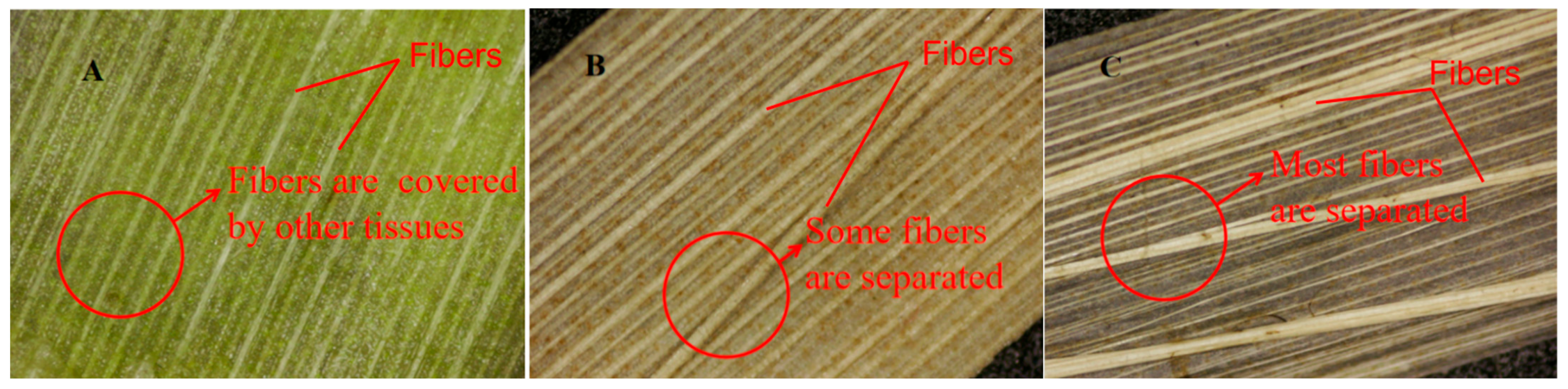
3.4.2. Microscopic Observation on the Outer Epidermis of Stem Slices
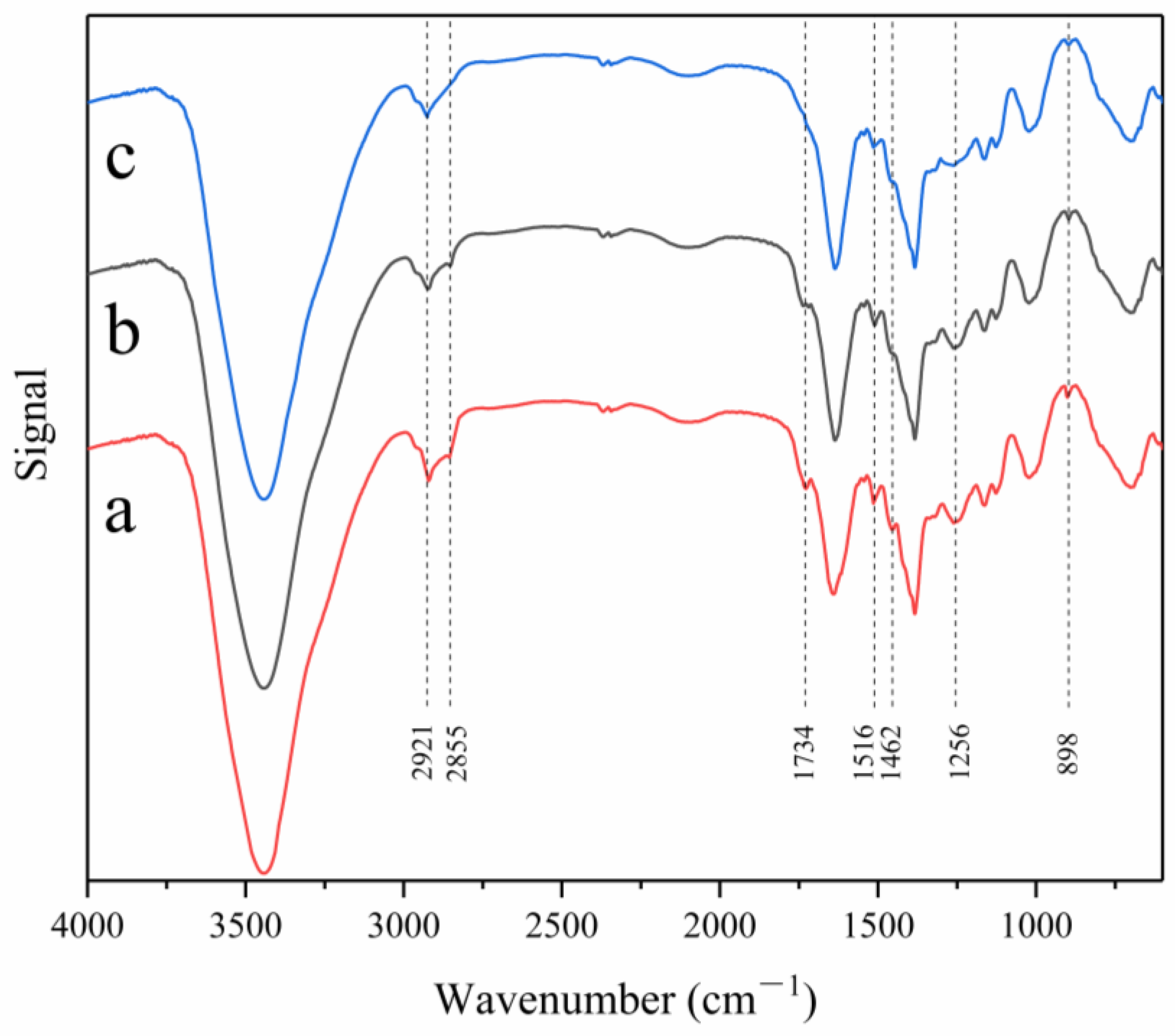
3.5. FTIR Spectral Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmud, S.; Hasan, K.M.F.; Jahid, A.; Mohiuddin, K.; Zhang, R.; Zhu, J. Comprehensive review on plant fiber-reinforced polymeric biocomposites. J. Mater. Sci. 2021, 56, 7231–7264. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, X.; Sun, T.; Chu, J. Experimental study of plant fiber-polymer composite for indirect evaporative cooler application. Appl. Therm. Eng. 2021, 199, 117543. [Google Scholar] [CrossRef]
- Sampath, B.; Naveenkumar, N.; Sampathkumar, P.; Silambarasan, P.; Venkadesh, A.; Sakthivel, M. Experimental comparative study of banana fiber composite with glass fiber composite material using Taguchi method. Mater. Today Proc. 2022, 49, 1475–1480. [Google Scholar] [CrossRef]
- Paul, S.A.; Boudenne, A.; Ibos, L.; Candau, Y.; Joseph, K.; Thomas, S. Effect of fiber loading and chemical treatments on thermophysical properties of banana fiber/polypropylene commingled composite materials. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1582–1588. [Google Scholar] [CrossRef]
- Sakthivel, M.; Ramesh, S. Mechanical properties of natural fibre (banana, coir, sisal) polymer composites. Sci. Park. 2013, 1, 1–6. [Google Scholar]
- Alzate Acevedo, S.; Díaz Carrillo, Á.J.; Flórez-López, E.; Grande-Tovar, C.D. Recovery of banana waste-loss from production and processing: A contribution to a circular economy. Molecules 2021, 26, 5282. [Google Scholar] [CrossRef]
- Maache, M.; Bezazi, A.; Amroune, S.; Scarpa, F.; Dufresne, A. Characterization of a novel natural cellulosic fiber from Juncus effusus L. Carbohydr. Polym. 2017, 171, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Akatwijuka, O.; Gepreel, M.A.; Abdel-Mawgood, A.; Yamamoto, M.; Saito, Y.; Hassanin, A.H. Overview of banana cellulosic fibers: Agro-biomass potential, fiber extraction, properties, and sustainable applications. Biomass Convers. Biorefinery 2022, 1–17. [Google Scholar] [CrossRef]
- Vadivel, K.; Vijayakumar, A.; Solomon, S.; Santhoshkumar, R.A. A review paper on design and fabrication of banana fiber extraction machine and evaluation of banana fiber properties. Int. J. Adv. Res. Electr. Electron. Instrum. Eng. 2017, 63. [Google Scholar] [CrossRef]
- Oreko, B.U.; Okiy, S.; Emagbetere, E.; Okwu, M. Design and development of plantain fibre extraction machine. Niger. J. Technol. 2018, 37, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Kumari, S.; Surah, S.S.; Rai, B.; Kumar, R.; Sirohi, S.; Kumar, G. A simple approach for the isolation of cellulose nanofibers from banana fibers. Mater. Res. Express 2019, 6, 105601. [Google Scholar] [CrossRef]
- Bekraoui, N.; El Qoubaa, Z.; Chouiyakh, H.; Faqir, M.; Essadiqi, E. Banana Fiber Extraction and Surface Characterization of Hybrid Banana Reinforced Composite. J. Nat. Fibers 2022, 1–14. [Google Scholar] [CrossRef]
- Twebaze, C.; Zhang, M.; Zhuang, X.; Kimani, M.; Zheng, G.; Wang, Z. Banana Fiber Degumming by Alkali Treatment and Ultrasonic Methods. J. Nat. Fibers 2022, 1–13. [Google Scholar] [CrossRef]
- Chauhan, S.; Sharma, A.K. Utilization of pectinases for fiber extraction from banana plant’s waste. Int. J. Waste Resour. 2014, 162, 2. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.L.; Tal, X.Y. Screening of banana fiber degumming bacteria and preliminary study on its enzyme producing conditions. J. Trop. Crops. 2011, 32, 71–75. [Google Scholar]
- Cai, Y.; Wei, S.Y. Screening of Biodegumming Strain of Banana Pseudostem and Its Application Effect. J. South Agric. 2011, 42, 944–947. [Google Scholar]
- Vishnu Vardhini, K.J.; Murugan, R. Effect of laccase and xylanase enzyme treatment on chemical and mechanical properties of banana fiber. J. Nat. Fibers 2017, 14, 217–227. [Google Scholar] [CrossRef]
- Kaur, A.; Varghese, L.M.; Battan, B.; Patra, A.K.; Mandhan, R.P.; Mahajan, R. Bio-degumming of banana fibers using eco-friendly crude xylano-pectinolytic enzymes. Prep. Biochem. Biotechnol. 2020, 50, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Aktar, N.; Kabir, S.M.; Honi, U.; Halim, A.; Islam, R.; Sarker, M.D.H.; Haque, M.; Alam, M.; Islam, M. Pectinolytic bacterial consortia reduce jute retting period and improve fibre quality. Sci. Rep. 2020, 10, 5174. [Google Scholar] [PubMed] [Green Version]
- Nicemol, J.; Prema, P. Novel process for the simultaneous extraction and degumming of banana fibers undersolid-state cultivation. Braz. J. Microbiol. 2008, 39, 115–121. [Google Scholar]
- Charlet, K.; Baley, C.; Morvan, C.; Jernot, J.; Gomina, M.; Bréard, J. Characteristics of Hermès flax fibres as a function of their location in the stem and properties of the derived unidirectional composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1912–1921. [Google Scholar] [CrossRef]
- Huang, X.; Sun, H.; Xie, D.; Peng, J.; Li, J.; Li, X. Microbial degumming technology and theoretical research of flax in South China II. The influence of main external factors on pectinase and flax degumming effect. China Hemp Ind. 2003, 25, 4–6. [Google Scholar] [CrossRef]
- Fernando, D.; Thygesen, A.; Meyer, A.S.; Daniel, G. Elucidating field retting mechanisms of hemp fibres for biocomposites: Effects of microbial actions and interactions on the cellular micro-morphology and ultrastructure of hemp stems and bast fibres. BioResources 2019, 14, 4047–4084. [Google Scholar] [CrossRef]
- Djemiel, C.; Goulas, E.; Badalato, N.; Chabbert, B.; Hawkins, S.; Grec, S. Targeted metagenomics of retting in flax: The beginning of the quest to harness the secret powers of the microbiota. Front. Genet. 2020, 11, 581664. [Google Scholar] [CrossRef]
- Karaduman, Y.; Gokcan, D.; Onal, L. Effect of enzymatic pretreatment on the mechanical properties of jute fiber-reinforced polyester. J. Compos. Mater. 2013, 47, 1293–1302. [Google Scholar] [CrossRef]
- Balda, S.; Sharma, A.; Capalash, N.; Sharma, P. Banana fibre: A natural and sustainable bioresource for eco-friendly applications. Clean Technol. Environ. Policy 2021, 23, 1389–1401. [Google Scholar] [CrossRef]
- Duval, A.; Bourmaud, A.; Augier, L.; Baley, C. Influence of the sampling area of the stem on the mechanical properties of hemp fibers. Mater. Lett. 2011, 65, 797–800. [Google Scholar] [CrossRef]
- Abdollahzadeh, R.; Pazhang, M.; Najavand, S.; Fallahzadeh-Mamaghani, V.; Amani-Ghadim, A.R. Screening of pectinase-producing bacteria from farmlands and optimization of enzyme production from selected strain by RSM. Folia Microbiol. 2020, 65, 705–719. [Google Scholar] [CrossRef]
- Mahjoub, R.; Yatim, J.M.; Sam, A.R.M.; Hashemi, S.H. Tensile properties of kenaf fiber due to various conditions of chemical fiber surface modifications. Constr. Build. Mater. 2014, 55, 103–113. [Google Scholar] [CrossRef]
- Charlet, K.; Eve, S.; Jernot, J.; Gomina, M.; Bréard, J. Tensile deformation of a flax fiber. Procedia Eng. 2009, 1, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.Q.; Wang, Y.; Xiao, Q. Effects of hemicellulose removal on cellulose fiber structure and recycling characteristics of eucalyptus pulp. Bioresour. Technol. 2010, 101, 4577–4583. [Google Scholar] [CrossRef] [PubMed]
- Samrat, M.; Raul, F.; Yusuf, A.; Ulku, S. Banana fibers–Variability and fracture behaviour. J. Eng. Fibers Fabr. 2008, 3, 39–45. [Google Scholar]
- Marrot, L.; Lefeuvre, A.; Pontoire, B.; Bourmaud, A.; Baley, C. Analysis of the hemp fiber mechanical properties and their scattering (Fedora 17). Ind. Crops Prod. 2013, 51, 317–327. [Google Scholar] [CrossRef]
- Banik, N.; Dey, V.; Sastry, G.R.K. An overview of lignin & hemicellulose effect upon biodegradable bamboo fiber composites due to moisture. Mater. Today Proc. 2017, 4, 3222–3232. [Google Scholar]
- Chen, Y.; Su, N.; Zhang, K.; Zhu, S.; Zhu, Z.; Qin, W.; Guo, Y. Effect of fiber surface treatment on structure, moisture absorption and mechanical properties of luffa sponge fiber bundles. Ind. Crop. Prod. 2018, 123. [Google Scholar] [CrossRef]
- Dey, P.; Mahapatra, B.; Pramanick, B.; Kumar, A.; Negi, M.; Paul, J.; Shukla, D.; Singh, S. Quality optimization of flax fibre through durational management of water retting technology under sub-tropical climate. Ind. Crop. Prod. 2021, 162, 113277. [Google Scholar] [CrossRef]
- Rodríguez, L.J.; Álvarez-Láinez, M.L.; Orrego, C.E. Optimization of processing conditions and mechanical properties of banana fiber-reinforced polylactic acid/high-density polyethylene biocomposites. J. Appl. Polym. Sci. 2022, 139, 51501. [Google Scholar] [CrossRef]
- Jonoobi, M.; Khazaeian, A.; Tahir, P.; Azry, S.S.; Oksman, K. Characteristics of cellulose nanofibers isolated from rubberwood and empty fruit bunches of oil palm using chemo-mechanical process. Cellulose 2011, 18, 1085–1095. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Vázquez, A. Influence of fiber chemical modification procedure on the mechanical properties and water absorption of MaterBi-Y/sisal fiber composites. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1672–1680. [Google Scholar] [CrossRef]
- Sun, X.-F.; Xu, F.; Sun, R.C.; Fowler, P.; Baird, M.S. Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr. Res. 2005, 340, 97–106. [Google Scholar] [CrossRef]
- Belouadah, Z.; Ati, A.; Rokbi, M. Characterization of new natural cellulosic fiber from Lygeum spartum L. Carbohydr. Polym. 2015, 134, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Sgriccia, N.; Hawley, M.; Misra, M. Characterization of natural fiber surfaces and natural fiber composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1632–1637. [Google Scholar] [CrossRef]
- Punyamurthy, R.; Sampathkumar, D.; Srinivasa, C.V.; Bennehalli, B. Effect of alkali treatment on water absorption of single cellulosic abaca fibre. Bioresources 2012, 7, 3515–3524. [Google Scholar]
- Alhayat, G.T.; Sahu, O. Process ability enhancement of false banana fibre for rural development. J. Agric. Econ. Ext. Rural. Dev. 2014, 1, 64–73. [Google Scholar]



| Position | AI | BI | CI | ||||
|---|---|---|---|---|---|---|---|
| Nature | Regain | REGAIN—Standard Deviation | Regain | Regain—Standard Deviation | Regain | Regain—Standard Deviation | |
| Time (Day) | |||||||
| 5 | 15.92 | 0.64 | 15.25 | 0.61 | 14.28 | 0.57 | |
| 7 | 15.84 | 0.64 | 15.30 | 0.60 | 14.42 | 0.58 | |
| 9 | 16.07 | 0.65 | 15.28 | 0.61 | 14.31 | 0.58 | |
| 11 | 15.84 | 0.64 | 15.14 | 0.60 | 14.25 | 0.57 | |
| 13 | 15.72 | 0.63 | 14.87 | 0.59 | 14.09 | 0.55 | |
| 15 | 15.44 | 0.63 | 14.56 | 0.58 | 14.15 | 0.57 | |
| 17 | 15.28 | 0.61 | 14.26 | 0.58 | 14.14 | 0.57 | |
| 19 | 14.80 | 0.60 | 13.91 | 0.56 | 13.86 | 0.55 | |
| 21 | 14.58 | 0.59 | 13.61 | 0.55 | 13.66 | 0.56 | |
| 23 | 14.42 | 0.60 | 13.32 | 0.53 | 13.35 | 0.53 | |
| 25 | 13.93 | 0.59 | 13.01 | 0.52 | 13.04 | 0.52 | |
| 27 | 13.63 | 0.58 | 13.00 | 0.53 | 12.81 | 0.51 | |
| 29 | 13.42 | 0.58 | 13.06 | 0.52 | 12.58 | 0.49 | |
| 31 | 13.18 | 0.58 | 12.98 | 0.51 | 12.25 | 0.50 | |
| 33 | 12.85 | 0.53 | 13.04 | 0.53 | 11.99 | 0.48 | |
| 35 | 12.72 | 0.53 | 13.05 | 0.51 | 11.82 | 0.46 | |
| Position | AII | BII | CII | ||||
|---|---|---|---|---|---|---|---|
| Nature | Regain | Regain—Standard Deviation | Regain | Regain—Standard Deviation | Regain | Regain—Standard Deviation | |
| Time (Day) | |||||||
| 5 | 15.83 | 0.63 | 15.25 | 0.61 | 14.17 | 0.58 | |
| 7 | 15.76 | 0.63 | 15.30 | 0.62 | 14.30 | 0.56 | |
| 9 | 15.96 | 0.65 | 15.28 | 0.61 | 14.20 | 0.58 | |
| 11 | 15.72 | 0.63 | 15.14 | 0.60 | 14.13 | 0.55 | |
| 13 | 15.59 | 0.62 | 14.87 | 0.59 | 14.00 | 0.56 | |
| 15 | 15.31 | 0.62 | 14.56 | 0.58 | 14.01 | 0.56 | |
| 17 | 15.16 | 0.61 | 14.26 | 0.57 | 13.75 | 0.53 | |
| 19 | 14.70 | 0.59 | 13.91 | 0.57 | 13.99 | 0.57 | |
| 21 | 14.44 | 0.57 | 13.61 | 0.54 | 13.76 | 0.55 | |
| 23 | 14.22 | 0.55 | 13.32 | 0.53 | 13.73 | 0.55 | |
| 25 | 13.74 | 0.55 | 13.01 | 0.52 | 13.53 | 0.53 | |
| 27 | 13.14 | 0.53 | 13.00 | 0.51 | 13.44 | 0.54 | |
| 29 | 12.40 | 0.50 | 13.06 | 0.52 | 13.24 | 0.53 | |
| 31 | 12.07 | 0.48 | 12.98 | 0.53 | 13.23 | 0.52 | |
| 33 | 12.17 | 0.50 | 13.04 | 0.52 | 13.22 | 0.53 | |
| 35 | 12.13 | 0.50 | 13.05 | 0.51 | 13.36 | 0.53 | |
| Position | AⅢ | BⅢ | CⅢ | ||||
|---|---|---|---|---|---|---|---|
| Nature | Regain | Regain—Standard Deviation | Regain | Regain—Standard Deviation | Regain | Regain—Standard Deviation | |
| Time (Day) | |||||||
| 5 | 15.47 | 0.63 | 14.89 | 0.60 | 13.94 | 0.57 | |
| 7 | 15.51 | 0.61 | 14.91 | 0.62 | 14.02 | 0.56 | |
| 9 | 15.54 | 0.63 | 14.92 | 0.61 | 13.93 | 0.55 | |
| 11 | 15.56 | 0.62 | 14.71 | 0.59 | 13.88 | 0.56 | |
| 13 | 15.31 | 0.61 | 14.49 | 0.58 | 13.81 | 0.55 | |
| 15 | 15.09 | 0.61 | 14.12 | 0.55 | 13.71 | 0.55 | |
| 17 | 14.84 | 0.58 | 13.83 | 0.55 | 13.60 | 0.53 | |
| 19 | 14.48 | 0.58 | 13.52 | 0.56 | 13.55 | 0.55 | |
| 21 | 14.20 | 0.57 | 13.21 | 0.53 | 13.51 | 0.54 | |
| 23 | 13.81 | 0.56 | 12.92 | 0.52 | 13.38 | 0.54 | |
| 25 | 13.36 | 0.53 | 12.61 | 0.51 | 13.25 | 0.55 | |
| 27 | 12.89 | 0.51 | 12.59 | 0.50 | 13.11 | 0.51 | |
| 29 | 12.12 | 0.48 | 12.61 | 0.49 | 12.99 | 0.52 | |
| 31 | 11.85 | 0.48 | 12.62 | 0.50 | 12.92 | 0.52 | |
| 33 | 11.84 | 0.49 | 12.61 | 0.51 | 12.86 | 0.52 | |
| 35 | 11.86 | 0.46 | 12.60 | 0.50 | 12.88 | 0.50 | |
| Functional Group | Vibration Form | Wave Number (cm−1) | Source |
|---|---|---|---|
| OH | VOH | 3100–3800 | cellulose, lignin, hemicellulose |
| CH2 | VasCH | 2921–2931 | cellulose, hemicellulose |
| CH | VsCH | 2855–2895 | cellulose, hemicellulose |
| COOR | VC=O | 1750–1730 | hemicellulose |
| C=C | VasC=C | 1650–1450 | lignin |
| CH2 | δCH | 1445–1485 | lignin |
| C-OH | VC-O | 1200–1300 | hemicellulose |
| COOR | VC-O-C | 1000–1300 | lignin |
| Glucose ring | β- Glycosidic bond | 898 | cellulose |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Xia, Y.; Liang, D.; Fu, W.; Yin, C. Effect of Warm-Water Retting Pretreatment on the Physical Properties of Banana Stem and Its Fibre. Materials 2022, 15, 8462. https://doi.org/10.3390/ma15238462
Yu X, Xia Y, Liang D, Fu W, Yin C. Effect of Warm-Water Retting Pretreatment on the Physical Properties of Banana Stem and Its Fibre. Materials. 2022; 15(23):8462. https://doi.org/10.3390/ma15238462
Chicago/Turabian StyleYu, Xiangyu, Yuyang Xia, Dong Liang, Wei Fu, and Chenghai Yin. 2022. "Effect of Warm-Water Retting Pretreatment on the Physical Properties of Banana Stem and Its Fibre" Materials 15, no. 23: 8462. https://doi.org/10.3390/ma15238462
APA StyleYu, X., Xia, Y., Liang, D., Fu, W., & Yin, C. (2022). Effect of Warm-Water Retting Pretreatment on the Physical Properties of Banana Stem and Its Fibre. Materials, 15(23), 8462. https://doi.org/10.3390/ma15238462







